Comparative Analysis of PRV-1 in Atlantic Salmon and PRV-3 in Coho Salmon: Host-Specific Immune Responses and Apoptosis in Red Blood Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Overall Description
2.2. Isolation and Purification of the PRV Inoculums
2.3. Blood Sampling and Isolation of RBC
2.4. RBC Culture and Treatments
2.5. Alamar Blue Assay
2.6. Total RNA Extraction, cDNA Synthesis, and Relative Gene Expression in RBC
2.7. PRV Detection and Kinetics in Salmonid RBC
2.8. Flow Cytometry
2.9. Immunofluorescence Microscopy
2.10. Statistical Analysis
3. Results
3.1. PRV-3 and PRV-1 Genotypes Infect Salmonids RBC Ex Vivo
3.2. PRV-3 Affects Cell Metabolism but Does Not Induce Cell Lysis in the Coho Salmon RBC Ex Vivo
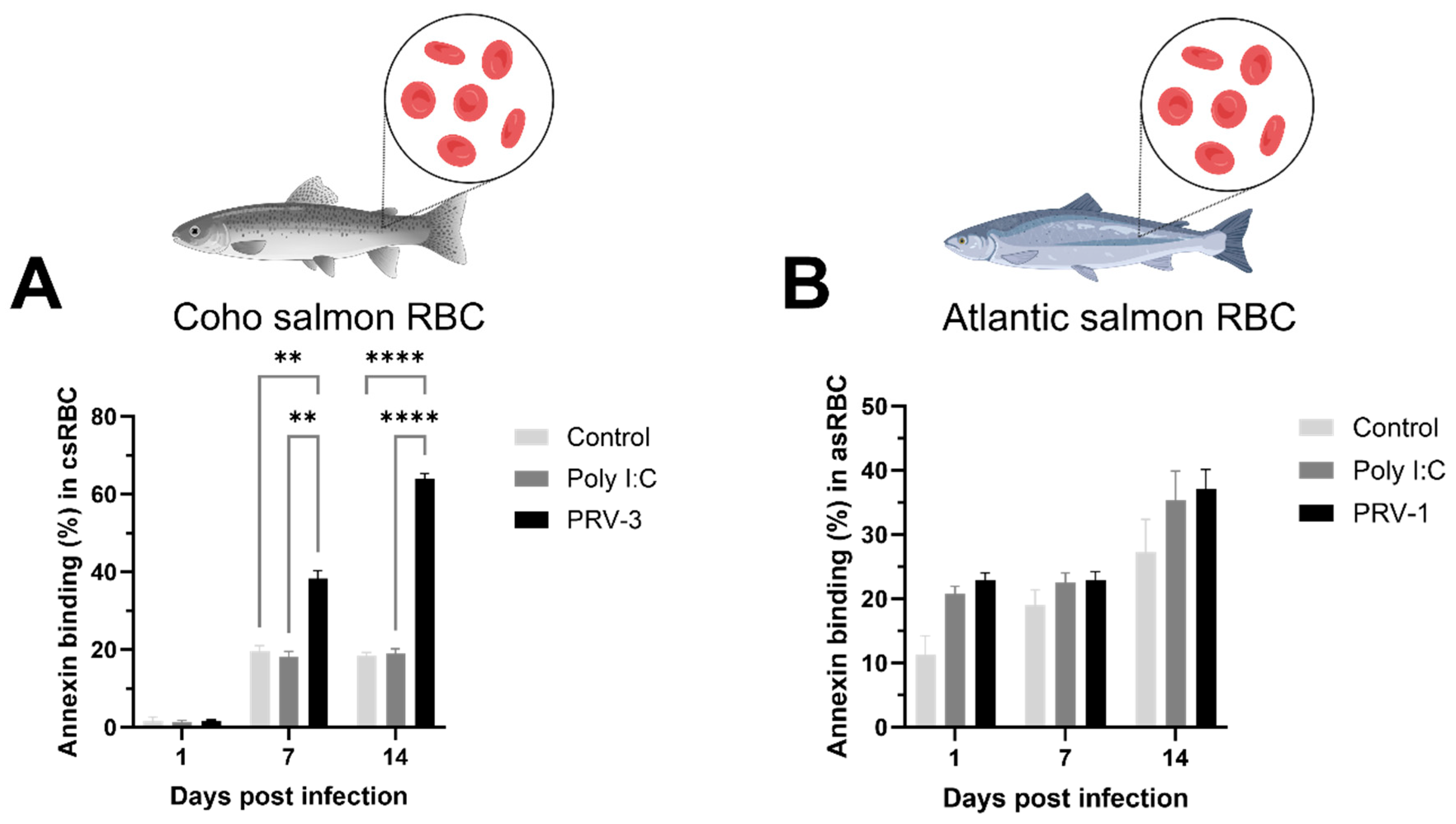
3.3. PRV-3 Infection Induces Apoptosis in Coho Salmon RBC Ex Vivo
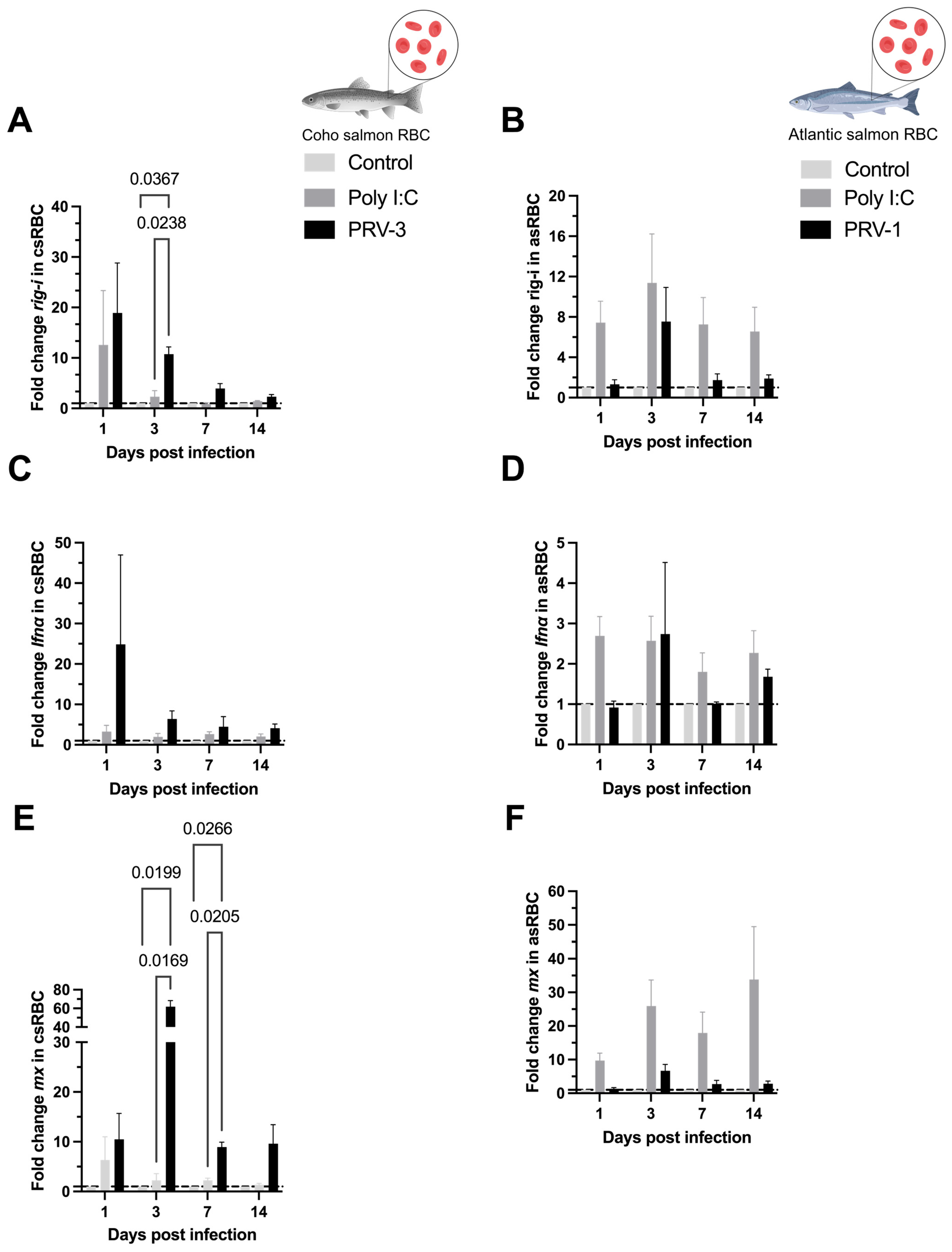
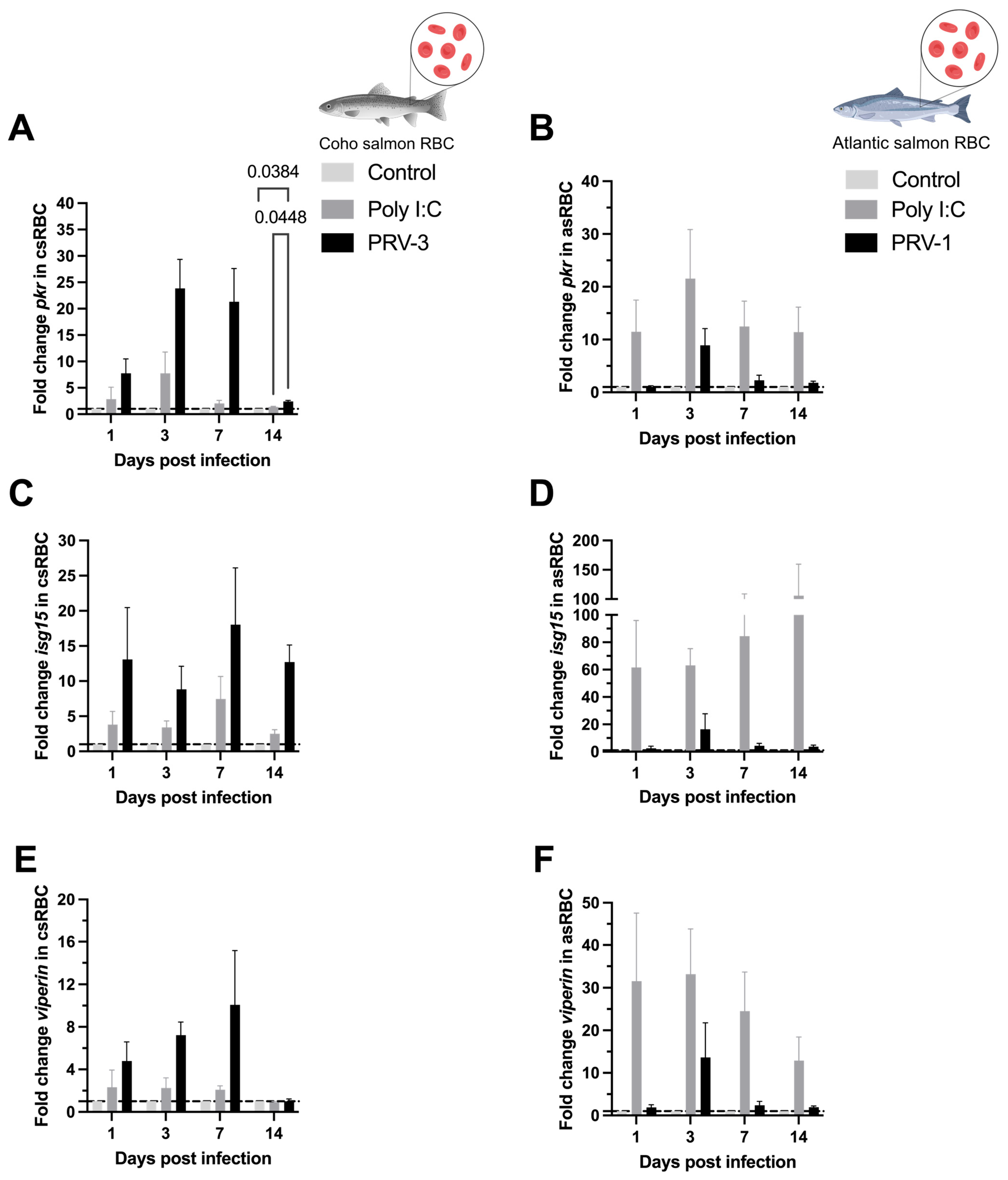
3.4. Purified PRV-3 Elicits Strong Antiviral Immune Response in Coho Salmon RBC Ex Vivo
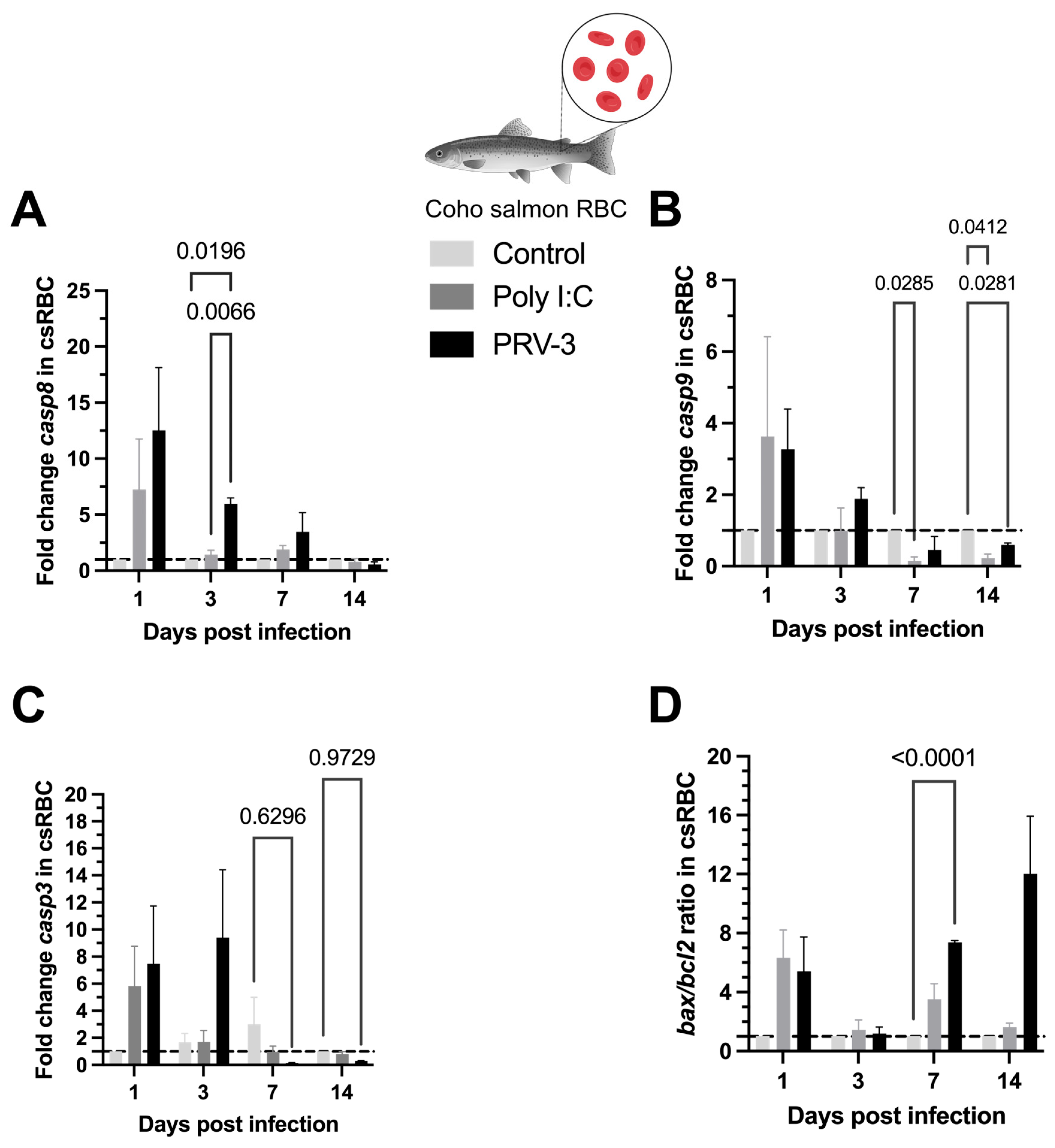
3.5. Gene Expression of Caspases and Apoptotic Regulators During PRV-3-Induced Apoptosis in Coho Salmon RBC
4. Discussion
4.1. Description of the PRV Inoculums
4.2. Replication Kinetics of PRV in RBC
4.3. Cell Viability and Apoptosis
4.4. Antiviral Immune Response in RBC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iversen, A.; Asche, F.; Hermansen, Ø.; Nystøyl, R. Production cost and competitiveness in major salmon farming countries 2003–2018. Aquaculture 2020, 522, 735089. [Google Scholar] [CrossRef]
- Senthamarai, M.D.; Rajan, M.R.; Bharathi, P.V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef]
- Flores-Kossack, C.; Montero, R.; Köllner, B.; Maisey, K. Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification. Fish Shellfish Immunol. 2020, 98, 52–67. [Google Scholar] [CrossRef]
- Valero, Y.; Cuesta, A. Reassortant viruses threatening fish aquaculture. Rev. Aquac. 2023, 15, 1720–1731. [Google Scholar] [CrossRef]
- Kannimuthu, D.; Roh, H.; Peñaranda, M.M.D.; Wessel, Ø.; Mæhle, S.; Berhe, G.D.; Nordbø, J.; Kvamme, B.O.; Morton, H.C.; Grove, S. Long-term persistence of Piscine orthoreovirus-1 (PRV-1) infection during the pre-smolt stages of Atlantic salmon in freshwater. Vet. Res. 2023, 54, 69. [Google Scholar] [CrossRef]
- Malik, M.S.; Teige, L.H.; Braaen, S.; Olsen, A.B.; Nordberg, M.; Amundsen, M.M.; Dhamotharan, K.; Svenning, S.; Edholm, E.S.; Takano, T.; et al. Piscine orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon. Vaccines 2021, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, N.; Kannimuthu, D.; Olsen, A.B.; Cuenca, A.; Teige, L.H.; Wessel, Ø.; Iburg, T.M.; Dahle, M.K.; Rimstad, E.; Olesen, N.J. Piscine orthoreovirus subtype 3 (PRV-3) causes heart inflammation in rainbow trout (Oncorhynchus mykiss). Vet. Res. 2019, 50, 14. [Google Scholar] [CrossRef]
- ICTV. Virus Taxonomy. The ICTV Report on Virus Classification and Taxon Nomenclatura. Family: Spinareoviridae Genus: Orthoreovirus. 2023. Available online: https://ictv.global/report/chapter/spinareoviridae/spinareoviridae/orthoreovirus (accessed on 31 March 2023).
- Palacios, G.; Lovoll, M.; Tengs, T.; Hornig, M.; Hutchison, S.; Hui, J.; Kongtorp, R.-T.; Savji, N.; Bussetti, A.V.; Solovyov, A.; et al. Heart and Skeletal Muscle Inflammation of Farmed Salmon Is Associated with Infection with a Novel Reovirus. PLoS ONE 2010, 5, e11487. [Google Scholar] [CrossRef]
- Godoy, M.; Medina, D.A.; Suarez, R.; Valenzuela, S.; Romero, J.; Kibenge, M.; Wang, Y.; Kibenge, F. Extensive Phylogenetic Analysis of Piscine orthoreovirus Genomic Sequences Shows the Robustness of Subgenotype Classification. Pathogens 2021, 10, 41. [Google Scholar] [CrossRef]
- Takano, T.; Nawata, A.; Sakai, T.; Matsuyama, T.; Ito, T.; Kurita, J.; Terashima, S.; Yasuike, M.; Nakamura, Y.; Fujiwara, A.; et al. Full-Genome Sequencing and Confirmation of the Causative Agent of Erythrocytic Inclusion Body Syndrome in Coho Salmon Identifies a New Type of Piscine orthoreovirus. PLoS ONE 2016, 11, e0165424. [Google Scholar] [CrossRef]
- Olsen, A.B.; Hjortaas, M.; Tengs, T.; Hellberg, H.; Johansen, R. First Description of a New Disease in Rainbow Trout (Oncorhynchus mykiss (Walbaum)) Similar to Heart and Skeletal Muscle Inflammation (HSMI) and Detection of a Gene Sequence Related to Piscine orthoreovirus (PRV). PLoS ONE 2015, 10, e0131638. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.G.; Kibenge, M.J.T.; Wang, Y.; Suarez, R.; Leiva, C.; Vallejos, F.; Kibenge, F.S.B. First description of clinical presentation of Piscine orthoreovirus (PRV) infections in salmonid aquaculture in Chile and identification of a second genotype (Genotype II) of PRV. Virol. J. 2016, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, J.; Jiménez, C.; Spencer, E. Detection of Piscine orthoreoviruses (PRV-1b AND PRV-3a) in farmed Coho salmon with jaundice syndrome from Chile. Aquaculture 2020, 528, 735480. [Google Scholar] [CrossRef]
- Solarte-Murillo, L.; Romero, A.; Cárcamo, J.G.; Godoy, M.; Morera, F.J.; Loncoman, C.A. Piscine orthoreovirus 3a detected from farmed coho salmon with jaundice syndrome displays positive selection and polymorphisms in S1 and M2 viral segment. Aquaculture 2023, 574, 739709. [Google Scholar] [CrossRef]
- Sommerset, I.; Wiik-Nielsen, J.; Moldal, T.; de Oliveira, V.H.S.; Svendsen, J.; Haukaas, A.; Brun, E. Norwegian Fish Health Report 2023. Available online: https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2024/fishhealthreport-2023 (accessed on 14 May 2025).
- SERNAPESCA. Informe Con Antecedentes Sanitarios de Agua Dulce y Mar. Servicio Nacional de Pesca y Acuicultura. Subdirección de Acuicultura Departamento de Salud Animal. Septiembre, 2024. 2024. Available online: https://www.sernapesca.cl/app/uploads/2024/09/Informe-Sanitario-ANO-2023.pdf (accessed on 14 May 2025).
- Løvoll, M.; Alarcón, M.; Jensen, B.B.; Taksdal, T.; Kristoffersen, A.; Tengs, T. Quantification of piscine reovirus (PRV) at different stages of Atlantic salmon Salmo salar production. Dis. Aquat. Org. 2012, 99, 7–12. [Google Scholar] [CrossRef]
- Kumagai, A.; Takano, T.; Matsuyama, T.; Sakai, T.; Nochiri, Y.; Honda, R.; Kikuta, T.; Honjo, M. Detection of Erythrocytic Inclusion Body Syndrome (EIBS) Virus (Piscine orthoreovirus 2) from Ovarian Fluid of Coho Salmon Survivor. Fish. Pathol. 2019, 54, 20–23. [Google Scholar] [CrossRef]
- Eckstrand, C.D.; Torrevillas, B.K.; Wolking, R.M.; Bradway, D.S.; Reno, J.L.; McMenamin-Snekvik, K.M.; Snekvik, K.R. Detection, sequencing, and tissue distribution of Piscine orthoreovirus 2–like virus in diseased coho salmon in Alaska. J. Vet. Diagn. Investig. 2024, 36, 338–345. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Ildefonso, R.; Peña, A.; Jaramillo, V.; Correa, R.; Barrientos, S.; Muñoz, A.; Maldonado, L.; Peñaloza, E. PRV-1b and PRV-3a infection is associated with the same clinical disease in coho salmon (Oncorhynchus kisutch) farmed in Chile: Unraveling the pathogenesis of the orthoreoviral cardiomyopathy and hemolytic jaundice (OCHJ). Vet. Res. 2025, 56, 17. [Google Scholar] [CrossRef]
- Pham, P.H.; Misk, E.; Papazotos, F.; Jones, G.; Polinski, M.P.; Contador, E.; Russell, S.; Garver, K.A.; Lumsden, J.S.; Bols, N.C. Screening of Fish Cell Lines for Piscine orthoreovirus-1 (PRV-1) Amplification: Identification of the Non-Supportive PRV-1 Invitrome. Pathogens 2020, 9, 833. [Google Scholar] [CrossRef]
- Polinski, M.P.; Marty, G.D.; Snyman, H.N.; Garver, K.A. Piscine orthoreovirus demonstrates high infectivity but low virulence in Atlantic salmon of Pacific Canada. Sci. Rep. 2019, 9, 3297. [Google Scholar] [CrossRef]
- Wessel, Ø.; Olsen, C.; Rimstad, E.; Dahle, M. Piscine orthoreovirus (PRV) replicates in Atlantic salmon (Salmo salar L.) erythrocytes ex vivo. Vet. Res. 2015, 46, 26. [Google Scholar] [CrossRef]
- Puente-Marin, S.; Thwaite, R.; Mercado, L.; Coll, J.; Roher, N.; Ortega-Villaizan, M.D.M. Fish Red Blood Cells Modulate Immune Genes in Response to Bacterial Inclusion Bodies Made of TNFα and a G-VHSV Fragment. Front. Immunol. 2019, 10, 1055. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Z.; Li, F.; Shi, F.; Zhan, F.; Zhao, L.; Li, Y.; Li, J.; Lin, L.; Qin, Z. Escherichia coli induced ferroptosis in red blood cells of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2021, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G.; De Haro, N.Á.; Fernando, A.J.; Desbois, A.P.; Robb, C.T.; Rossi, A.G. Fish Erythrocyte Extracellular Traps (FEETs) are an evolutionarily conserved cellular process triggered by different stimuli. Fish Shellfish Immunol. 2023, 136, 108638. [Google Scholar] [CrossRef]
- Nombela, I.; Requena-Platek, R.; Morales-Lange, B.; Chico, V.; Puente-Marin, S.; Ciordia, S.; Mena, M.; Coll, J.; Perez, L.; Mercado, L.; et al. Rainbow Trout Red Blood Cells Exposed to Viral Hemorrhagic Septicemia Virus Up-Regulate Antigen-Processing Mechanisms and MHC I&II, CD86, and CD83 Antigen-presenting Cell Markers. Cells 2019, 8, 386. [Google Scholar] [CrossRef]
- Haatveit, H.; Wessel, Ø.; Markussen, T.; Lund, M.; Thiede, B.; Nyman, I.; Braaen, S.; Dahle, M.; Rimstad, E. Viral Protein Kinetics of Piscine orthoreovirus Infection in Atlantic Salmon Blood Cells. Viruses 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, E.; Ferguson, H.W.; Kaukinen, K.H.; Schulze, A.D.; Li, S.; Tabata, A.; Günther, O.P.; Mordecai, G.; Suttle, C.A.; Miller, K.M. The same strain of Piscine orthoreovirus (PRV-1) is involved in the development of different, but related, diseases in Atlantic and Pacific Salmon in British Columbia. FACETS 2018, 3, 599–641. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Røsæg, M.V.; Lund, M.; Nyman, I.B.; Markussen, T.; Aspehaug, V.; Sindre, H.; Dahle, M.K.; Rimstad, E. Immunological interactions between Piscine orthoreovirus and Salmonid alphavirus infections in Atlantic salmon. Fish Shellfish Immunol. 2017, 64, 308–319. [Google Scholar] [CrossRef]
- Li, H.; Jiang, W.; Liu, Y.; Jiang, J.; Zhang, Y.; Wu, P.; Zhao, J.; Duan, X.; Zhou, X.; Feng, L. The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting mitochondria and calcium ion involved pathways in fish erythrocytes. Free Radic. Biol. Med. 2016, 92, 126–140. [Google Scholar] [CrossRef]
- FlowJoTM Software (for Windows), version 10.8; Becton, Dickinson and Company: Ashland, OR, USA, 2023.
- Dhamotharan, K.; Tengs, T.; Wessel, Ø.; Braaen, S.; Nyman, I.B.; Hansen, E.F.; Christiansen, D.H.; Dahle, M.K.; Rimstad, E.; Markussen, T. Evolution of the Piscine orthoreovirus Genome Linked to Emergence of Heart and Skeletal Muscle Inflammation in Farmed Atlantic Salmon (Salmo salar). Viruses 2019, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.; Parker, C.J.; Prchal, J.T. How Do Red Blood Cells Die? Front. Physiol. 2021, 12, 655393. [Google Scholar] [CrossRef]
- Chico, V.; Puente-Marin, S.; Nombela, I.; Ciordia, S.; Mena, M.; Carracedo, B.; Villena, A.; Mercado, L.; Coll, J.; Ortega-Villaizan, M. Shape-Shifted Red Blood Cells: A Novel Red Blood Cell Stage? Cells 2018, 7, 31. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, M.; Zhang, K.; Zhan, F.; Li, F.; Shi, F.; Li, Y.; Zhao, L.; Li, J.; Lin, L.; et al. Aeromonas hydrophila infection activates death receptor apoptosis pathway in the red blood cells of grass carp (Ctenopharyngodon idellus). Aquaculture 2021, 532, 735956. [Google Scholar] [CrossRef]
- Key, T.; Read, J.; Nibert, M.L.; Duncan, R. Piscine reovirus encodes a cytotoxic, non-fusogenic, integral membrane protein and previously unrecognized virion outer-capsid proteins. J. General. Virol. 2013, 94, 1039–1050. [Google Scholar] [CrossRef]
- Joseph, T.; Cepica, A.; Brown, L.; Ikede, B.O.; Kibenge, F.S.B. Mechanism of cell death during infectious salmon anemia virus infection is cell type-specific. J. Gen. Virol. 2004, 85, 3027–3036. [Google Scholar] [CrossRef]
- Dopazo, C.P. The Infectious Pancreatic Necrosis Virus (IPNV) and its Virulence Determinants: What is Known and What Should be Known. Pathogens 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Hou, Z.-S.; Zhao, H.-K.; Xin, Y.-R.; Liu, M.-Q.; Yang, X.-D.; Wen, H.-S.; Li, J.-F. Identification and characterization of caspases genes in rainbow trout (Oncorhynchus mykiss) and their expression profiles after Aeromonas salmonicida and Vibrio anguillarum infection. Dev. Comp. Immunol. 2021, 118, 103987. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Li, S.; Zheng, G.; Zou, S. Effects of hypoxia on the gill morphological structure, apoptosis and hypoxia-related gene expression in blunt snout bream (Megalobrama amblycephala). Fish Physiol. Biochem. 2023, 49, 939–949. [Google Scholar] [CrossRef]
- Tsoulia, T.; Sundaram, A.Y.M.; Braaen, S.; Jørgensen, J.B.; Rimstad, E.; Wessel, Ø.; Dahle, M.K. Transcriptomics of early responses to purified Piscine orthoreovirus-1 in Atlantic salmon (Salmo salar L.) red blood cells compared to non-susceptible cell lines. Front. Immunol. 2024, 15, 1359552. [Google Scholar] [CrossRef]
- Ortega-Villaizan, D.; Chico, V.; Perez, L. Fish Innate Immune Response to Viral Infection—An Overview of Five Major Antiviral Genes. Viruses 2022, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.; Chaves-Pozo, E. Antigen Presentation and Autophagy in Teleost Adaptive Immunity. Int. J. Mol. Sci. 2022, 23, 4899. [Google Scholar] [CrossRef]
- Jung, M.-H.; Chico, V.; Ciordia, S.; Mena, M.C.; Jung, S.-J.; Ortega-Villaizan, M.D.M. The Megalocytivirus RBIV Induces Apoptosis and MHC Class I Presentation in Rock Bream (Oplegnathus fasciatus) Red Blood Cells. Front. Immunol. 2019, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, J.; Tambley, C.; Sandino, A.M.; Spencer, E.; Tello, M. Detection of Piscine orthoreovirus in farmed rainbow trout from Chile. Aquaculture 2018, 493, 79–84. [Google Scholar] [CrossRef]
- Garseth, Å.H.; Moldal, T.; Gåsnes, S.K.; Hjortaas, M.J.; Sollien, V.P.; Gjevre, A.-G. Piscine orthoreovirus-3 is prevalent in wild seatrout (Salmo trutta L.) in Norway. J. Fish Dis. 2019, 42, 391–396. [Google Scholar] [CrossRef]
- Sørensen, J.; Vendramin, N.; Priess, C.; Kannimuthu, D.; Henriksen, N.H.; Iburg, T.M.; Olesen, N.J.; Cuenca, A. Emergence and Spread of Piscine orthoreovirus Genotype 3. Pathogens 2020, 9, 823. [Google Scholar] [CrossRef]
- Sørensen, J.; Cuenca, A.; Olsen, A.B.; Skovgaard, K.; Iburg, T.M.; Olesen, N.J.; Vendramin, N. Decreased water temperature enhance Piscine orthoreovirus genotype 3 replication and severe heart pathology in experimentally infected rainbow trout. Front. Vet. Sci. 2023, 10, 1112466. [Google Scholar] [CrossRef]
- Li, D.; Mo, R.; Li, X.; Cheng, R.; Xie, J.; Li, H.; Yang, Y.; Li, S.; Li, H.; Yan, Z.; et al. Mammalian orthoreovirus capsid protein σ3 antagonizes RLR-mediated antiviral responses by degrading MAVS. mSphere 2024, 9, e00236-24. [Google Scholar] [CrossRef]




| Gene | Sequence | Amplicon Size | Reference |
|---|---|---|---|
| Immune genes | |||
| ifnα (both experiments) | Fw: ACTGAAACGCTACTTCAAGAAGTTGA | 104 | [24] |
| Rv: GCAGATGACGTTTTGTCTCTTTCCT | |||
| rig-i (both experiments) | Fw: ACGCCTTGAAGAGCTGGATA | 89 | [24] |
| Rv: CTGGCTGGACTTGTGTCCTC | |||
| pkr (both experiments) | Fw: CAGGATGCAACACCATCATC | 162 | [24] |
| Rv: GGTCTTGACCGGTGACATCT | |||
| mx (experiment I) | Fw: GATGCTGCACCTCAAGTCCTATTA | 82 | [24] |
| Rv: CACCAGGTAGCGGATCACCAT | |||
| mx (experiment II) | Fw: GGTGATAGGGGACCAGAGT | 173 | [32] |
| Rv: CTCCTCACGGTCTTGGTAGC | |||
| isg15 (experiment I) | Fw: GGCCTGCATTCAGGATCTAA | 120 | [29] |
| Rv: TACAGTCTCACCAGGCACCA | |||
| isg15 (experiment II) | Fw: ATATCTACTGAACATATATCTATCATGGAACTC | 150 | [32] |
| Rv: CCTCTGCTTTGTTGTGGCCACTT | |||
| viperin (both experiments) | Fw: AGCAATGGCAGCATGATCAG | 101 | [6] |
| Rv: TGGTTGGTGTCCTCGTCAAAG | |||
| mhc-i | Fw: CCAGAGGATGTATGGTTGTGAG | 124 | [28] |
| Rv: TGGAGCGATCCATGTCTTTGTC | |||
| ef1α (experiment II) | Fw: TGCCCCTCCAGGATGTCTAC | 57 | [18] |
| Rv: CACGGCCCACAGGTACTG | |||
| β-actinA (experiment I) | Fw: GGACTTTGAGCAGGAGATGG | 91 | This study |
| Rv: ATGATGGAGTTGTAGGTGGTC | |||
| Piscine orthoreovirus detection | |||
| PRV3-S1 (experiment I) | Fw: AATGACAGACCAGACCGACG | 115 | This study |
| Rv: CCATCCAGCCACTGAAACCA | |||
| PRV1-S1 (experiment II) | Fw: TGCGTCCTGCGTATGGCACC | 143 | [24] |
| Rv: GGCTGGCATGCCCGAATAGCA | |||
| Probe: 6FAM- ATCACAACGCCTACCT –MGBNFQ | |||
| Apoptosis genes in csRBC | |||
| casp8 | Fw: GTCAGGGGATCCAGTGTGTG | 100 | This study |
| Rv: TCTCTGCACAGGAAGCACAG | |||
| casp9 | Fw: CCAGGGCAACAGGAAGAGTT | 135 | This study |
| Rv: TGGACCCTGTGCGGTTATTC | |||
| casp3 | Fw: GGATGAGTTGGGCCAGTGAA | 99 | This study |
| Rv: TTGATGTGTGCAGAGGGTCC | |||
| bax | Fw: TGGCCTTCTACATAGTGTTCTTTC | 59 | This study |
| Rv: GTGGCTTCGTTTTGCTCGTT | |||
| bcl2 | Fw: TTCTCAGCTACCAACCCTGG | 84 | This study |
| Rv: ACGTCAGTTCCTTACCGACAC | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solarte-Murillo, L.V.; Salgado, S.; Gatica, T.; Cárcamo, J.G.; Tsoulia, T.; Dahle, M.K.; Loncoman, C. Comparative Analysis of PRV-1 in Atlantic Salmon and PRV-3 in Coho Salmon: Host-Specific Immune Responses and Apoptosis in Red Blood Cells. Microorganisms 2025, 13, 1167. https://doi.org/10.3390/microorganisms13051167
Solarte-Murillo LV, Salgado S, Gatica T, Cárcamo JG, Tsoulia T, Dahle MK, Loncoman C. Comparative Analysis of PRV-1 in Atlantic Salmon and PRV-3 in Coho Salmon: Host-Specific Immune Responses and Apoptosis in Red Blood Cells. Microorganisms. 2025; 13(5):1167. https://doi.org/10.3390/microorganisms13051167
Chicago/Turabian StyleSolarte-Murillo, Laura V., Sebastián Salgado, Tomás Gatica, Juan Guillermo Cárcamo, Thomais Tsoulia, Maria K. Dahle, and Carlos Loncoman. 2025. "Comparative Analysis of PRV-1 in Atlantic Salmon and PRV-3 in Coho Salmon: Host-Specific Immune Responses and Apoptosis in Red Blood Cells" Microorganisms 13, no. 5: 1167. https://doi.org/10.3390/microorganisms13051167
APA StyleSolarte-Murillo, L. V., Salgado, S., Gatica, T., Cárcamo, J. G., Tsoulia, T., Dahle, M. K., & Loncoman, C. (2025). Comparative Analysis of PRV-1 in Atlantic Salmon and PRV-3 in Coho Salmon: Host-Specific Immune Responses and Apoptosis in Red Blood Cells. Microorganisms, 13(5), 1167. https://doi.org/10.3390/microorganisms13051167









