Abstract
The global emergence of carbapenem-resistant Acinetobacter baumanii (CRAB) represents a significant public health threat. In the summer of 2022, a polyclonal CRAB outbreak occurred in our hospital, marking the first detection of an NDM-1 plus OXA-23 co-producing A. baumannii strain in Spain. The aim of this study was to phenotypically and genotypically characterize the clonal spread of NDM-1 and OXA-23 co-producing A. baumannii isolates and to describe the infection control measures implemented to contain the outbreak. Patients with multidrug-resistant A. baumannii isolates (July 2022–May 2023) were included in the study. Isolates were identified via MALDI-TOF, and antimicrobial susceptibility was tested using a broth microdilution method (DKMGN SensititreTM panels). Whole-genome sequencing was performed on 24 representative isolates. Phylogenetic analysis was performed using Ridom SeqSphere+ (cgMLST), while sequence typing was performed using ARIBA (Pasteur and Oxford schemes). A. baumannii isolates from the affected patients belonged to five different sequence types. The two main STs were ST1Pas/ST231Oxf (NDM-1- and OXA-23-co-producing), which accounted for 58%, and ST136Pas/ST406Oxf (OXA-23-producing), which accounted for 21%. All isolates were resistant to fluoroquinolones, trimethoprim/sulfamethoxazole, aminoglycosides, and carbapenems. In addition, 8% were resistant to colistin and 17% to cefiderocol. Finally, the affected patients were cohorted, and a thorough cleaning of the affected units was carried out. This study documents the clonal spread of an NDM-1- and OXA-23-co-producing A. baumannii strain in Spain, linked to a Libyan patient, highlighting the risk of cross-border spread. Although infection control measures successfully contained the outbreak, surveillance is essential as the incidence of CRAB infections is expected to increase.
1. Introduction
Antimicrobial resistance is emerging as one of the most important threats to global public health, threatening the effective treatment of an increasing number of bacterial infections in various healthcare settings [1]. Multidrug-resistant Acinetobacter baumannii (MDRAB) has been identified as one of the most important pathogens, with high rates of resistance to multiple classes of antibiotics [2]. Recent genomic and phenotypic analyses of A. baumannii have identified several virulence factors responsible for its pathogenicity, including porins, capsular polysaccharides, lipopolysaccharides, phospholipases, outer membrane vesicles, metal acquisition systems, and protein secretion systems [3,4]
The rapid emergence of MDRAB has led to a worrying situation worldwide. MDRAB is an opportunist pathogen that, in recent decades, has emerged as one of the main causes of nosocomial infection, mainly affecting patients admitted to intensive care units (ICUs) and burn units.
A multinational study of ICUs revealed that the prevalence of MDRAB was 14.8% in Africa, 5.6% in Western Europe, 3.7% in North America, 13.8% in Central and South America, 17.1% in Eastern Europe, 4.4% in Oceania, and 19.2% in Asia [5]. MDRAB can be resistant to all currently available antibiotics, limiting treatment options and resulting in prolonged hospital stays, excess morbidity and mortality, and a significant economic burden [6]. In this regard, the rise of carbapenem-resistant Acinetobacter baumannii (CRAB) has been highly significant, resulting in a globally widespread and alarming problem [7]. In this sense, in 2017, the World Health Organization (WHO) published its first-ever list of antibiotic-resistant “priority pathogens”, among which CRAB was categorized as a critical-priority microorganism [8].
According to the European Antimicrobial Resistance Surveillance Network (EARS-Net), the resistance to carbapenems in invasive isolates during 2021 was 39.9%, and the combined resistance to carbapenems, fluoroquinolones, and aminoglycosides was 36.8% [9]. Other non-exclusively European data sources report 68–82% of CRAB isolates from Saudi Arabia, Egypt, South Africa, Argentina, Brazil, Iran, Pakistan, and Italy [10]; 80–91% of CRAB isolates from Russia, Ukraine, and Belarus [11]; and similarly, 82% CRAB isolates in China [12].
Carbapenem resistance is mainly due to the production of carbapenemases of the OXA-type hydrolyzing class D (class β-lactamases, CHDLs, oxacillinases), the chromosomal carbapenemase OXA-51, and acquisitions such as OXA-23, OXA-24/40, and OXA-58. Less frequently, it is due to class B metallo-β-lactamases such as VIM (Verona Integron-encoded metallo-β-lactamase) and IMP (imipenemase metallo-β-lactamase) [13] or NDM-1 (New Delhi metallo-β-lactamase) [14], the latter of which has recently been noted for the worrying possibility of gene expression without any fitness cost [15]. The co-production of OXA-23 and NDM-1 is infrequent, having been initially detected in African [16,17,18,19,20,21] and Asian [22,23,24,25] countries. In Europe, its presence has only been detected in the Czech Republic and Serbia [26,27].
The main objective of this study was to conduct phenotypic and genotypic characterization of the clonal spread of NDM-1- and OXA-23-co-producing A. baumannii isolates for the first time in Spain, which was initiated in a burn unit of a secondary care hospital during a CRAB polyclonal outbreak context.
2. Materials and Methods
2.1. Study Design
This study was a retrospective cohort observational analytical study.
During the second half of 2022, an increase in the isolation of CRAB isolates with atypical antibiotic susceptibility profiles was noted at the University Hospital Getafe (HUG) in Madrid, Spain. This observation gave us the starting point to initiate the present investigation. HUG is a secondary care hospital with 510 inpatient beds, 18 intensive care beds, 6 burn care beds, and over 1000 hospital admissions per year.
All the patients infected and/or colonized by CRAB isolates between July 2022 and May 2023 were included in this study. The bacterial isolates were obtained from different samples taken from patients admitted to ICU/burn units as well as from different departments in the hospital. An infected case caused by CRAB was defined according to CDC criteria [28], and a colonized case was defined as a patient carrying CRAB without clinical evidence of infection.
A total of 75 samples (41 diagnostic and 34 colonization samples) were collected from the 24 patients affected. One representative CRAB isolate from each patient was selected for phenotypic and genotypic studies, prioritizing isolates implicated in infections according to the criteria established by the CDC, with greater clinical relevance and pathogenic potential.
2.2. Identification of Bacterial Isolates and Extraction of Carbapenemases Gene
Presumptive Acinetobacter species were isolated on MacConkey agar and sheep blood agar (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) (https://www.bd.com/es-es/products-and-solutions/products/productfamilies/dehydrated-culture-media-and-additives (accessed on 4 May 2025)).
Bacterial identification was performed using a MALDI-TOF Biotyper instrument (Bruker Daltonics GmbH, Leipzig, Germany) by comparing the unique mass spectra of bacterial proteins with those in a database. All isolates were identified with a score higher than 2, indicating more reliable identification, typically at the species level. Carbapenemase production was tested by a PCR assay (CarbaR+, Novodiag, Hologic, Marlborough, MA, USA), a platform for the multiplex qualitative detection of carbapenemases blaKPC, blaNDM, blaVIM, blaIMP, blaOXA-23, blaOXA-24, blaOXA-48/181, and blaOXA-58, and of colistin resistance gene mcr-1. Both the extraction protocol and PCR conditions were strictly in accordance with the manufacturer’s instructions. The automated software interpretation of the sample results is based on the validation of the internal extraction/inhibition control result (https://www.hologic.com/molecular-diagnostics (accessed on 4 May 2025)). For sample processing, one overnight single colony grown on a MacConkey agar plate was taken with a sterile loop and directly transferred to the eNat preservation medium provided by the manufacturer. The colony was thoroughly suspended, and the eNat tube was vortexed for about 5 s and incubated for 30 min at room temperature for DNA release. After vortexing, 600 µL of the eNat suspension was added to the cartridge that was run on the Novodiag system according to the recommendations (Mobidiag, Espoo, Finland) [29,30]. All the isolates were stored at −80 °C until used.
All the molecular results performed by PCR were confirmed by whole-genome sequencing (WGS) at the national reference laboratory (Centro Nacional de Microbiología, Instituto de Salud Carlos III).
2.3. Antibiotic Susceptibility Tests
Antibiotic susceptibility testing (AST) was performed for the selected isolates with a broth microdilution method using the DKMGN SensititreTM Gram-Negative panels (Thermo Fisher, Waltham, MA, USA), with ATCC 27853 Pseudomonas aeruginosa as the quality control strain [31]. In addition, disk diffusion assays were performed for cefiderocol (Oxoid, Thermofisher, Waltham, MA, USA). Susceptibility results for colistin were confirmed by broth microdilution with a UMIC panel (Bruker, Billerica, MA, USA).
All the susceptibility results were interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [32]. The exceptions were susceptibilities to ceftazidime, cefotaxime, and piperacillin/tazobactam, which were interpreted according to CLSI guidelines [33].
2.4. Whole-Genome Sequencing and Read Assembly
Paired-end (2 × 150) libraries were prepared using the Nextera DNA Flex Preparation Kit and sequenced using Illumina HiSeq 500 (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s instructions. Fastp (version 0.23.4) [34] was used to trim the input data that had been assessed using FASTQC (version 0.11.9) for quality (Q20 threshold), followed by de novo assembly using Unicycler (version 0.4.8) [35]. The quality of the assembly was assessed with QUAST (version 5.2.0), and Prokka v1.14 beta was used for automatic genome annotation [36].
2.5. Phylogenetic Analysis and Diversity
Ridom SeqSphere+ (version 8.3.1; Ridom, Münsten, Germany) was used to perform core genome Multi-Locus Sequence-Typing analysis (cgMLST), using a built-in scheme for A. baumannii containing 2390 core genes, and to construct a minimum spanning tree based on allelic differences. ARIBA (version 2.6.2) [37] was used to determine STs according to the Pasteur (Pas) and Oxford (Oxf) schemes [38,39].
2.6. Antibiotic Resistance Genes, Virulence-Associated Genes, and Plasmids
Antibiotic resistance genes were analyzed via ARIBA (Versión 2-6.2) using the CARD database and ResFinder, with ID thresholds of 100% for β-lactamase variants and 98% for other genes. The presence of acquired resistance genes was considered when the full length of the gene was detected with a mean read depth higher than 25.
The Virulence Finder tool was used to detect genes associated with virulence and their respective sets [40]; PlasmidID was used to map the reads against a curated plasmid database, perform de novo plasmid assemblies, and determine the presence of resistance and replicon genes [41].
The Kaptive (Version 0.0.7–2.0.0) [42] was used to study the capsule polysaccharide K locus (KL) and the outer-core OC locus (OCL virulence-associated genes) of carbapenemase-producing A. baumannii isolates.
2.7. Insertion Sequences in Carbapenem-Resistant A. baumannii
ISMapper V2.0.2.26 [43] was used to describe copy locations of ISAba1, ISAba10, and ISAba125 [44]. All query sequences were obtained from ISFinder reference sequences. These query sequences, together with paired-end Illumina reads of all isolates and the reference genome (CP010781 https://www.ncbi.nlm.nih.gov/nuccore/CP010781 (accessed on 14 May 2024)), were used as inputs for ISMapper [45].
3. Results
3.1. Patients and Description of the Outbreak
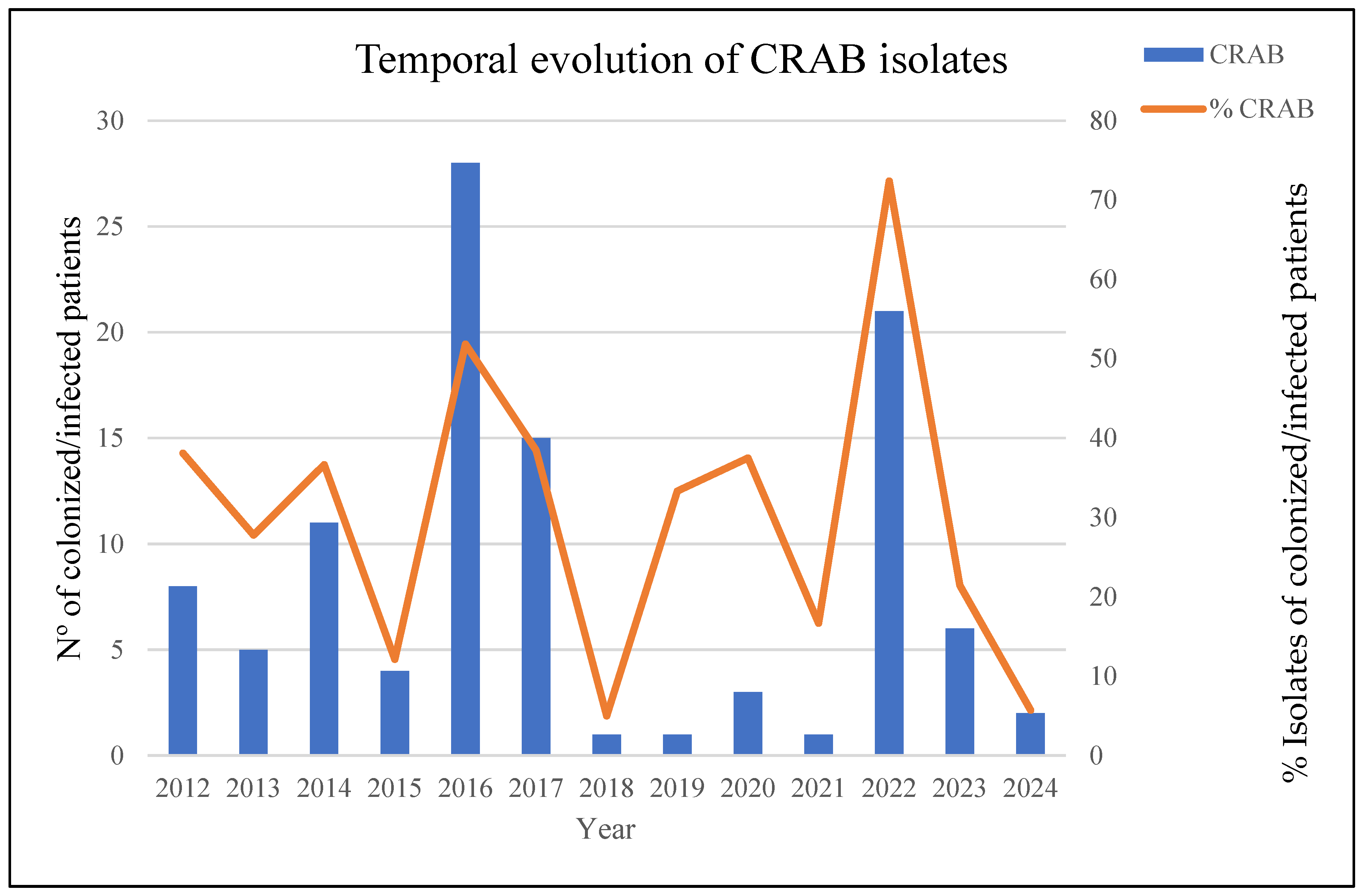
In July 2022, we detected an unusual increase in the number of cases of infection/colonization with CRAB compared with previous years (Figure 1). The initial analyzed isolates of CRAB came from the Angiology and Vascular Surgery Department, and three weeks later, new CRAB isolates, with similar antibiotic resistance profiles, were detected at the hospital’s burn unit. This detection was related to the admission of four severely burned patients as a consequence of a tanker truck explosion in Libya. Subsequently, we began to detect patients infected/colonized with MDRAB in other hospital wards.
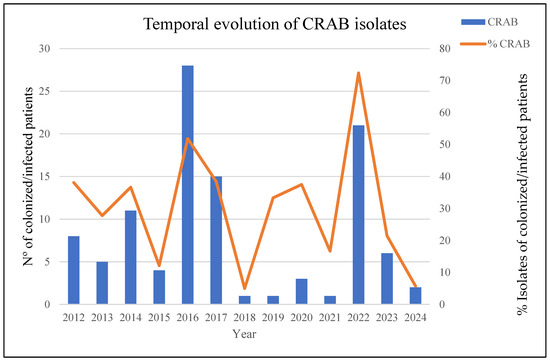
Figure 1.
The temporal evolution of CRAB-colonized/infected patients over the last 12 years: left axis—absolute number of CRAB isolates; right axis—percentage of CRAB isolates relative to total A. baumannii isolates.
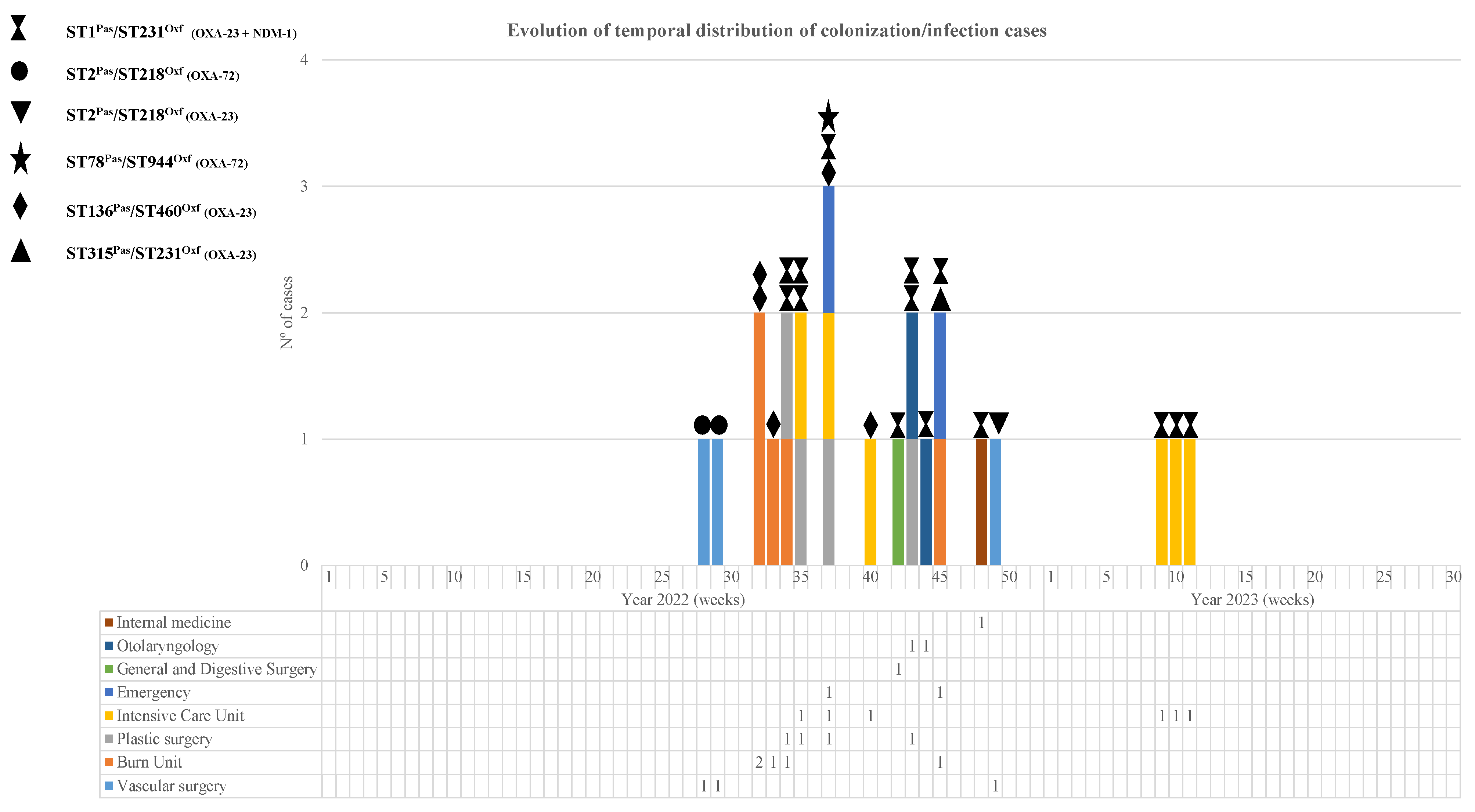
In total, the outbreak affected 24 patients, of which 75 samples were studied in the laboratory, including 41 diagnostic samples (54.6%) and 34 colonization samples (45.4%). Descriptions of the affected patients and temporal outbreak evolution are shown in Table 1 and Figure 1 and Figure 2.

Table 1.
Chronological order of representative CRAB isolates selected from each patient.
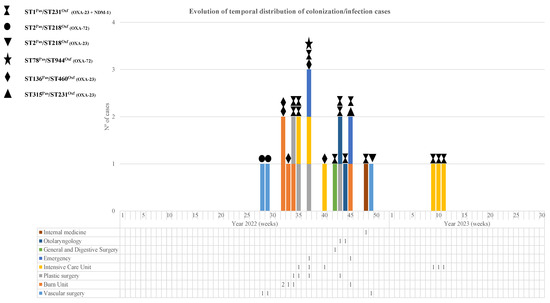
Figure 2.
Weekly temporal distribution of CRAB sequencing types (STPas/STOxf) and carbapenemase types during the study period.
3.2. Carbapenemase Types and Phylogenetic Analysis of CRAB Isolates
The carbapenemase genes detected were blaOXA-23 (21), blaNDM-1 (14), and blaOXA-72 (variant of blaOXA-24) (3); 14 isolates harbored both blaOXA-23 and blaNDM-1 (Table 2).

Table 2.
Clonal lineages (Pasteur and Oxford schemes) and β-lactamase genes were identified through sequencing experiments.
MLST analysis revealed five and six sequence types (STs) according to the Pasteur and Oxford schemes, respectively (Table 2 and Figure 2), with ST1Pas/ST231Oxf (14 isolates, 58%) and ST136Pas/ST460Oxf (5 isolates, 21%) being the most frequently occurring.
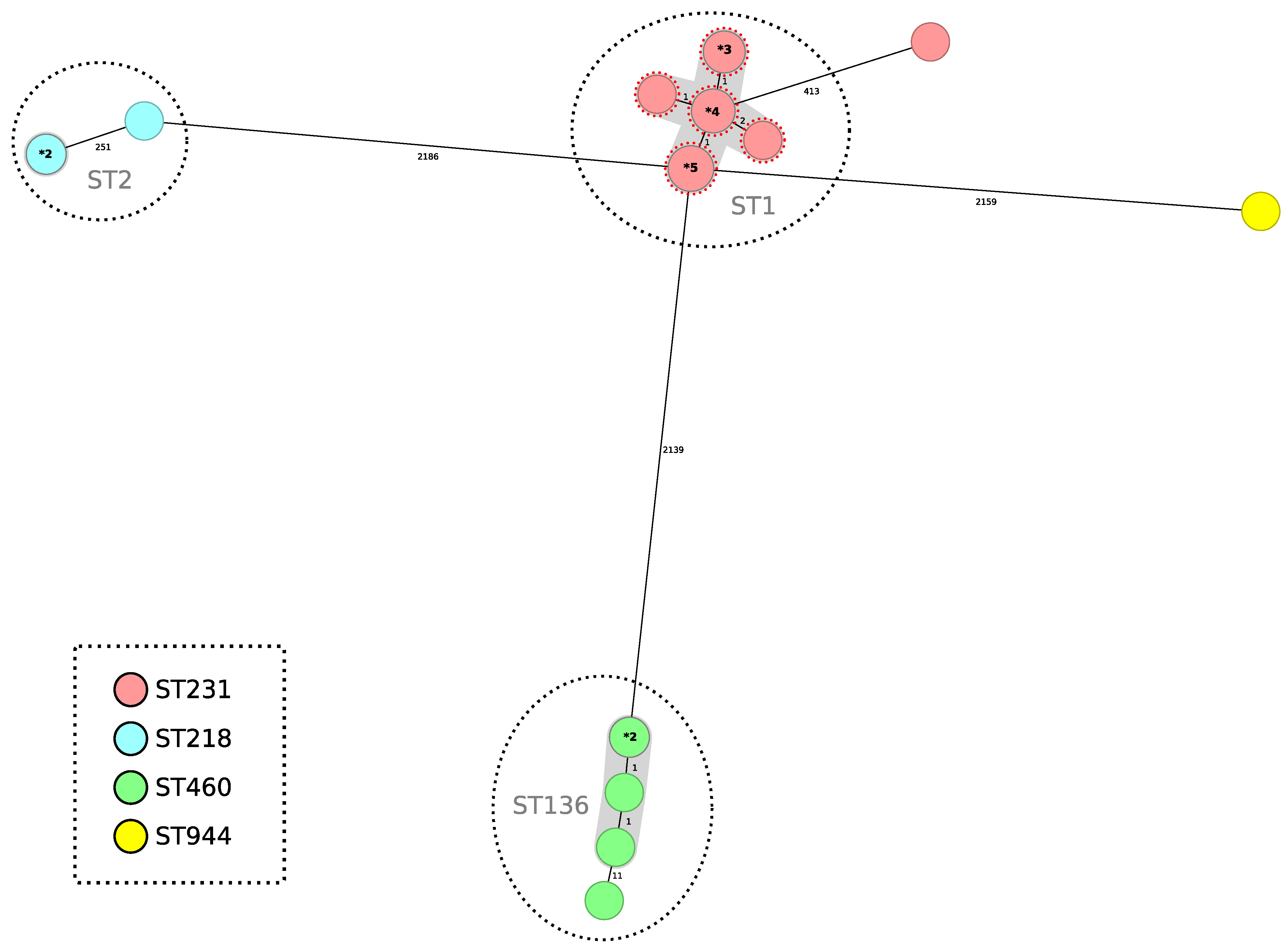
A minimum spanning tree was constructed for all 24 isolates included in this study using the gene-by-gene approach, with allelic distance calculated using cgMLST (Figure 3). Applying a relatedness threshold of five alleles, two groups with more than three related isolates were detected. The average allelic distance between pairs of isolates for both clusters was one allele (Cluster 1 ST1Pas/ST231Oxf range: 0–3; Cluster 2 ST136Pas/ST460Oxf range: 0–2). The first cluster comprised fourteen isolates producing OXA-23 and NDM-1, and the second group included five isolates producing OXA-23 (Figure 3).
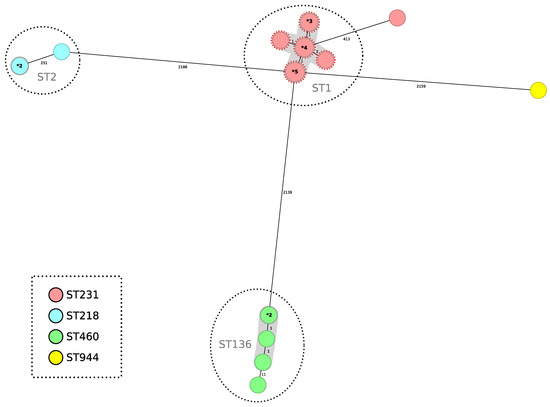
Figure 3.
Minimum spanning tree representing distances between A. baumannii genomes by applying cgMLST of 2390 genes. Colors in each circle indicate STOxf ST and gray ovals represent groups (more than two isolates) with the same STPas. A dotted red circle indicates that the strain has NDM-1. Where a circle corresponds to more than one isolate, the number of isolates is indicated in bold type preceded by an asterisk. Gray shadows represent a cluster of strains; a threshold of 5 alleles was applied.
All 14 ST1Pas/ST231Oxf OXA-23- and NDM-1-co-producing isolates had the blaOXA-69 chromosomal carbapenemase gene (a variant of blaOXA-51), while the ST136Pas/ST406Oxf isolates harbored the chromosomal variant blaOXA-409.
Finally, one blaOXA-23-producing isolate belonged to the ST2Pas/ST218Oxf. The other two isolates belonged to the ST2Pas/ST218Oxf sequence type and were blaOXA-72 producers indistinguishable by cgMLST, while the other blaOXA-72-producing isolate belonged to the ST78Pas/ST_776_SLVOxf sequence type.
When analyzing the chronological evolution of the cases (Figure 2), we observed that, before the emergence of the main clone involved in the outbreak (OXA-23/NDM-1; ST1Pas/ST231Oxf), there were already cases of carbapenemase-producing MDRAB infection belonging to the OXA-72; ST2Pas/ST218Oxf clone. This clone, detected for the first time in the Angiology and Vascular Surgery Unit, appeared 6 months later in the same unit, although this new isolate was not an OXA-72 producer but an OXA-23 producer.
During the following month, four patients from Libya were admitted to the burn unit with severe third-degree burns (burns with 40–70% involvement depending on the patient) caused by a tanker explosion. All of them required mechanical ventilation, and CRAB was detected in tracheal aspirates as well as in burn exudates and colonization samples (rectal swab and pharyngeal exudate). Three of them were colonized by CRAB-producing OXA-23 (ST136Pas/ST406Oxf) at admission to the Burns Unit, and the fourth one was colonized by CRAB, co-producing OXA-23 and NDM-1 (ST1Pas/ST231Oxf).
3.3. Antibiotic Susceptibility Testing of CRAB Isolates
One isolate from each patient involved in the outbreak was tested for AST. All the isolates were resistant to ciprofloxacin, trimethoprim/sulfamethoxazole, aminoglycosides (gentamicin, tobramycin), and carbapenems (imipenem and meropenem). Only one of them was susceptible to amikacin. Likewise, and according to CLSI susceptibility guidelines, all the isolates were resistant to cephalosporins (ceftazidime and cefotaxime) and β-lactam combinations (piperacillin/tazobactam and ampicillin/sulbactam). Overall, 8% of isolates were resistant to colistin (2 out of 24 isolates), and 17% were resistant to cefiderocol (4 out of 24 isolates) (Table 3).

Table 3.
Antibiotic susceptibility of 24 carbapenemase-producing Acinetobacter baumannii isolates as determined by the microdilution method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI).
In five patients with an initial colistin-susceptible CRAB isolate, a second colistin-resistant isolate was detected more than fifteen days later. However, after genotypic analysis, no colistin resistance genes (pmrA and pmrB) were found in these isolates, suggesting that there was a different gene. The same occurred in the case of another patient, in whom the first isolate of CRAB detected was susceptible to cefiderocol and the second isolate detected was resistant, but the iron uptake mutation system (pirA/B), which is the most commonly associated mechanism of resistance to this antibiotic, was not detected, nor was the presence of the blaNDM-1carbapenemases.
All isolates belonging to the ST136Pas/ST460Oxf were susceptible to colistin, and only two of them were resistant to cefiderocol. Likewise, the isolates belonging to ST2Pas/ST218Oxf (two carriers of blaOXA-72, the other being blaOXA-23) were susceptible to both colistin and cefiderocol. Lastly, two of the fourteen isolates belonging to the ST1Pas7ST231Oxf sequence type were resistant to colistin, while another two isolates were resistant to cefiderocol. The only amikacin-susceptible (MIC 4 mg/L) isolate belonged to ST315Pas/ST231Oxf.
3.4. Resistome and Virulome of Carbapenemases-Producing CRAB
Resistome analysis included genes associated with acquired resistance to carbapenems, aminoglycosides, sulfonamides, fluoroquinolones, and phenicols, as well as genes associated with chromosomal resistance to β-lactams (cephalosporins and carbapenems). Table S1 shows the acquired antibiotic resistance genes (ARGs) detected, where a mean of 12.2 ARGs was observed (range of 6–15, including acquired carbapenemase genes and excluding chromosomal genes and mutations).
Isolates with dual acquired carbapenemases (blaOXA-23/blaNDM-1 (n = 14)) had more ARGs (mean = 14.6; range 14–15) than isolates with one acquired carbapenemase (blaOXA-23; n = 9 and blaOXA-72; n = 1) (mean = 8; range: 6–10).
Chromosomal constitutive genes, blaOXA-69 carbapenemases and blaADC-191 betalactamases, were detected in ST1Pas/ST231Oxf CRAB isolates, while blaOXA-409 carbapenemases and blaADC-88 betalactamases were detected in ST136Pas/ST460Oxf isolates.
No acquired resistance genes, encoding resistance to fluoroquinolones, were detected. Isolates belonging to ST136Pas/ST406Oxf expressed the mutations gyrA codon 81 TCA (Ser) → TTA (Leu) and parC codon 84 TCA (Ser) → TTA (Leu), whereas in ST1Pas/ST231Oxf isolates only gyrA codon 81 TCA (Ser) → TTA (Leu) mutations were found (Table S1).
Regarding non β-lactamase ARGs, 100% of the genes analyzed were detected with aminoglycoside resistance, the most predominant being aac (3)-IIa, (100%; n = 24); ant (2``)-Ia (83.3%, n = 20) and ant (3″)-Ia (50%, n = 12). No association was found between the aminoglycoside resistance genes and the sequence types of the isolates. In addition, the 16s rRNA methylase gene armA, which confers resistance to all aminoglycosides, was present in 71% (n = 17) of the isolates, including all the isolates belonging to the ST1Pas/ST231Oxf cluster (Table S1).
Resistance to trimethoprim-sulfamethoxazole was associated with the sul1, sul2, and dfrA1 genes. All ST1Pas/ST231Oxf isolates produced sul1/sul2 and sfrA1 genes, while only the sul2 gene was detected in ST136Pas/ST406Oxf and ST2Pas/ST218Oxf isolates (Table S1).
The analysis also includes information on the role of upstream insertion sequences disrupting the outer membrane protein gene carO as an additional mechanism of resistance to carbapenems due to their nonspecific and passive diffusion properties [46]. The CRAB isolates, affected by carO disruption resulting from insertion sequences ISAba125 and ISAba10, are shown (Table S1). All the isolates belonging to ST1Pas/ST231Oxf showed disruptions in carO mediated by ISAba10, and this was also true for 10 out of 14 isolated mediated by ISAba125. No ISAba1-mediated disruption was observed in any of the isolates analyzed.
3.5. Characterization of the Virulence-Associated Genes
In the 24 genomes of A. baumannii, 17 genes associated with virulence factors were studied (Table S1). All isolates presented the 17 virulence genes, including the gene encoding OmpA, which is involved in host cell adhesion and invasion [47]; the aba1 inducer, which is involved in quorum sensing [48]; and the pgaABC locus, which is associated with polysaccharide biosynthesis and biofilm formation [49]. Virulence genes responsible for iron uptake through the production of the siderophore acinetobactin entE [50] and the csuA/B ABCDE operons were involved in pili synthesis and assembly [51].
3.6. Capsular Exopolysaccharide in CRAB Isolates
We also determined the capsular polysaccharide K locus (KL) and the outer-core OC locus (OCL) types. Six types of K locus were detected, where the main types were KL17 (n = 14, 58%) and KL25 (n = 5, 21%). All isolates with KL7 belonged to the ST1Pas/ST231Oxf sequence type. The KL25 type was expressed by isolates belonging to the ST136Pas/ST406Oxf sequence type.
The other four K locus types were KL9 (in ST2Pas/ST218Oxf isolates producers of OXA-72 carbapenemases), KL7 (in ST2Pas/ST218Oxf isolate producer of OXA-23 carbapenemases), KL91 (in ST315Pas/ST231Oxf isolate), and KL3 (in ST78Pas/ST944Oxf isolate). As for the outer-core capsule, OCL1 was the most common type of capsule (15 isolates, 63%). Fourteen out of fifteen isolates belonged to the ST1Pas/ST231Oxf sequence type, while one isolate belonged to the ST78Pas/ST944Oxf sequence type. OCL3 was detected in all the ST136Pas/ST406Oxf sequence-type isolates. The remaining isolates exhibited variable OCLs (Table S1). Both KLs and OCLs exhibited a coverage of 100% and an identity above 98%.
3.7. Detection of Plasmids in CRAB Isolates
Plasmids were detected in 21 of 24 CRAB isolates. As seen in the results, the pS32-1 plasmid was detected in 13 of 14 isolates belonging to the ST1Pas/ST231Oxf clone. Another plasmid was only detected in one of them (pA297-1 (pRAY*)). The most common plasmid replicon was R3-T1, detected in 14 isolates (13 of which belonged to clone ST1Pas/ST231Oxf). pD4 and pD72-2 plasmids were detected in ST136Pas/ST406Oxf isolates (Table S1).
3.8. Infection Control Measures and Outcome
Infection prevention and control strategies aimed at preventing the spread of these microorganisms included contact precautions, daily chlorhexidine baths, patient cohorting, environmental disinfection, and active rectal screening. In addition, we decided to look for a reservoir that could explain the rapid dissemination of microorganisms. For this purpose, surface samples were taken at critical points in the different ICU and burn boxes, but no conclusion could be reached. Finally, an effective and aggressive intervention during the last quarter of 2022, in which the patients were cohorted, was necessary, and an exhaustive cleaning of the units involved was carried out. These measures were sufficient to resolve the outbreak; no more cases of CRAB, co-producing OXA-23 and NDM-1, (ST1Pas/ST231Oxf) were detected, and the general incidence fell to pre-outbreak levels (Figure 1).
4. Discussion
In this study, we describe the clonal dissemination of the OXA-23- and NDM-1-co-producing CRAB ST1 clone in a Spanish hospital after the admission of a severely burned patient from Libya infected with this bacterium. This co-production of carbapenemases is an uncommon combination that could generate diagnostic and therapeutic challenges. There are some descriptions of NDM-1- and OXA-23-co-producing CRAB isolates in African, Asian, and European countries, but they mainly implicate sporadic isolates that have been emerging recently [16,17,18,19,20,21,22,23,24,25,26,27]. To the best of our knowledge, this is the first description of this CRAB genotype in Spain following cross-border dissemination.
The incidence of infections caused by MDRAB in HUG has been infrequent during the last 10 years, with fewer than 10 cases per year of infected/colonized patients detected (for an average of 7 cases/year). A unique significant outbreak was detected during 2016–2017, which involved 43 ICU patients (Figure 1) and was largely caused by an OXA-23/ST2Pas-ST2164Oxf cluster, unrelated to the current one; the outbreak was confined to the ICU, and no further cases were detected in the rest of the hospital.
The event described in the present study, which occurred in the second half of 2022, showed a 20-fold increase in incidence compared to the previous 4-year period, with a prevalence of MDRAB strains close to 75%. Unlike what occurred in 2016–2017, it affected multiple units. This inter-unit dissemination could have occurred as a consequence of the need for these patients to heal their burns, involving a transfer to different units. The dissemination capacity of A. baumannii among medical facilities, mainly due to its ability to persist on dry surfaces and to acquire resistance to different classes of antibiotics, is well documented [52].
It is important to highlight the importance of integrating whole-genomic sequencing into CRAB surveillance, as advised by the ECDC, and for which coordination with national reference laboratories takes on special importance [53].
Numerous studies have recently reports outbreaks caused by MDRAB with carbapenemase production in ICU and major burn units [54,55,56,57,58,59]. MDRAB is among the ten most frequently isolated microorganisms in ICU-acquired healthcare-associated infections [55]. A recent study provided a global view on CRAB, showing that the situation in Europe reflects an increase in these kinds of strains, among which the production of the metallo-beta-lactamases, although rare, is gaining some importance [60].
Regarding AST, the results indicate that NDM-1 is detected in both colistin-resistant and colistin-susceptible isolates belonging to ST1Pas/ST231Oxf, and in ST2Pas/ST218Oxf, it is detected in colistin-susceptible isolates. This finding is not in agreement with recent studies in which NDM is only detected in colistin-resistant ST1 isolates [61]. Cefiderocol is an alternative in treatment for MDRAB infections. Guidance documents from various American and European scientific societies recommend cefiderocol for treating CRAB infections. Our results show that 17% of the isolates analyzed were resistant to this antibiotic. These results are in agreement with previous studies describing the decreased efficacy of this antibiotic in MDRAB isolates [62].
We also detected OmpA protein in 100% of the isolates. It is known that this protein plays various roles related to virulence and bacteria’s survival under harsh conditions, such as adhesion, invasion, apoptosis, and antibiotic resistance. It also plays an important role in biofilm formation [63]. All isolates, except one, were resistant to the aminoglycosides gentamicin, tobramycin, and amikacin. In the amikacin-susceptible isolate (MIC ≤4 mg/L), no genes such as aph (3)-VI or 16s rRNA armA methylase gene, both associated with amikacin resistance, were detected [64,65]. The armA methylase gene was detected in all ST1Pas/ST231Oxf isolates. These findings are consistent with previous reports describing the co-occurrence of 16S rRNA methylase ArmA with blaNDM-1 and blaOXA-23 in A. baumannii clinical isolates [66].
Some results related to resistance to aminoglycosides in strains co-producing OXA-23 and NDM carbapenemases have been recently reported. This association has been observed in MDRAB clinical isolates from Egypt, although our results differ from those reported by these investigators since they describe the greater variability of high-risk clones [67]. Although not all ST1 isolates showed the same mechanism of resistance to aminoglycosides, the same mechanism of resistance to the rest of the antibiotics studied (excluding beta-lactams) was detected in all the ST1 isolates, with quinolone resistance being related to the expression of the mutation in the gyrA_S81L gene, trimethoprim-sulfamethoxazole resistance being related to the presence of sul1/sul2 and dfrA1 genes, and cmlA5 gene expression (chloramphenicol resistance) and sat2 gene expression being related to resistance to macrolides, lincosamines, and streptogramins. Regarding colistin resistance, no resistance genes to this antibiotic were detected in isolates that showed a change in colistin susceptibility. This could be due to adaptive resistance, probably because of the use of colistin to treat these patients [68].
In this case, the infection control measures carried out were sufficient to manage and control the outbreak. These measures were previously proven to be the most effective measures for permanently eliminating the spread of MDRAB [69], although we must be aware that the problem may increase in the coming years.
The cross-border dissemination of MDRAB high-risk clones may increase over time as we are witnessing a worldwide increase in the incidence of MDRAB infection, with Asia and Africa being the most affected continents [70,71,72,73]. This is likely to lead to a higher probability of spread to other countries such as those in Europe [74,75,76,77]. In this case, the index case was a patient from Libya, an African country of the Arab League, where there is a high prevalence of MDRAB infections, reaching 88% of the multiresistant isolates studied [70]. It is not only important to know the prevalence of these microorganisms in other countries, but also to take into account that there are certain events that increase frequency, such as wars that impose direct consequences such as the movement of refugees or evacuated patients from the country involved, which can lead to a change in the local ecology with the emergence of previously undetected multidrug-resistant microorganisms [78]. European structured surveys, including WGS analysis, that allow for the identification of successful clones of CRAB and the extent of their spread provide a better understanding of predominant resistance mechanisms to carbapenems and detect potential cross-border spread [53].
In conclusion, this study documents the clonal spread of an emerging NDM-1- and OXA-23-co-producing A. baumannii strain in Spain, belonging to the high-risk clone ST1 and linked to a Libyan patient. This highlights the risk of cross-border spread of multidrug-resistant microorganisms. Although infection control measures were successful in containing the outbreak, the integration of whole-genome sequencing into CRAB surveillance in coordination with national reference laboratories was essential.
It is important to maintain surveillance strategies as the incidence of CRAB infections is expected to increase in the coming years.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13051149/s1, Table S1: Clonal lineages (Pasteur and Oxford schemes), β-lactamase genes, plasmids, resistome and virulome identified through sequencing experiments.
Author Contributions
D.M.A. and J.O.-I. were responsible for the organization, coordination, and design of the study. M.P.-V., E.H., Á.I., J.E.C.-G., O.V., B.A. and J.S. were responsible for the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by CIBER—Consorcio Centro de Investigación Biomédica en Red (CIBERINFEC), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, and Unión Europea-NextGenerationEU; and by the Antibiotic Resistance Surveillance Programs of the National Center for Microbiology, Instituto de Salud Carlos III. This research was also supported by Personalized and precision medicine grant from the Instituto de Salud Carlos III (MePRAM Project, PMP22/00092) and by two additional grants from the Instituto de Salud Carlos III (PI21CIII/00039 and PI24CIII/00044).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank the Genomics Unit of the Centro Nacional de Microbiología for performing the DNA sequencing.
Conflicts of Interest
The authors declare that this research was conducted without any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDRAB | Multidrug-resistant Acinetobacter baumannii |
| ICU | intensive care unit |
| CRAB | Carbapenem-resistant Acinetobacter baumannii |
| EARS-Net | European Antimicrobial Resistance Surveillance Network |
| HUG | University Hospital Getafe |
| AST | Antibiotic susceptibility testing |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| CgMLST | Multi-Locus Sequence-Typing analysis |
| ARGs | Acquired antibiotic resistance genes |
| KL | K locus |
| OCL | OC locus |
References
- Restrepo-Arbeláez, N.; García-Betancur, J.C.; Pallares, C.J.; El Ayoubi, L.W.; Kiratisin, P.; Kanj, S.S.; Villegas, M.V. Can Risk Factors and Risk Scores Help Predict Colonization and Infection in Multidrug-Resistant Gram-Negative Bacteria? Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e196. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter Baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter Baumannii: Human Infections, Factors Contributing to Pathogenesis and Animal Models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef]
- Gautam, D.; Dolma, K.G.; Khandelwal, B.; Mitsuwan, W.; Mahboob, T.; Pereira, M.L.; Nawaz, M.; Wiart, C.; Ardebili, A.; Siyadatpanah, A.; et al. Acinetobacter Baumannii: An Overview of Emerging Multidrug-Resistant Pathogen. Med. J. Malays. 2022, 77, 357–370. [Google Scholar]
- National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Girerd-Genessay, I.; Bénet, T.; Vanhems, P. Multidrug-Resistant Bacterial Outbreaks in Burn Units: A Synthesis of the Literature According to the ORION Statement. J. Burn Care Res. 2016, 37, 172–180. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022.
- WHO. GLASS, 2019 WHO. Global Antimicrobial Resistance Surveillance System; WHO: Geneva, Switzerland, 2019.
- WHO. CAESAR, 2019. Central Asian and European Surveillance of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2019.
- WHO. CHINET, 2017. China Antimicrobial Surveillance Network; WHO: Geneva, Switzerland, 2017.
- Amudhan, M.S.; Sekar, U.; Kamalanathan, A.; Balaraman, S. Bla(IMP) and Bla(VIM) Mediated Carbapenem Resistance in Pseudomonas and Acinetobacter Species in India. J. Infect. Dev. Ctries. 2012, 6, 757–762. [Google Scholar] [CrossRef]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-β-Lactamase (NDM): A Threat to Public Health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- López, C.; Ayala, J.A.; Bonomo, R.A.; González, L.J.; Vila, A.J. Protein Determinants of Dissemination and Host Specificity of Metallo-β-Lactamases. Nat. Commun. 2019, 10, 3617. [Google Scholar] [CrossRef]
- Mathlouthi, N.; El Salabi, A.A.; Ben Jomàa-Jemili, M.; Bakour, S.; Al-Bayssari, C.; Zorgani, A.A.; Kraiema, A.; Elahmer, O.; Okdah, L.; Rolain, J.-M.; et al. Early Detection of Metallo-β-Lactamase NDM-1- and OXA-23 Carbapenemase-Producing Acinetobacter Baumannii in Libyan Hospitals. Int. J. Antimicrob. Agents 2016, 48, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Maamar, E.; Alonso, C.A.; Ferjani, S.; Jendoubi, A.; Hamzaoui, Z.; Jebri, A.; Saidani, M.; Ghedira, S.; Torres, C.; Boubaker, I.B.-B. NDM-1- and OXA-23-Producing Acinetobacter Baumannii Isolated from Intensive Care Unit Patients in Tunisia. Int. J. Antimicrob. Agents 2018, 52, 910–915. [Google Scholar] [CrossRef]
- Revathi, G.; Siu, L.K.; Lu, P.-L.; Huang, L.-Y. First Report of NDM-1-Producing Acinetobacter Baumannii in East Africa. Int. J. Infect. Dis. 2013, 17, e1255–e1258. [Google Scholar] [CrossRef]
- Uwingabiye, J.; Lemnouer, A.; Roca, I.; Alouane, T.; Frikh, M.; Belefquih, B.; Bssaibis, F.; Maleb, A.; Benlahlou, Y.; Kassouati, J.; et al. Clonal Diversity and Detection of Carbapenem Resistance Encoding Genes among Multidrug-Resistant Acinetobacter Baumannii Isolates Recovered from Patients and Environment in Two Intensive Care Units in a Moroccan Hospital. Antimicrob. Resist. Infect. Control 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ragueh, A.A.; Aboubaker, M.H.; Mohamed, S.I.; Rolain, J.-M.; Diene, S.M. Emergence of Carbapenem-Resistant Gram-Negative Isolates in Hospital Settings in Djibouti. Antibiotics 2023, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Abouelfetouh, A.; Torky, A.S.; Aboulmagd, E. Phenotypic and Genotypic Characterization of Carbapenem-Resistant Acinetobacter Baumannii Isolates from Egypt. Antimicrob. Resist. Infect. Control 2019, 8, 185. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Han, A.; Celiloglu, H.; Karam Sarkis, D. Detection of OXA-23, GES-11 and NDM-1 among Carbapenem-Resistant Acinetobacter Baumannii in Dubai: A Preliminary Study. J. Glob. Antimicrob. Resist. 2021, 24, 27–28. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Thirunarayan, M.A.; Krishnan, P. Coexistence of blaOXA-23 with blaNDM-1 and armA in Clinical Isolates of Acinetobacter Baumannii from India. J. Antimicrob. Chemother. 2010, 65, 2253–2254. [Google Scholar] [CrossRef]
- Joshi, P.R.; Acharya, M.; Kakshapati, T.; Leungtongkam, U.; Thummeepak, R.; Sitthisak, S. Co-Existence of blaOXA-23 and blaNDM-1 Genes of Acinetobacter Baumannii Isolated from Nepal: Antimicrobial Resistance and Clinical Significance. Antimicrob. Resist. Infect. Control 2017, 6, 21. [Google Scholar] [CrossRef]
- Leungtongkam, U.; Thummeepak, R.; Wongprachan, S.; Thongsuk, P.; Kitti, T.; Ketwong, K.; Runcharoen, C.; Chantratita, N.; Sitthisak, S. Dissemination of blaOXA-23, blaOXA-24, blaOXA-58, and blaNDM-1 Genes of Acinetobacter Baumannii Isolates from Four Tertiary Hospitals in Thailand. Microb. Drug Resist. 2018, 24, 55–62. [Google Scholar] [CrossRef]
- Krizova, L.; Bonnin, R.A.; Nordmann, P.; Nemec, A.; Poirel, L. Characterization of a Multidrug-Resistant Acinetobacter Baumannii Strain Carrying the blaNDM-1 and blaOXA-23 Carbapenemase Genes from the Czech Republic. J. Antimicrob. Chemother. 2012, 67, 1550–1552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The First Nationwide Multicenter Study of Acinetobacter Baumannii Recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-Producing Isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC Definitions for Nosocomial Infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Girlich, D.; Bogaerts, P.; Bouchahrouf, W.; Bernabeu, S.; Langlois, I.; Begasse, C.; Arangia, N.; Dortet, L.; Huang, T.-D.; Glupczynski, Y.; et al. Evaluation of the Novodiag CarbaR+, a Novel Integrated Sample to Result Platform for the Multiplex Qualitative Detection of Carbapenem and Colistin Resistance Markers. Microb. Drug Resist. 2021, 27, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Holma, T.; Antikainen, J.; Haiko, J. Evaluation of Three Molecular Carbapenemase Tests: Eazyplex SuperBug Complete B, Novodiag CarbaR+, and Amplidiag CarbaR+MCR. J. Microbiol. Methods 2021, 180, 106105. [Google Scholar] [CrossRef]
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization: Geneva, Switzerland, 2019.
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 12.0; EUCAST: Växjö, Sweden, 2022. [Google Scholar]
- M100; Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Hunt, M.; Mather, A.E.; Sánchez-Busó, L.; Page, A.J.; Parkhill, J.; Keane, J.A.; Harris, S.R. ARIBA: Rapid Antimicrobial Resistance Genotyping Directly from Sequencing Reads. Microb. Genom. 2017, 3, e000131. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The Population Structure of Acinetobacter Baumannii: Expanding Multiresistant Clones from an Ancestral Susceptible Genetic Pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef]
- Bartual, S.G.; Seifert, H.; Hippler, C.; Luzon, M.A.D.; Wisplinghoff, H.; Rodríguez-Valera, F. Development of a Multilocus Sequence Typing Scheme for Characterization of Clinical Isolates of Acinetobacter Baumannii. J. Clin. Microbiol. 2005, 43, 4382–4390. [Google Scholar] [CrossRef]
- Lucidi, M.; Visaggio, D.; Migliaccio, A.; Capecchi, G.; Visca, P.; Imperi, F.; Zarrilli, R. Pathogenicity and Virulence of Acinetobacter Baumannii: Factors Contributing to the Fitness in Healthcare Settings and the Infected Host. Virulence 2024, 15, 2289769. [Google Scholar] [CrossRef]
- Pérez-Vazquez, M.; Oteo-Iglesias, J.; Sola-Campoy, P.J.; Carrizo-Manzoni, H.; Bautista, V.; Lara, N.; Aracil, B.; Alhambra, A.; Martínez-Martínez, L.; Campos, J.; et al. Characterization of Carbapenemase-Producing Klebsiella Oxytoca in Spain, 2016–2017. Antimicrob. Agents Chemother. 2019, 63, e02529-18. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Cahill, S.M.; Holt, K.E.; Hall, R.M.; Kenyon, J.J. Identification of Acinetobacter Baumannii Loci for Capsular Polysaccharide (KL) and Lipooligosaccharide Outer Core (OCL) Synthesis in Genome Assemblies Using Curated Reference Databases Compatible with Kaptive. Microb. Genom. 2020, 6, e000339. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, J.; Hamidian, M.; Wick, R.R.; Edwards, D.J.; Billman-Jacobe, H.; Hall, R.M.; Holt, K.E. ISMapper: Identifying Transposase Insertion Sites in Bacterial Genomes from Short Read Sequence Data. BMC Genom. 2015, 16, 667. [Google Scholar] [CrossRef]
- Adams, M.D.; Bishop, B.; Wright, M.S. Quantitative Assessment of Insertion Sequence Impact on Bacterial Genome Architecture. Microb. Genom. 2016, 2, e000062. [Google Scholar] [CrossRef]
- Holt, K.E.; Hamidian, M.; Kenyon, J.J.; Wynn, M.T.; Hawkey, J.; Pickard, D.; Hall, R.M. Genome Sequence of Acinetobacter Baumannii Strain A1, an Early Example of Antibiotic-Resistant Global Clone 1. Genome Announc. 2015, 3, e00032-15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, C.-K.; Lee, H.; Jeong, S.H.; Yong, D.; Lee, K. A Novel Insertion Sequence, ISAba10, Inserted into ISAba1 Adjacent to the Bla(OXA-23) Gene and Disrupting the Outer Membrane Protein Gene carO in Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2011, 55, 361–363. [Google Scholar] [CrossRef]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the Multidrug Resistance Phenotype of Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Sun, X.; Ni, Z.; Tang, J.; Ding, Y.; Wang, X.; Li, F. The abaI/abaR Quorum Sensing System Effects on Pathogenicity in Acinetobacter Baumannii. Front. Microbiol. 2021, 12, 679241. [Google Scholar] [CrossRef]
- Choi, A.H.K.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD Locus of Acinetobacter Baumannii Encodes the Production of Poly-Beta-1-6-N-Acetylglucosamine, Which Is Critical for Biofilm Formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Neres, J.; Wilson, D.J.; Celia, L.; Beck, B.J.; Aldrich, C.C. Aryl Acid Adenylating Enzymes Involved in Siderophore Biosynthesis: Fluorescence Polarization Assay, Ligand Specificity, and Discovery of Non-Nucleoside Inhibitors via High-Throughput Screening. Biochemistry 2008, 47, 11735–11749. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and Biofilm Formation on Abiotic Surfaces by Acinetobacter Baumannii: Involvement of a Novel Chaperone-Usher Pili Assembly System. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Singh, M.; De Silva, P.M.; Al-Saadi, Y.; Switala, J.; Loewen, P.C.; Hausner, G.; Chen, W.; Hernandez, I.; Castillo-Ramirez, S.; Kumar, A. Characterization of Extremely Drug-Resistant and Hypervirulent Acinetobacter Baumannii AB030. Antibiotics 2020, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, F.; Albiger, B.; Monnet, D.L.; Struelens, M.J.; Seifert, H.; Kohlenberg, A.; European Antimicrobial Resistance Genes Surveillance Network (EURGen-Net) Carbapenem-Resistant Acinetobacter Baumannii Capacity Survey Group; EURGen-Net Carbapenem-Resistant Acinetobacter Baumannii Capacity Survey Group. Epidemiological Situation, Laboratory Capacity and Preparedness for Carbapenem-Resistant Acinetobacter Baumannii in Europe, 2019. Euro Surveill. 2020, 25, 2001735. [Google Scholar] [CrossRef] [PubMed]
- Doughty, E.L.; Liu, H.; Moran, R.A.; Hua, X.; Ba, X.; Guo, F.; Chen, X.; Zhang, L.; Holmes, M.; van Schaik, W.; et al. Endemicity and Diversification of Carbapenem-Resistant Acinetobacter Baumannii in an Intensive Care Unit. Lancet Reg. Health West. Pac. 2023, 37, 100780. [Google Scholar] [CrossRef] [PubMed]
- Medioli, F.; Bacca, E.; Faltoni, M.; Burastero, G.J.; Volpi, S.; Menozzi, M.; Orlando, G.; Bedini, A.; Franceschini, E.; Mussini, C.; et al. Is It Possible to Eradicate Carbapenem-Resistant Acinetobacter baumannii (CRAB) from Endemic Hospitals? Antibiotics 2022, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Bedenić, B.; Bratić, V.; Mihaljević, S.; Lukić, A.; Vidović, K.; Reiner, K.; Schöenthaler, S.; Barišić, I.; Zarfel, G.; Grisold, A. Multidrug-Resistant Bacteria in a COVID-19 Hospital in Zagreb. Pathogens 2023, 12, 117. [Google Scholar] [CrossRef]
- Huang, W.; Qiao, F.; Cai, L.; Zong, Z.; Zhang, W. Effect of Daily Chlorhexidine Bathing on Reducing Infections Caused by Multidrug-Resistant Organisms in Intensive Care Unit Patients: A Semiexperimental Study with Parallel Controls. J. Evid. Based Med. 2023, 16, 32–38. [Google Scholar] [CrossRef]
- Mangioni, D.; Fox, V.; Chatenoud, L.; Bolis, M.; Bottino, N.; Cariani, L.; Gentiloni Silverj, F.; Matinato, C.; Monti, G.; Muscatello, A.; et al. Genomic Characterization of Carbapenem-Resistant Acinetobacter Baumannii (CRAB) in Mechanically Ventilated COVID-19 Patients and Impact of Infection Control Measures on Reducing CRAB Circulation during the Second Wave of the SARS-CoV-2 Pandemic in Milan, Italy. Microbiol. Spectr. 2023, 11, e0020923. [Google Scholar] [CrossRef]
- Xiong, L.; Deng, C.; Yang, G.; Shen, M.; Chen, B.; Tian, R.; Zha, H.; Wu, K. Molecular Epidemiology and Antimicrobial Resistance Patterns of Carbapenem-Resistant Acinetobacter Baumannii Isolates from Patients Admitted at ICUs of a Teaching Hospital in Zunyi, China. Front. Cell Infect. Microbiol. 2023, 13, 1280372. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reuter, S.; Wille, J.; Xanthopoulou, K.; Stefanik, D.; Grundmann, H.; Higgins, P.G.; Seifert, H. A Global View on Carbapenem-Resistant Acinetobacter Baumannii. mBio 2023, 14, e0226023. [Google Scholar] [CrossRef]
- Ejaz, H.; Ahmad, M.; Younas, S.; Junaid, K.; Abosalif, K.O.A.; Abdalla, A.E.; Alameen, A.A.M.; Elamir, M.Y.M.; Bukhari, S.N.A.; Ahmad, N.; et al. Molecular Epidemiology of Extensively-Drug Resistant Acinetobacter Baumannii Sequence Type 2 Co-Harboring Bla NDM and Bla OXA From Clinical Origin. Infect. Drug Resist. 2021, 14, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread Cefiderocol Heteroresistance in Carbapenem-Resistant Gram-Negative Pathogens. Lancet Infect. Dis. 2021, 21, 597–598. [Google Scholar] [CrossRef]
- Shahryari, S.; Mohammadnejad, P.; Noghabi, K.A. Screening of Anti-Acinetobacter Baumannii Phytochemicals, Based on the Potential Inhibitory Effect on OmpA and OmpW Functions. R. Soc. Open Sci. 2021, 8, 201652. [Google Scholar] [CrossRef]
- Lioy, V.S.; Goussard, S.; Guerineau, V.; Yoon, E.-J.; Courvalin, P.; Galimand, M.; Grillot-Courvalin, C. Aminoglycoside Resistance 16S rRNA Methyltransferases Block Endogenous Methylation, Affect Translation Efficiency and Fitness of the Host. RNA 2014, 20, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Rather, P.N.; Hare, R.S.; Miller, G.H. Molecular Genetics of Aminoglycoside Resistance Genes and Familial Relationships of the Aminoglycoside-Modifying Enzymes. Microbiol. Rev. 1993, 57, 138–163. [Google Scholar] [CrossRef]
- El-Sayed-Ahmed, M.A.E.-G.; Amin, M.A.; Tawakol, W.M.; Loucif, L.; Bakour, S.; Rolain, J.-M. High Prevalence of Bla(NDM-1) Carbapenemase-Encoding Gene and 16S rRNA armA Methyltransferase Gene among Acinetobacter Baumannii Clinical Isolates in Egypt. Antimicrob. Agents Chemother. 2015, 59, 3602–3605. [Google Scholar] [CrossRef]
- Sánchez-Urtaza, S.; Ocampo-Sosa, A.; Molins-Bengoetxea, A.; El-Kholy, M.A.; Hernandez, M.; Abad, D.; Shawky, S.M.; Alkorta, I.; Gallego, L. Molecular Characterization of Multidrug Resistant Acinetobacter Baumannii Clinical Isolates from Alexandria, Egypt. Front. Cell. Infect. Microbiol. 2023, 13, 1208046. [Google Scholar] [CrossRef]
- Genteluci, G.L.; de Souza, P.A.; Gomes, D.B.C.; Sousa, V.S.; de Souza, M.J.; Abib, J.R.L.; de Castro, E.A.R.; Rangel, K.; Villas Bôas, M.H.S. Polymyxin B Heteroresistance and Adaptive Resistance in Multidrug- and Extremely Drug-Resistant Acinetobacter Baumannii. Curr. Microbiol. 2020, 77, 2300–2306. [Google Scholar] [CrossRef]
- Meschiari, M.; Lòpez-Lozano, J.-M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A Five-Component Infection Control Bundle to Permanently Eliminate a Carbapenem-Resistant Acinetobacter Baumannii Spreading in an Intensive Care Unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Moghnieh, R.A.; Kanafani, Z.A.; Tabaja, H.Z.; Sharara, S.L.; Awad, L.S.; Kanj, S.S. Epidemiology of Common Resistant Bacterial Pathogens in the Countries of the Arab League. Lancet Infect. Dis. 2018, 18, e379–e394. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Apisarnthanarak, A.; Khan, E.; Suwantarat, N.; Ghafur, A.; Tambyah, P.A. Carbapenem-Resistant Acinetobacter Baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2017, 30, 1–22. [Google Scholar] [CrossRef]
- Wareth, G.; Linde, J.; Nguyen, N.H.; Nguyen, T.N.M.; Sprague, L.D.; Pletz, M.W.; Neubauer, H. WGS-Based Analysis of Carbapenem-Resistant Acinetobacter Baumannii in Vietnam and Molecular Characterization of Antimicrobial Determinants and MLST in Southeast Asia. Antibiotics 2021, 10, 563. [Google Scholar] [CrossRef]
- Aldali, J.A. Acinetobacter Baumannii: A Multidrug-Resistant Pathogen, Has Emerged in Saudi Arabia. Saudi Med. J. 2023, 44, 732–744. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Carbonara, S.; Marino, A.; Di Caprio, G.; Carretta, A.; Mularoni, A.; Mariani, M.F.; Maraolo, A.E.; Scotto, R.; et al. Mortality Attributable to Bloodstream Infections Caused by Different Carbapenem-Resistant Gram-Negative Bacilli: Results From a Nationwide Study in Italy (ALARICO Network). Clin. Infect. Dis. 2023, 76, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Goic-Barisic, I.; Music, M.S.; Drcelic, M.; Tuncbilek, S.; Akca, G.; Jakovac, S.; Tonkić, M.; Hrenovic, J. Molecular Characterisation of Colistin and Carbapenem-Resistant Clinical Isolates of Acinetobacter Baumannii from Southeast Europe. J. Glob. Antimicrob. Resist. 2023, 33, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Janssen, H.; Hey-Hadavi, J.H.; Hackel, M.; Sahm, D. Multidrug-Resistant Gram-Negative Bacilli Recovered from Respiratory and Blood Specimens from Adults: The ATLAS Surveillance Program in European Hospitals, 2018–2020. Int. J. Antimicrob. Agents 2023, 61, 106724. [Google Scholar] [CrossRef]
- Reich, S.; Adler, A. Introduction and Spread of NDM-Producing Enterobacterales and Acinetobacter Baumannii into Middle Eastern Countries: A Molecular-Based Hypothesis. Expert. Rev. Anti Infect. Ther. 2023, 21, 749–758. [Google Scholar] [CrossRef]
- Sandfort, M.; Hans, J.B.; Fischer, M.A.; Reichert, F.; Cremanns, M.; Eisfeld, J.; Pfeifer, Y.; Heck, A.; Eckmanns, T.; Werner, G.; et al. Increase in NDM-1 and NDM-1/OXA-48-Producing Klebsiella Pneumoniae in Germany Associated with the War in Ukraine, 2022. Euro Surveill. 2022, 27, 2200926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).