Gut Microbiota and Lipid Metabolism in Bullfrog Tadpoles: A Comparative Study Across Nutritional Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Aquaculture Management
2.3. Sampling Procedure
2.4. Biochemical and Enzymatic Analysis
2.5. Histological Analysis
2.6. qRT-PCR Analysis
2.7. Microbiological Community Analysis
2.8. Statistical Analysis
3. Results
3.1. Histological Observations of Organisms at Different Nutritional Stages
3.2. Differences in the Biochemical and Enzymatic Indicators of Bullfrog Tadpoles
3.3. Differences in Lipid Metabolism-Related Gene Expression in Bullfrog Tadpoles
3.4. Sequencing Data and Diversity Analysis
3.5. An Analysis of Microbial Community Composition
3.6. An Analysis of the Similarities and Differences of Microbial Communities
3.7. Assembly Processes of Microbial Communities in Different Habitats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| TG | Triacylglycerol |
| TC | Total cholesterol |
| NEFA | Non-esterified fatty acid |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| ACTB | β-Actin |
| ppar-γ | Peroxisome proliferators activated receptor-γ |
| ppar-α | Peroxisome proliferators activated receptor-α |
| fas | Fatty acid synthase |
| dgat1 | Diacylglycerol O-acyltransferase 1 |
| hsl | Hormone-sensitive lipase |
| cpt1 | Carnitine O-palmitoyltransferase-1 |
| acox1 | Acyl-CoA oxidase |
| OTUs | Operational taxonomic units |
| PCoA | Principal coordinate analysis |
| LEfSe | Linear discriminant analysis effect size |
| LDA | Linear discriminant analysis |
| NCM | Neutral community model |
| βNTI | Beta nearest taxon index |
References
- Huo, X.; Wang, P.; Zhao, F.; Liu, Q.; Tian, Q.; Tang, L.; Lv, M.; Wei, Z.; Yang, C.; Su, J. Revitalizing pond culture system: Harnessing the power of composite nanopeptide CI20, 1,3-1,6-β-glucan, and anthocyanidin biotherapy as an antibiotic substitute for efficient management of bacterial diseases in bullfrogs. Aquaculture 2024, 581, 740394. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Lin, H.; Chen, K.; Qin, Z.; Jiang, B.; Li, W.; Wang, Q.; Su, Y.; Huang, Y.; et al. Identification and characterization of Streptococcus iniae from farmed American Bullfrogs (Aquarana catesbeiana). Aquac. Rep. 2024, 35, 101980. [Google Scholar] [CrossRef]
- Wake, M.H. Fetal adaptations for viviparity in amphibians. J. Morphol. 2015, 276, 941–960. [Google Scholar] [CrossRef]
- Huong, H.K.; Huang, C.; Nam, H.K.; My Nhan, N.T.; Diep, D.X. Effects of salinity on the egg fertilization, hatching, and tadpole growth and survival rates of the Thailand frog (Rana tigerina Dubois, 1981). J. Appl. Ichthyol. 2024, 2024, 6750783. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Yang, Y.; Cheng, R.; Wang, C.; He, D.; Li, G.; Ma, H.; Bi, J.; Chen, F.; et al. Study on artificial propagation and early development of Acrossocheilus longipinnis. S. China Fish. Sci. 2024, 20, 63–72. [Google Scholar]
- Gao, J.; Li, X.; Lu, K.; Song, K.; Zhang, J.; Wang, L.; Zhang, C. Optimal dietary protein level for the growth and metamorphosis of bullfrog (Lithobates catesbeianus) tadpoles. Aquaculture 2024, 580, 740265. [Google Scholar] [CrossRef]
- Ding, X.; Jin, F.; Xu, J.; Zhang, S.; Chen, D.; Hu, B.; Hong, Y. The impact of aquaculture system on the microbiome and gut metabolome of juvenile Chinese softshell turtle (Pelodiscus sinensis). iMeta 2022, 1, e17. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, S.; Wang, H.; Zhou, Y.; Peng, F.; Zhang, X.; Zhou, Y.; Guo, S.; You, Y. Transcriptome sequencing analysis reveals dynamic changes in major biological functions during the early development of clearhead icefish, Protosalanx chinensis. Fishes 2022, 7, 115. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Wu, D.; Lu, Z.; Xiao, J.; Huang, H.; Fu, Q.; Guo, Z. Multi-omics analysis revealed the differences in lipid metabolism of the gut between adult and juvenile yellowfin tuna (Thunnus albacares). Front. Microbiol. 2024, 14, 1326247. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, C.; Tan, F.; Limbu, S.M.; Chen, L.; Du, Z. Gnotobiotic models: Powerful tools for deeply understanding intestinal microbiota-host interactions in aquaculture. Aquaculture 2020, 517, 734800. [Google Scholar] [CrossRef]
- Gong, H.; Wang, T.; Wu, M.; Chu, Q.; Lan, H.; Lang, W.; Zhu, L.; Song, Y.; Zhou, Y.; Wen, Q.; et al. Maternal effects drive intestinal development beginning in the embryonic period on the basis of maternal immune and microbial transfer in chickens. Microbiome 2023, 11, 41. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Jiang, Y.; Wang, Y.; Liao, M.; Rong, X.; Liu, Q. The intestine of artificially bred larval turbot (Scophthalmus maximus) contains a stable core group of microbiota. Arch. Microbiol. 2020, 202, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, H.; Jiang, Y.; Gao, Q.; Tang, B.; Ling, J.; Yuan, X. Relationships between the gut microbiota of juvenile black sea bream (Acanthopagrus schlegelii) and associated environment compartments in different habitats. Microorganisms 2021, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Su, S.; Zhang, C.; Zhu, J.; Hou, Y.; Li, Z.; Yang, X.; Zhou, X.; He, X.; Munganga, B.P.; et al. Dynamic changes in microbial community structure in farming pond water and their effect on the intestinal microbial community profile in juvenile common carp (Cyprinus carpio L.). Genomics 2021, 113, 2547–2560. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Z.; Zhong, X.; Wang, H.; Liu, Y.; Li, X. Manipulation of cadmium and diethylhexyl phthalate on Rana chensinensis tadpoles affects the intestinal microbiota and fatty acid metabolism. Sci. Total Environ. 2022, 821, 153455. [Google Scholar] [CrossRef]
- Mourente, G.; Vázquez, R. Changes in the content of total lipid, lipid classes and their fatty acids of developing eggs and unfed larvae of the Senegal sole, Solea senegalensis Kaup. Fish Physiol. Biochem. 1996, 15, 221–235. [Google Scholar] [CrossRef]
- Liu, Z.; Qiuqian, L.; Yao, Z.; Wang, X.; Huang, L.; Zheng, J.; Wang, K.; Li, L.; Zhang, D. Effects of a commercial microbial agent on the bacterial communities in shrimp culture system. Front. Microbiol. 2018, 9, 2430. [Google Scholar] [CrossRef]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Heilig, H.; Benvenuti, G.; Verreth, J.; Verdegem, M. The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci. Rep. 2015, 5, 18206. [Google Scholar] [CrossRef]
- Zeng, A.; Tan, K.; Gong, P.; Lei, P.; Guo, Z.; Wang, S.; Gao, S.; Zhou, Y.; Shu, Y.; Zhou, X.; et al. Correlation of microbiota in the gut of fish species and water. 3 Biotech 2020, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dai, W.; Qiu, Q.; Dong, C.; Zhang, J.; Xiong, J. Contrasting ecological processes and functional compositions between intestinal bacterial community in healthy and diseased shrimp. Microb. Ecol. 2016, 72, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, K.K.; Rasmussen, B.B.; Castex, M.; Gram, L.; Bentzon-Tilia, M. The aquaculture microbiome at the centre of business creation. Microb. Biotechnol. 2017, 10, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, G.; Yu, E.; Xie, J.; Xia, Y.; Li, H.; Zhang, K.; Gong, W.; Li, Z.; Xie, W.; et al. Dietary deoxycholic acid decreases fat accumulation by activating liver farnesoid X receptor in grass crap (Ctenopharyngodon idella). Aquaculture 2024, 578, 740123. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Niu, S.; Xie, J.; Wang, G.; Li, Z.; Zhang, K.; Li, H.; Xia, Y.; Tian, J.; Yu, E.; Xie, W.; et al. Community assembly patterns and processes of bacteria in a field-scale aquaculture wastewater treatment system. Sci. Total Environ. 2024, 907, 167913. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, J.; Peng, C.; Song, J.; Xie, Z.; Jia, J.; Li, H.; Zhao, S.; Liang, Y.; Gong, B. The effect of the microalgae Chlorella vulgaris on the gut microbiota of juvenile nile tilapia (Oreochromis niloticus) is feeding-time dependent. Microorganisms 2023, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cao, H.; Li, H.; Li, K.; Yang, G.; Zhang, Y.; Chang, X.; Zhang, X.; Zhang, J. Effect of dietary honeysuckle (Lonicera caerulea L.) supplementation on lipid metabolism, immunity and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquac. Rep. 2022, 23, 101063. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Liu, X.; Deng, H.; Xu, Q.; Luo, K.; Zhou, J.; Gao, W.; Wang, Z.; Zhang, H.; Zhou, X. Effects of tea tree essential oil supplementation in low fish meal diet on growth, lipid metabolism, anti-oxidant capacity and immunity of largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 27, 101380. [Google Scholar] [CrossRef]
- Xie, R.; Amenyogbe, E.; Chen, G.; Huang, J. Effects of feed fat level on growth performance, body composition and serum biochemical indices of hybrid grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 2021, 530, 735813. [Google Scholar] [CrossRef]
- Cai, L.; Wang, L.; Song, K.; Lu, K.; Zhang, C.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Ning, Z.; Guo, X.; Liu, X.; Lu, C.; Wang, A.; Wang, X.; Wang, W.; Chen, H.; Qin, W.; Liu, X.; et al. USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat. Commun. 2022, 13, 2187. [Google Scholar] [CrossRef]
- Wang, X.; Bai, F.; Niu, X.; Sun, Y.; Ye, J. The lipid-lowering effect of dietary taurine in orange-spotted groupers (Epinephelus coioides) involves both bile acids and lipid metabolism. Front. Mar. Sci. 2022, 9, 859428. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; Shao, Y.; Li, Y.; Chen, C.; You, C.; Monroig, Ó.; Rahimnejad, S.; Tocher, D.R.; Wang, S. Impacts of dietary konjac glucomannan supplementation on growth, antioxidant capacity, hepatic lipid metabolism and inflammatory response in golden pompano (Trachinotus ovatus) fed a high fat diet. Aquaculture 2021, 545, 737113. [Google Scholar] [CrossRef]
- Chitraju, C.; Walther, T.C.; Farese, R.V. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef]
- Shen, J.; Sun, B.; Yu, C.; Cao, Y.; Cai, C.; Yao, J. Choline and methionine regulate lipid metabolism via the AMPK signaling pathway in hepatocytes exposed to high concentrations of nonesterified fatty acids. J. Cell. Biochem. 2019, 121, 3667–3678. [Google Scholar] [CrossRef]
- Thompson, A.M.; Trujillo, J.M. Dulaglutide: The newest GLP-1 receptor agonist for the management of type 2 diabetes. Ann. Pharmacother. 2015, 49, 351–359. [Google Scholar] [CrossRef]
- Schreiber, R.; Xie, H.; Schweiger, M. Of mice and men: The physiological role of adipose triglyceride lipase (ATGL). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef]
- Yang, W.; Wang, S.; Loor, J.J.; Lopes, M.G.; Zhao, Y.; Ma, X.; Li, M.; Zhang, B.; Xu, C. Role of diacylglycerol O-acyltransferase (DGAT) isoforms in bovine hepatic fatty acid metabolism. J. Dairy Sci. 2022, 105, 3588–3600. [Google Scholar] [CrossRef]
- Xiao, F.; Zhu, W.; Yu, Y.; He, Z.; Wu, B.; Wang, C.; Shu, L.; Li, X.; Yin, H.; Wang, J.; et al. Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota. npj Biofilms Microbiomes 2021, 7, 5. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringo, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Stephens, W.Z.; Burns, A.R.; Stagaman, K.; Wong, S.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2015, 10, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.F.; Riemann, L.; Bertilsson, S. Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J. 2009, 4, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Z.; Zhou, N.; Jiang, C.; Wang, B.; Cai, L.; Liu, S. Diversity, distribution and co-occurrence patterns of bacterial communities in a karst cave system. Front. Microbiol. 2019, 10, 1726. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Q.; Zhang, M.; Zhang, G.; Wu, T.; Liu, R.; Sui, W.; Zhang, J.; Yin, J.; Zhang, M. Leuconostoc pseudomesenteroides improves microbiota dysbiosis and liver metabolism imbalance and ameliorates the correlation between dihydroceramide and strains of Firmicutes and Proteobacteria in high fat diet obese mice. Food Funct. 2020, 11, 6855–6865. [Google Scholar] [CrossRef]
- Chen, Y.; Chiu, C.; Hung, S.; Huang, W.; Lee, Y.; Liu, J.; Huang, Y.; Chen, T.; Chuang, H. Gnotobiotic mice inoculated with Firmicutes, but not Bacteroidetes, deteriorate nonalcoholic fatty liver disease severity by modulating hepatic lipid metabolism. Nutr. Res. 2019, 69, 20–29. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, Q.; Liu, S.; Zhang, Q.; Chen, X.; Li, S.; Ran, C.; Yang, Y.; Teame, T.; Zhang, Z.; et al. The positive effects of postbiotic (SWF concentration®) supplemented diet on skin mucus, liver, gut health, the structure and function of gut microbiota of common carp (Cyprinus carpio) fed with high-fat diet. Fish Shellfish Immunol. 2023, 135, 108681. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Lu, K.; Song, K.; Mai, K.; Zhang, C.; Rahimnejad, S. Total replacement of fish meal with soybean meal in diets for bullfrog (Lithobates catesbeianus): Effects on growth performance and gut microbial composition. Aquaculture 2020, 524, 735236. [Google Scholar] [CrossRef]
- Wang, X.; Onchari, M.M.; Yang, X.; Xu, L.; Yin, X.; Wan, F.; Chen, Y.; Guan, M.; Li, B.; Luo, C. Genome analysis of Bacillus subtilis JCL16 and the synergistic relationship among its metabolites reveal its potential for biocontrol of Nocardia seriolae. Biol. Control 2022, 167, 104855. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Liu, Q.; Lai, Z.; Gao, Y.; Wang, C.; Zeng, Y.; Liu, E.; Mai, Y.; Yang, W.; Li, H. Connection between the Gut Microbiota of Largemouth Bass (Micropterus salmoides) and Microbiota of the Pond Culture Environment. Microorganisms 2021, 9, 1770. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; He, A.; Lin, Y.; Huang, X.; Liu, L.; Zhou, L. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquac. Rep. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Huang, G.; Qu, Q.; Wang, M.; Huang, M.; Zhou, W.; Wei, F. Global landscape of gut microbiome diversity and antibiotic resistomes across vertebrates. Sci. Total Environ. 2022, 838, 156178. [Google Scholar] [CrossRef]
- Rosindell, J.; Hubbell, S.P.; Etienne, R.S. The unified neutral theory of biodiversity and biogeography at age ten. Trends Ecol. Evol. 2011, 26, 340–348. [Google Scholar] [CrossRef]
- Sprockett, D.; Fukami, T.; Relman, D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 197–205. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, J.; Kong, J.; Sun, L.; Zhang, M.; Huang, Y.; Tang, L.; Zhang, S.; Yang, Z. Community assemblages and species coexistence of prokaryotes controlled by local environmental heterogeneity in a cold seep water column. Sci. Total Environ. 2023, 868, 161725. [Google Scholar] [CrossRef]
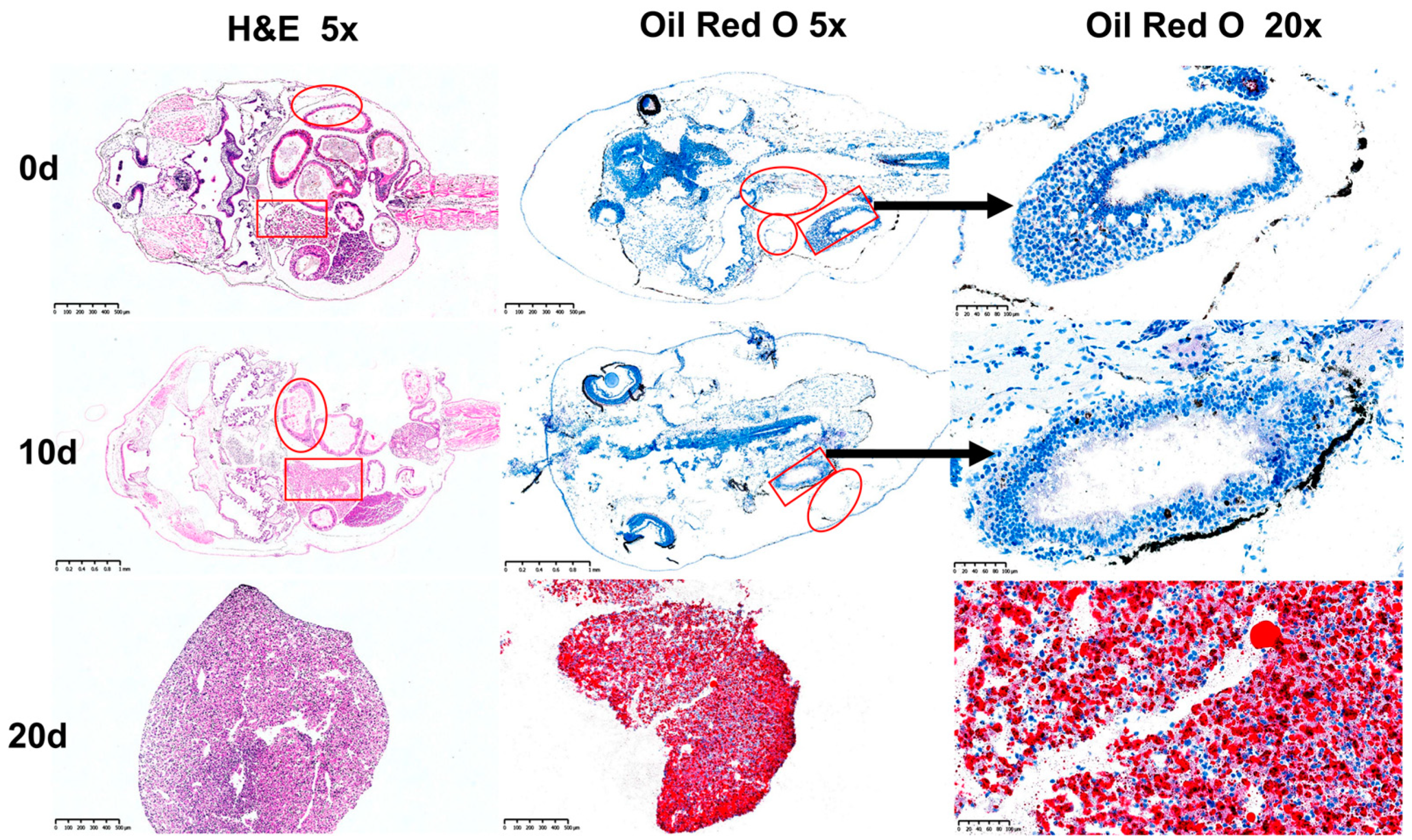

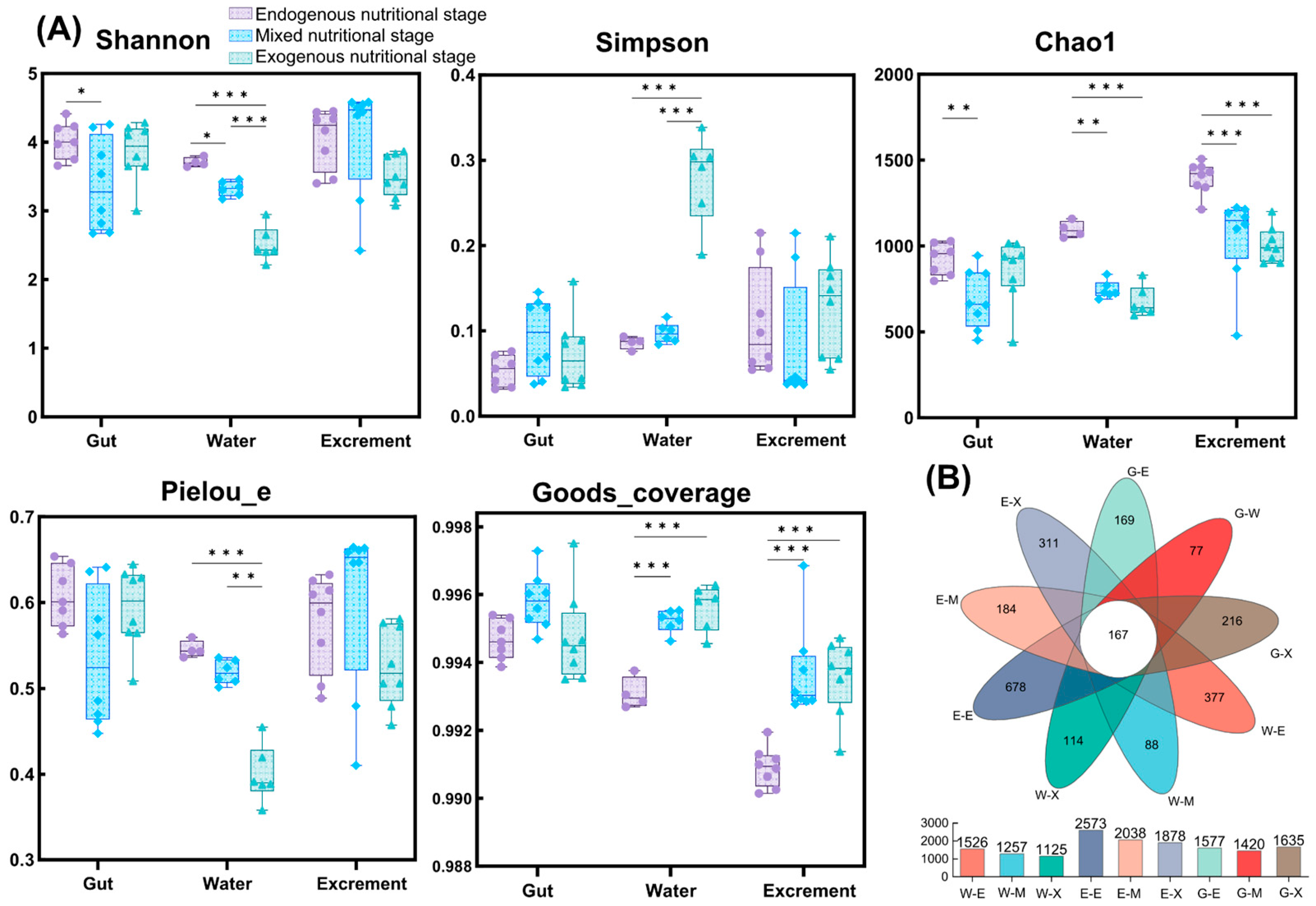
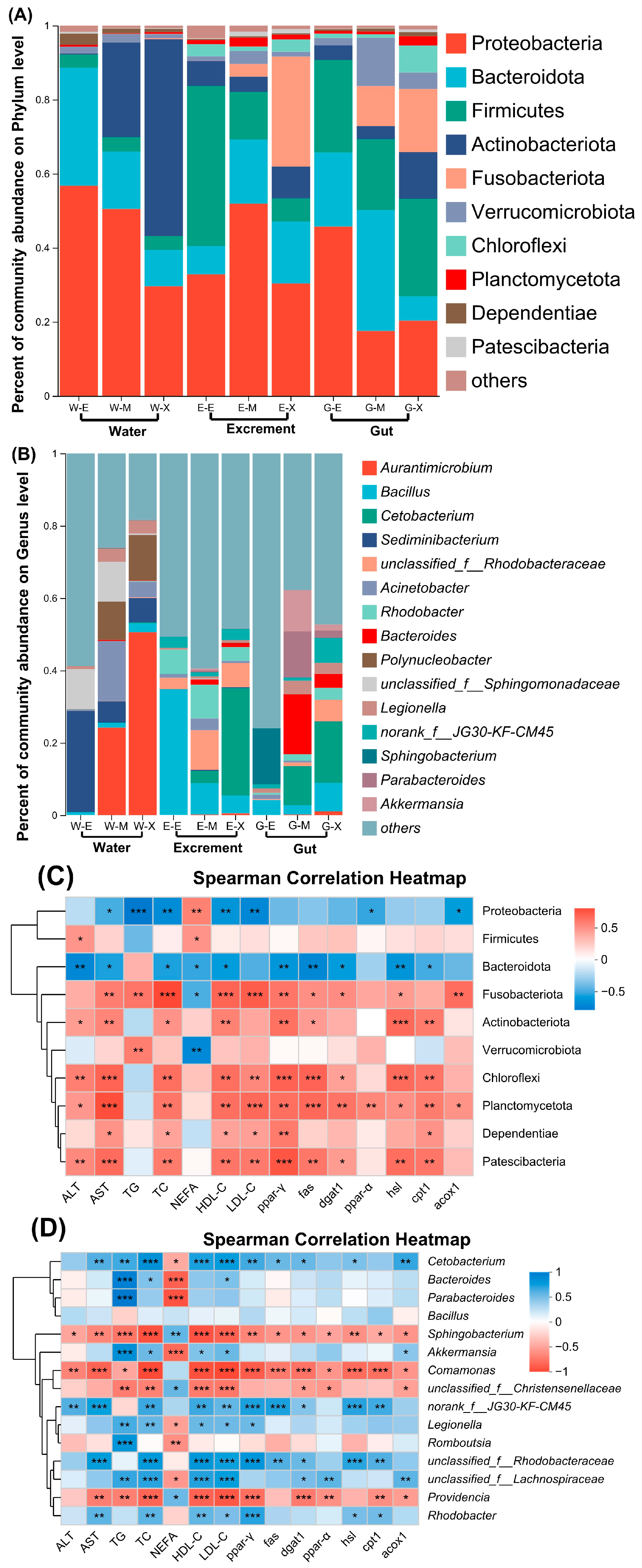
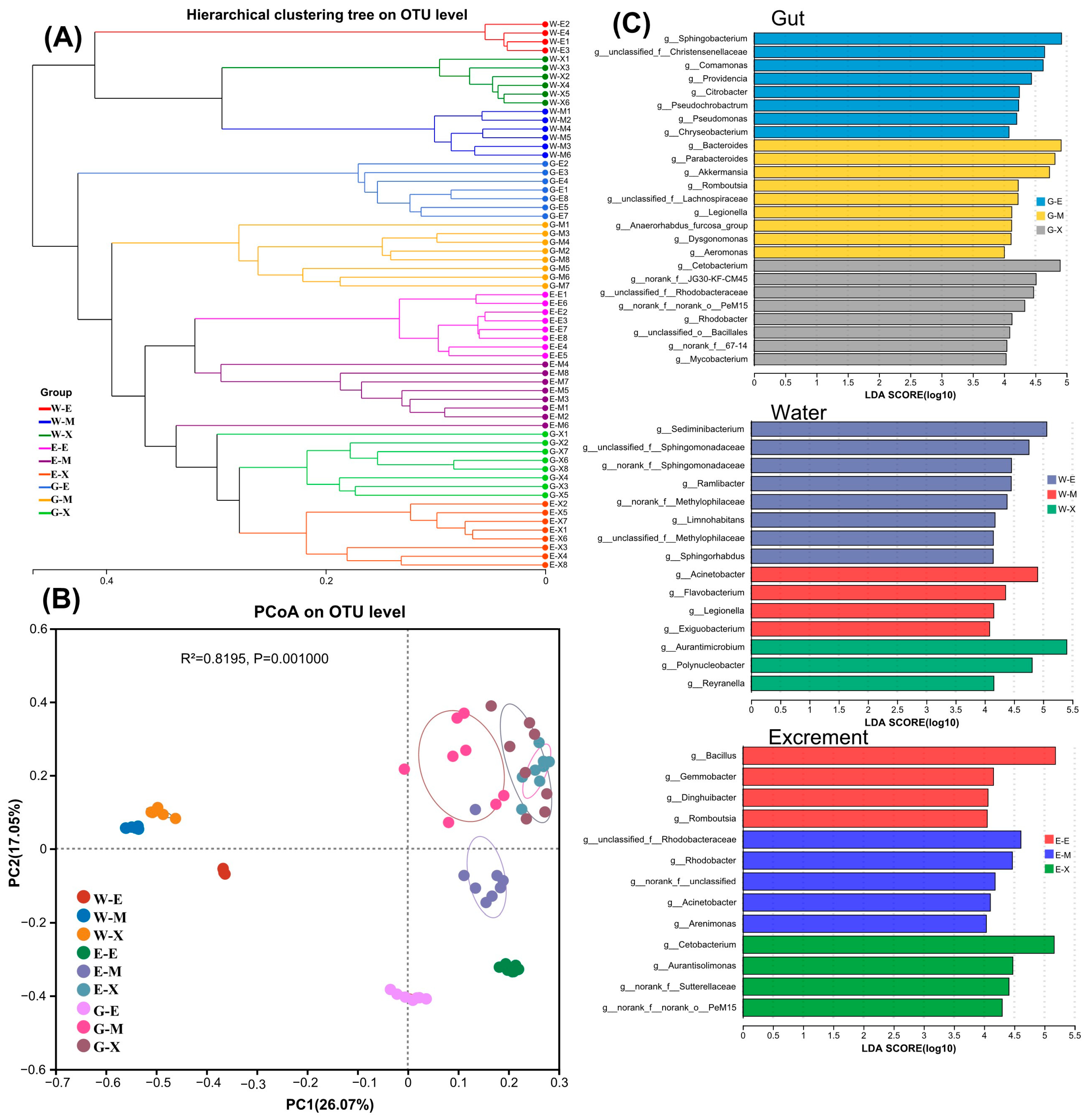

| Items | 0 d | 10 d | 20 d |
|---|---|---|---|
| ALT (U/gprot) | 29.90 ± 8.35 a | 28.02 ± 8.53 a | 55.41 ± 10.97 b |
| AST (U/gprot) | 15.62 ± 6.41 a | 18.70 ± 7.95 a | 35.01 ± 7.67 b |
| TG (mmol/gprot) | 0.08 ± 0.01 a | 0.31 ± 0.05 b | 0.44 ± 0.15 c |
| TC (mmol/gprot) | 0.07 ± 0.01 a | 0.18 ± 0.01 a | 0.38 ± 0.05 b |
| NEFA (mmol/gprot) | 0.09 ± 0.01 a | 0.21 ± 0.02 b | 0.27 ± 0.01 c |
| HDL-C (mmol/gprot) | 0.02 ± 0.01 a | 0.07 ± 0.02 a | 0.27 ± 0.08 b |
| LDL-C (mmol/gprot) | 0.01 ± 0.00 a | 0.05 ± 0.01 a | 0.39 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, P.; Xie, J.; Yang, H.; Wang, G.; Zhang, K.; Shu, R.; Li, Z.; Tian, J.; Li, H.; et al. Gut Microbiota and Lipid Metabolism in Bullfrog Tadpoles: A Comparative Study Across Nutritional Stages. Microorganisms 2025, 13, 1132. https://doi.org/10.3390/microorganisms13051132
Wang Z, Liu P, Xie J, Yang H, Wang G, Zhang K, Shu R, Li Z, Tian J, Li H, et al. Gut Microbiota and Lipid Metabolism in Bullfrog Tadpoles: A Comparative Study Across Nutritional Stages. Microorganisms. 2025; 13(5):1132. https://doi.org/10.3390/microorganisms13051132
Chicago/Turabian StyleWang, Zhilong, Pengxiang Liu, Jun Xie, Huirong Yang, Guangjun Wang, Kai Zhang, Rui Shu, Zhifei Li, Jingjing Tian, Hongyan Li, and et al. 2025. "Gut Microbiota and Lipid Metabolism in Bullfrog Tadpoles: A Comparative Study Across Nutritional Stages" Microorganisms 13, no. 5: 1132. https://doi.org/10.3390/microorganisms13051132
APA StyleWang, Z., Liu, P., Xie, J., Yang, H., Wang, G., Zhang, K., Shu, R., Li, Z., Tian, J., Li, H., Xie, W., Gong, W., & Xia, Y. (2025). Gut Microbiota and Lipid Metabolism in Bullfrog Tadpoles: A Comparative Study Across Nutritional Stages. Microorganisms, 13(5), 1132. https://doi.org/10.3390/microorganisms13051132








