Human Echinococcosis in the Russian Federation in the 21st Century: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Databases and Other Sources
2.3. Selection Criteria
2.4. Data Extraction and Generation
3. Results
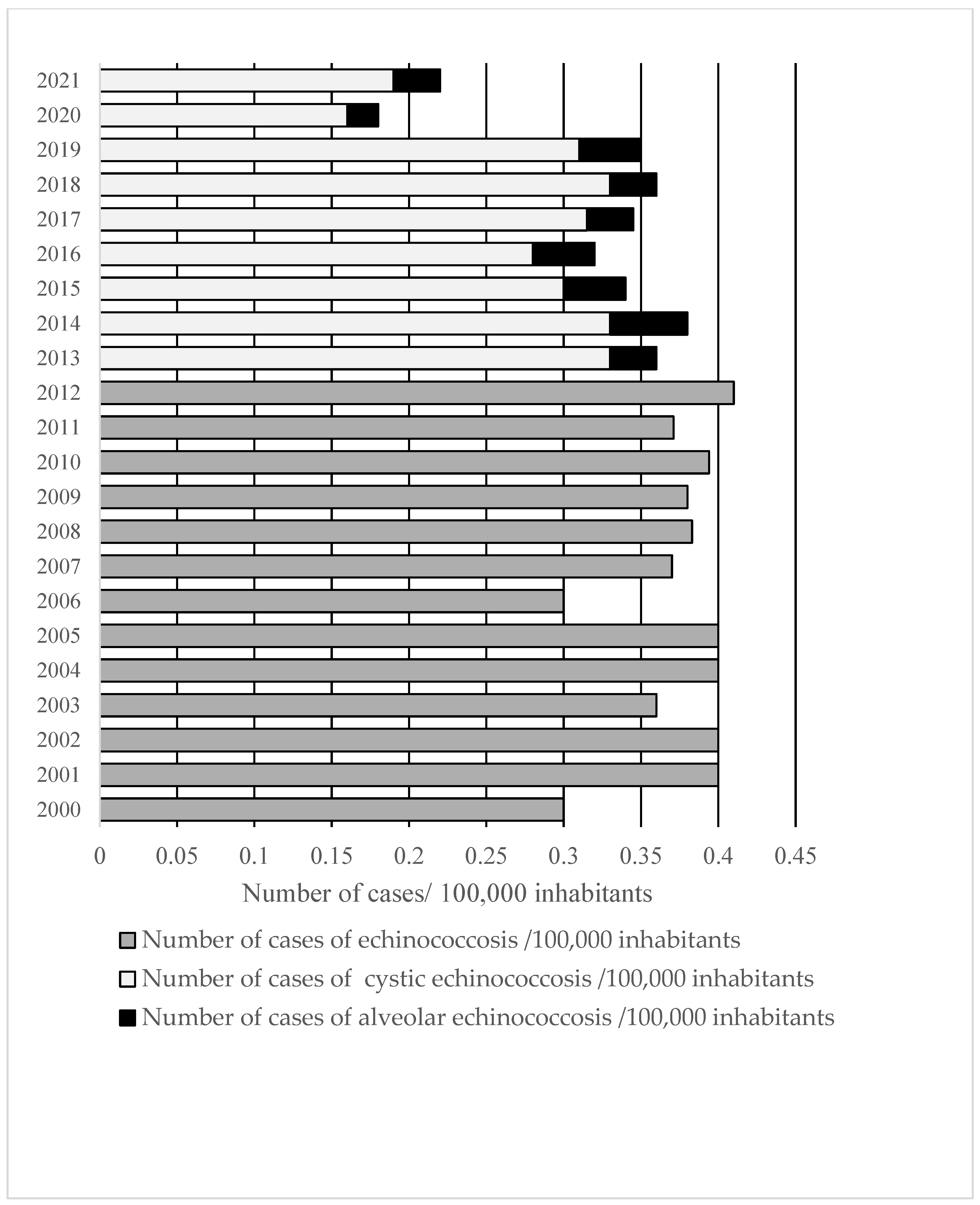
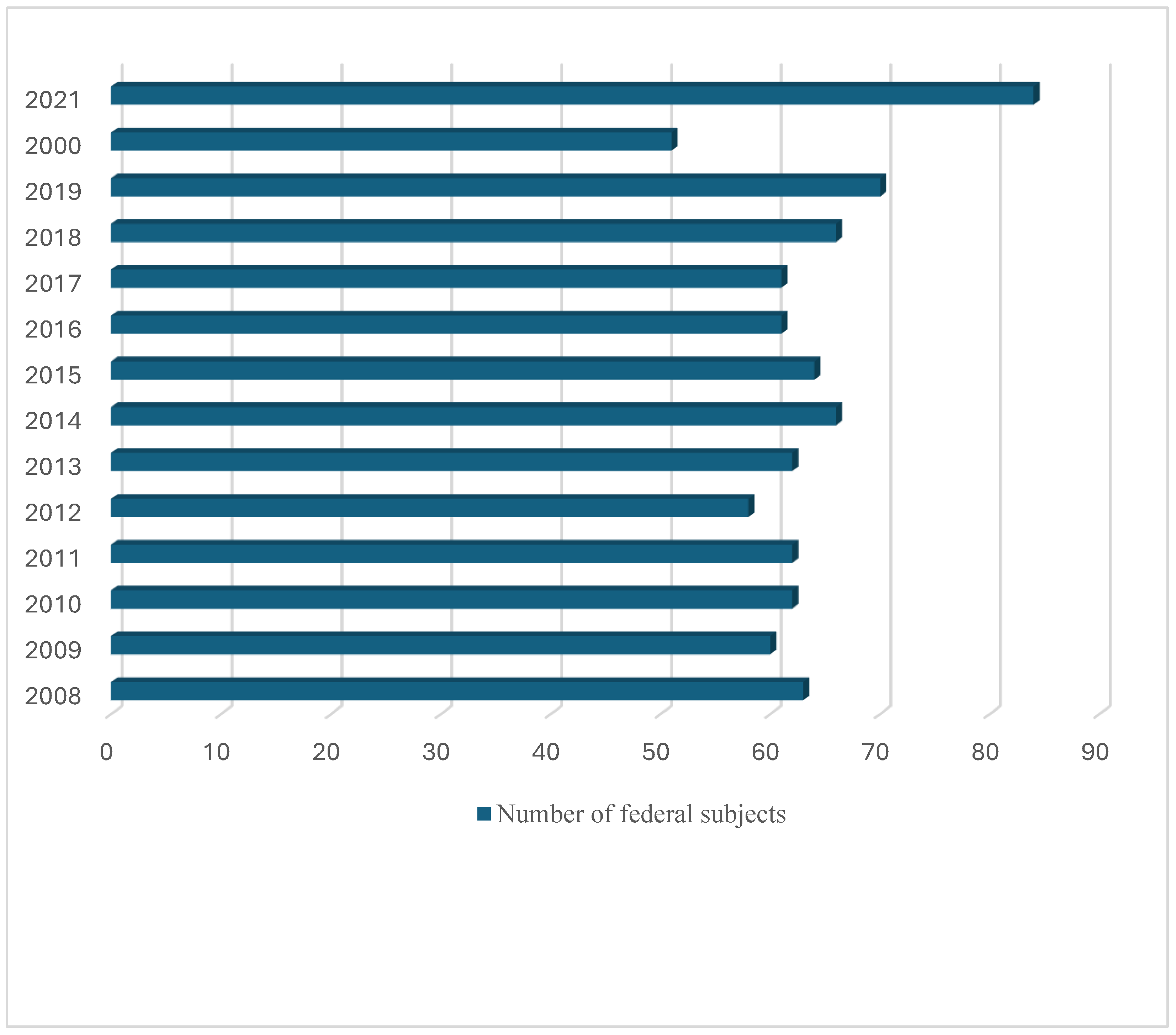
3.1. Incidence of Echinococcosis in RF
3.2. Cystic Echinococcosis
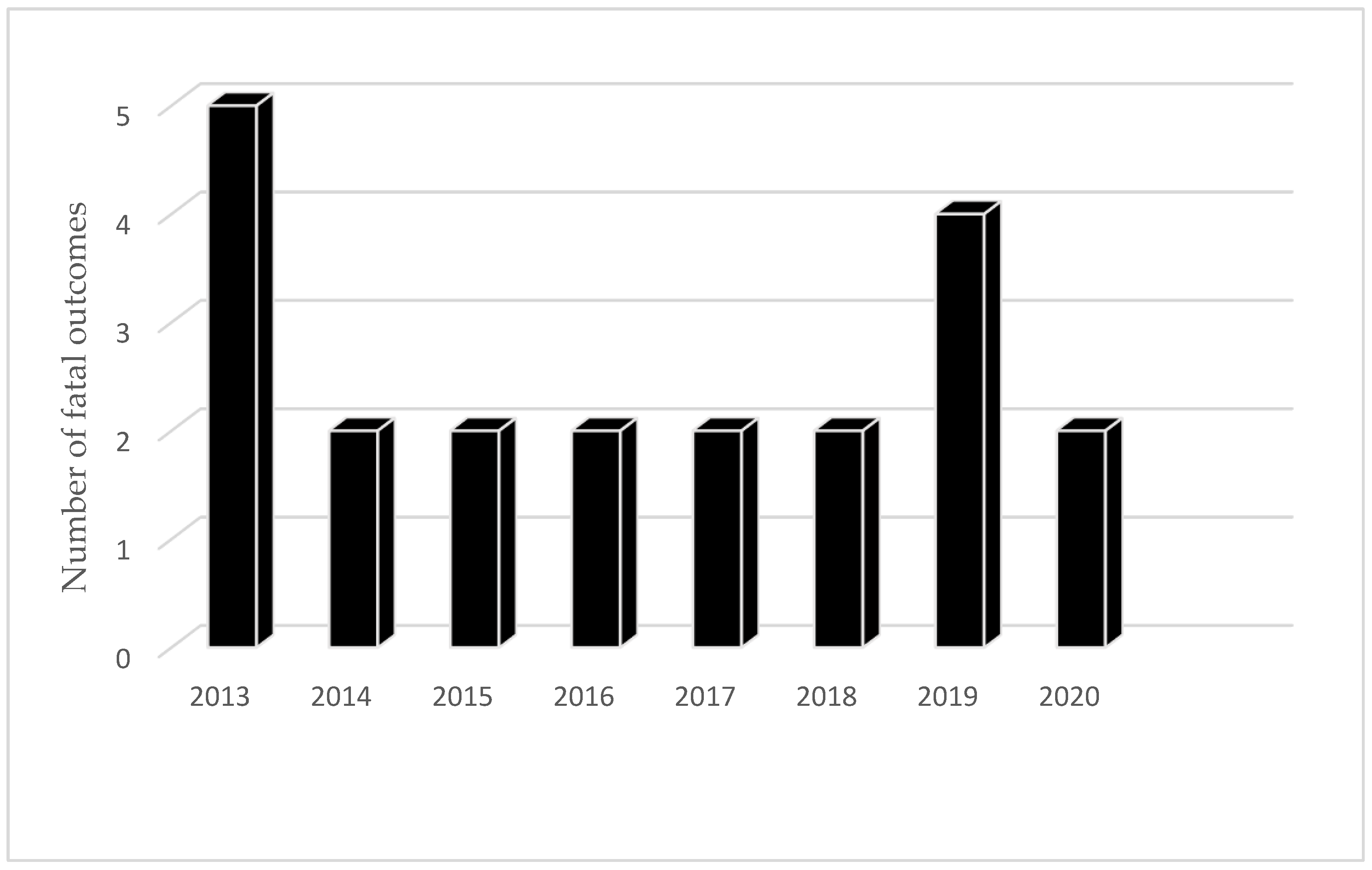
3.3. Alveolar Echinococcosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R.F. | Russian Federation |
| C.E. | cystic echinococcosis |
| E.G. | Echinococcus granulosus sensu lato |
| A.E. | alveolar echinococcosis |
| E.M. | Echinococcus multilocularis |
References
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2021.
- Kamiya, M. Collaborative Control Initiatives Targeting Zoonotic Agents of Alveolar Echinococcosis in the Northern Hemisphere. J. Vet. Sci. 2007, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar] [CrossRef]
- Muratova, E.T.; Khabirova, D.D. Laboratory-Practical Lesson. Methods for Diagnosing Trematodes, Cestodes, Nematodes in Animals; Veterinary Department of the Republic of Bashkortostan: Ufa, Russia, 2016; Available online: http://bashnpvl.ru/data/uploads/uc_m/ahinokokkoz.pdf (accessed on 25 December 2023). (In Russian)
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 315–493. [Google Scholar]
- Casulli, A.; Abela-Ridder, B.; Petrone, D.; Fabiani, M.; Bobić, B.; Carmena, D.; Šoba, B.; Zerem, E.; Gargaté, M.J.; Kuzmanovska, G.; et al. Unveiling the Incidences and Trends of the Neglected Zoonosis Cystic Echinococcosis in Europe: A Systematic Review from the MEmE Project. Lancet Infect. Dis. 2023, 23, e95–e107. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Legnardi, M.; Fittipaldo, A.; Drigo, M.; Cassini, R. Epidemiological Distribution of Echinococcus granulosus s.l. Infection in Human and Domestic Animal Hosts in European Mediterranean and Balkan Countries: A Systematic Review. PLoS Negl. Trop. Dis. 2020, 14, e0008519. [Google Scholar] [CrossRef]
- Ito, A.; Yamasaki, H.; Nakao, M.; Sako, Y.; Nakaya, K.; Mamuti, W.; Xiao, N.; Sato, M.O.; Ishikawa, Y. Echinococcosis and Cysticercosis in Asia: Evaluation of the Modern Technology for Epidemiological Study. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 2), 103–107. [Google Scholar] [PubMed]
- Bobić, B.; Ćirković, V.; Klun, I.; Štajner, T.; Srbljanović, J.; Bauman, N.; Djurković-Djaković, O. Epidemiology of Taenia solium Infection in the Russian Federation in the Last 20 Years: A Systematic Review. J. Helminthol. 2021, 95, e49. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Popova, A.Y. On the Incidence of Echinococcosis and Alveococcosis in the Russian Federation. Rospotrebnadzor Letter No. 01/7782-16-27, 20 June 2016. Available online: https://www.rospotrebnadzor.ru/upload/iblock/cca/ekhinokokkoz.pdf (accessed on 25 December 2023). (In Russian).
- Rospotrebnadzor (Federal Service for Supervision of Consumer Rights Protection and Human Well-Being). On the State of Sanitary and Epidemiological Well-Being of the Population in the Russian Federation: Annual State Reports 2008–2022. Available online: https://www.rospotrebnadzor.ru/documents/documents.php?arrFilter_ff%5BNAME%5D=%C3%EE%F1%F3%E4%E0%F0%F1%F2%E2%E5%ED%ED%FB%E9+%E4%EE%EA%EB%E0%E4+%CE+%F1%EE%F1%F2%EE%FF%ED%E8%E8+%F1%E0%ED%E8%F2%E0%F0%ED%EE-%FD%EF%E8%E4%E5%EC%E8%EE%EB%EE%E3%E8%F7%E5%F1%EA%EE%E3%EE+%E1%EB%E0%E3%EE%EF%EE%EB%F3%F7%E8%FF+%ED%E0%F1%E5%EB%E5%ED%E8%FF+%E2+%D0%EE%F1%F1%E8%E9%F1%EA%EE%E9+%D4%E5%E4%E5%F0%E0%F6%E8%E8+&arrFilter_pf%5BVID_DOC%5D=&arrFilter_pf%5BNUM_DOC%5D=&arrFilter_pf%5BDAT_DOC%5D=&arrFilter_pf%5BGOD%5D%5BLEFT%5D=2014&arrFilter_pf%5BGOD%5D%5BRIGHT%5D=2021&set_filter=%CD%E0%E9%F2%E8&set_filter=Y (accessed on 25 December 2023). (In Russian).
- Adoeva, E.Y.; Antykova, L.P.; Nikitin, A.F.; Rakhmanova, E.V. Socio-Epidemiological Characteristics of Cases of Echinococcosis in St. Petersburg for the Period from 2000 to 2011. Infect. Immun. 2012, 2, 350–351. [Google Scholar]
- Rospotrebnadzor Moscow (Federal Service for Supervision on Consumer Rights Protection and Human Wellbeing). State Report on the Condition of Sanitary and Epidemiological Welfare of the Population in the City of Moscow (Annual Reports 2011–2021). Available online: https://77.rospotrebnadzor.ru/index.php/doc/infdoc (accessed on 25 December 2023). (In Russian).
- Trishin, M.V. Epidemic Process of Echinococcosis and Epizootological Factors Conditioning Its Maintenance; Orenburg State Medical University: Orenburg, Russia, 2015; Available online: https://www.psma.ru/index.php?option=com_mtree&task=att_download&link_id=50&cf_id=2 (accessed on 25 December 2023). (In Russian)
- Abdulazizov, A.I. Modern Problems of Echinococcosis in Dagestan. Theory Pract. Combat. Parasit. Dis. 2011, 12, 6–10. Available online: https://cyberleninka.ru/article/n/sovremennye-problemy-ehinokokkoza-v-dagestane (accessed on 25 December 2023). (In Russian).
- Arakelyan, R.S.; Irdeeva, V.A.; Galimzyanov, K.M.; Akhmineeva, A.K.; Arakelyants, O.A. Clinical and Epidemiological Aspects of Human Echinococcosis in the Astrakhan Region for 2001–2020. Lechaschi Vrach 2023, 3, 58–63. (In Russian) [Google Scholar] [CrossRef]
- Peklo, G.N.; Stepanova, T.F. Echinococcosis in the Ural Federal District of Russia. Announcement 1: Epidemiological Aspects of the Problem. PH&LE 2017, 12, 51–56. Available online: https://cyberleninka.ru/article/n/ehinokokkozy-v-uralskom-federalnom-okruge-rossii-soobschenie-2-epizootologicheskie-aspekty-problemy (accessed on 25 December 2023). (In Russian).
- Rosselkhoznadzor (Federal Service for Veterinary and Phytosanitary Surveillance). Epizootic Situation in the Russian Federation: Annual Reports 2008–2021. Available online: https://fsvps.gov.ru/jepizooticheskaja-situacija/rossija/analiticheskij-ezhekvartalnyj-s-narastajushhim-itogom-otchet-po-jepidsituacii-v-strane-po-dannym-departamenta-veterinarii-msh (accessed on 25 December 2023). (In Russian)
- Rosstat (Federal State Statistics Service). Regions of Russia: Socio-Economic Indicators–2014. Available online: https://rosstat.gov.ru/bgd/regl/b14_14p/Main.htm (accessed on 25 December 2023). (In Russian)
- Popova, A.Y. On the Incidence of Echinococcosis and Alveococcosis in the Russian Federation. Federal Service for Surveillance in the Sphere of Consumer Rights Protection and Human Welfare (Rospotrebnadzor). Letter 24 June 2013 No. 01/14780-13-32. Available online: https://www.rospotrebnadzor.ru/documents/details.php?ELEMENT_ID=1097 (accessed on 25 December 2023). (In Russian).
- Dimidova, L.L.; Khutoryanina, I.V.; Chernikova, M.P.; Bolatchiev, K.K.; Tverdokhlebova, T.I. Analysis of Records of the Epidemiological Screening of Cases of Echinococcosis in Some Constituent Entities of the Russian Federation. Theory Pract. Combat. Parasit. Dis. 2021, 22, 162–167. Available online: http://www.spsl.nsc.ru/FullText/konfe/ТеoрПрБoрПаразитБoлВып22.pdf (accessed on 25 December 2023). (In Russian). [CrossRef]
- Minaev, S.V.; Mashchenko, A.N.; Aydemirov, A.N.; Anisimov, I.N.; Gerasimenko, I.N.; Rubanova, M.F. Epidemiological Characteristics of Echinococcosis in Adults and Pediatric Populations of Stavropol Region. Clin. Med. 2018, 7, 35–38. (In Russian) [Google Scholar]
- Panfilov, K.A. Choice of a Method for Surgical Treatment of Liver Hydatidosis; Samara State Medical University: Samara, Russia, 2020. (In Russian) [Google Scholar]
- Sharipov, R.H. Percutaneous Transhepatic Echinococcectomy: Dangers, Complications, and Prevention Strategies; Sechenov University: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Ministry of Health of Russia (Minzdrav). Prevention of Parasitic Diseases in the Russian Federation: Sanitary and Epidemiological Rules and Regulations (SanPiN 3.2.1333-03); Ministry of Health of Russia (Minzdrav): Moscow, Russia, 2003. (In Russian)
- Ministry of Health of the Russian Federation (Minzdrav). Prevention of Parasitic Diseases on the Territory of the Russian Federation: Sanitary and Epidemiological Rules and Regulations. SanPiN 3.2.3215-14; Ministry of Health of the Russian Federation (Minzdrav): Moscow, Russia, 2014. Available online: https://rospotrebnadzor.ru/documents/details.php?ELEMENT_ID=2890 (accessed on 25 December 2023). (In Russian)
- Ermakova, Y.A.; Dumbadze, O.S.; Chernikova, M.P.; Dimidova, Y.Y.; Tverdokhlebova, T.I. Analysis of the incidence of echinococcosis in the Russian Federation. Theory Pract. Combat. Parasit. Dis. 2023, 2, 177–183. (In Russian) [Google Scholar]
- Rospotrebnadzor (Federal Service for Surveillance in the Sphere of Consumer Rights Protection and Human Welfare). On the Incidence of Echinococcosis in the Russian Federation. Letter Dated 30 September 2010, No. 01/14090-0-32. Available online: https://www.garant.ru/products/ipo/prime/doc/4091138/ (accessed on 25 December 2023). (In Russian).
- Frider, B.; Larrieu, E. Treatment of Liver Hydatidosis: How to Treat an Asymptomatic Carrier? World J. Gastroenterol. 2010, 16, 4123–4129. [Google Scholar] [CrossRef]
- Ermakova, L.A.; Tverdokhlebova, T.I.; Nagorny, S.A.; Pshenichnaya, N.Y.; Boltachiev, K.K. Analysis of Incidence of Humans with Larvae Helminthiases (Echinococcosis, Toxocariasis, Dirofilariasis) in the Russian Federation. Epidemiol. Vaccinal Prev. 2017, 16, 43–46. (In Russian) [Google Scholar] [CrossRef]
- Rozhin, K.A.; Cristianovsky, P.I. The Dynamics of Epizootic Process of Echinococcosis in the Russian Federation and the Orenburg Region. Bull. Orenburg Sci. Cent. Ural Branch Russian Acad. Sci. 2014, 1, 1–6. (In Russian) [Google Scholar]
- Shishkanova, L.V.; Tverdokhlebova, T.I.; Ermakova, L.A.; Dumbadze, O.S. Analysis of the Incidence of Current Larval Helminthiases in the Population on the Territory of the Russian Federation. Theory Pract. Combat. Parasit. Dis. 2016, 17, 535–538. [Google Scholar]
- Weekly Annual Average of the Population of Constituent Entities of the Russian Federation Federation, 2015–2017. Available online: https://www.demoscope.ru/weekly/archives.php (accessed on 25 December 2023).
- Andreyanov, O.N. Examining Echinococcus multilocularis Infection in Some Midland Russia Predatory Animal Species. Russian J. Infect. Immun. 2020, 10, 193–196. Available online: https://cyberleninka.ru/article/n/alveolyarnyy-ehinokokkoz-u-promyslovyh-zhivotnyh-v-ryazanskoy-oblasti (accessed on 25 December 2023). (In Russian). [CrossRef]
- Rosstat-Federal State Statistics Service of the Russian Federation. Russian Statistical Yearbook 2003–2023. Available online: https://rosstat.gov.ru/folder/210/document/12994 (accessed on 25 October 2024).
- Doronin-Dorgelinsky, E.; Sivkova, T. The Management of Prevention and Control of Cystic Echinococcosis in the Territory of the Russian Federation. Bull. Voronezh State Agrar. Univ. 2017, 3, 67–75. Available online: http://vestnik.vsau.ru/wp-content/uploads/2017/12/67-74.pdf (accessed on 25 December 2023). (In Russian). [CrossRef]
- Rospotrebnadzor (Federal Service for Supervision of Consumer Rights Protection and Human Well-Being). On the State of Sanitary and Epidemiological Well-Being of the Population in the Russian Federation in 2018: State Report. 2019. Available online: https://rospotrebnadzor.ru/upload/iblock/798/gosudarstvennyy-doklad-o-sostoyanii-sanitarno_epidemiologicheskogo-blagopoluchiya-naseleniya-v-rossiyskoy-federatsii-v-2018-godu.pdf (accessed on 25 December 2023). (In Russian).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobić, B.; Štajner, T.; Ćirković, V.; Srbljanović, J.; Lijeskić, O.; Bauman, N.; Zlatković, Đ. Human Echinococcosis in the Russian Federation in the 21st Century: A Systematic Review. Microorganisms 2025, 13, 1122. https://doi.org/10.3390/microorganisms13051122
Bobić B, Štajner T, Ćirković V, Srbljanović J, Lijeskić O, Bauman N, Zlatković Đ. Human Echinococcosis in the Russian Federation in the 21st Century: A Systematic Review. Microorganisms. 2025; 13(5):1122. https://doi.org/10.3390/microorganisms13051122
Chicago/Turabian StyleBobić, Branko, Tijana Štajner, Vladimir Ćirković, Jelena Srbljanović, Olivera Lijeskić, Neda Bauman, and Đorđe Zlatković. 2025. "Human Echinococcosis in the Russian Federation in the 21st Century: A Systematic Review" Microorganisms 13, no. 5: 1122. https://doi.org/10.3390/microorganisms13051122
APA StyleBobić, B., Štajner, T., Ćirković, V., Srbljanović, J., Lijeskić, O., Bauman, N., & Zlatković, Đ. (2025). Human Echinococcosis in the Russian Federation in the 21st Century: A Systematic Review. Microorganisms, 13(5), 1122. https://doi.org/10.3390/microorganisms13051122





