Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface
Abstract
1. Introduction
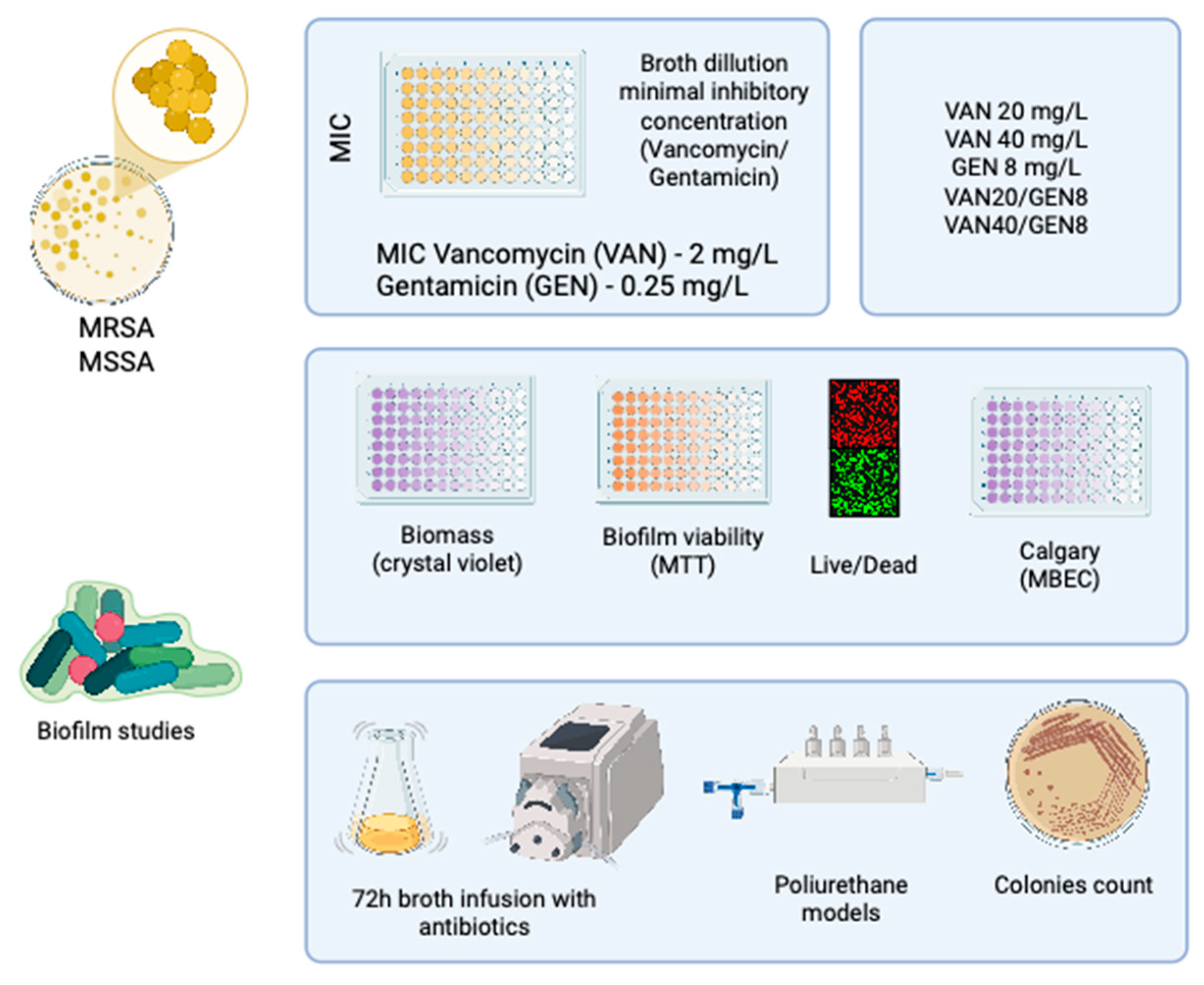
2. Materials and Methods
2.1. Ethical Considerations and Study Design
2.2. Bacterial Strains and Identification
2.3. Antimicrobial Agents and Preparation
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Evaluation of Growth Inhibition in Combination Therapy
2.6. Biofilm Formation and Quantification
2.7. Minimum Biofilm Eradication Concentration (MBEC) Assay
2.8. Live/Dead Fluorescence Microscopy
2.9. Dynamic Biofilm Model Under Continuous Flow
2.10. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC) Results
3.2. In Vitro Growth Inhibition by Monotherapy and Combination Therapy
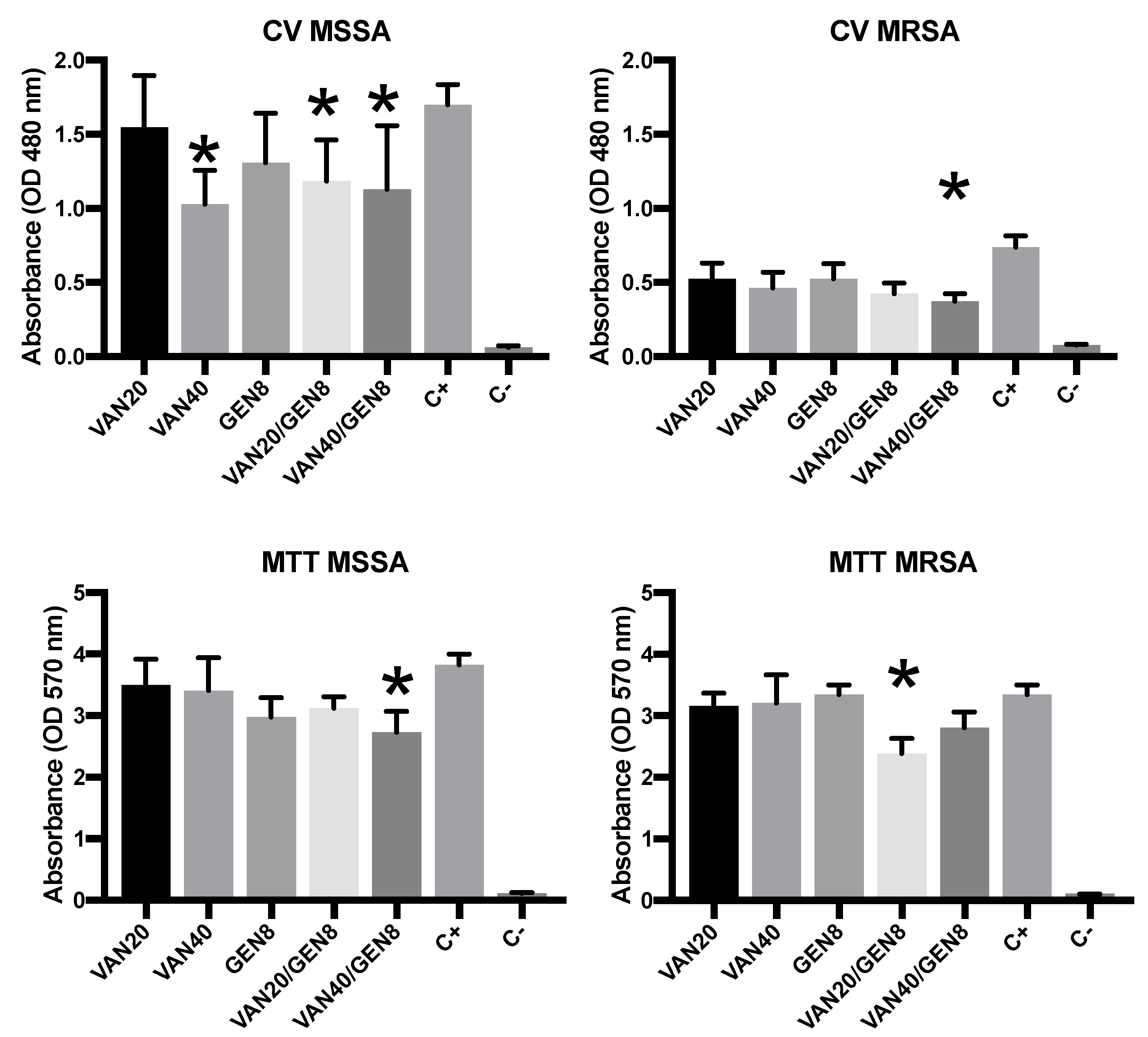
3.3. Biofilm Biomass Quantification and MTT
3.4. Minimum Biofilm Eradication Concentration (MBEC)
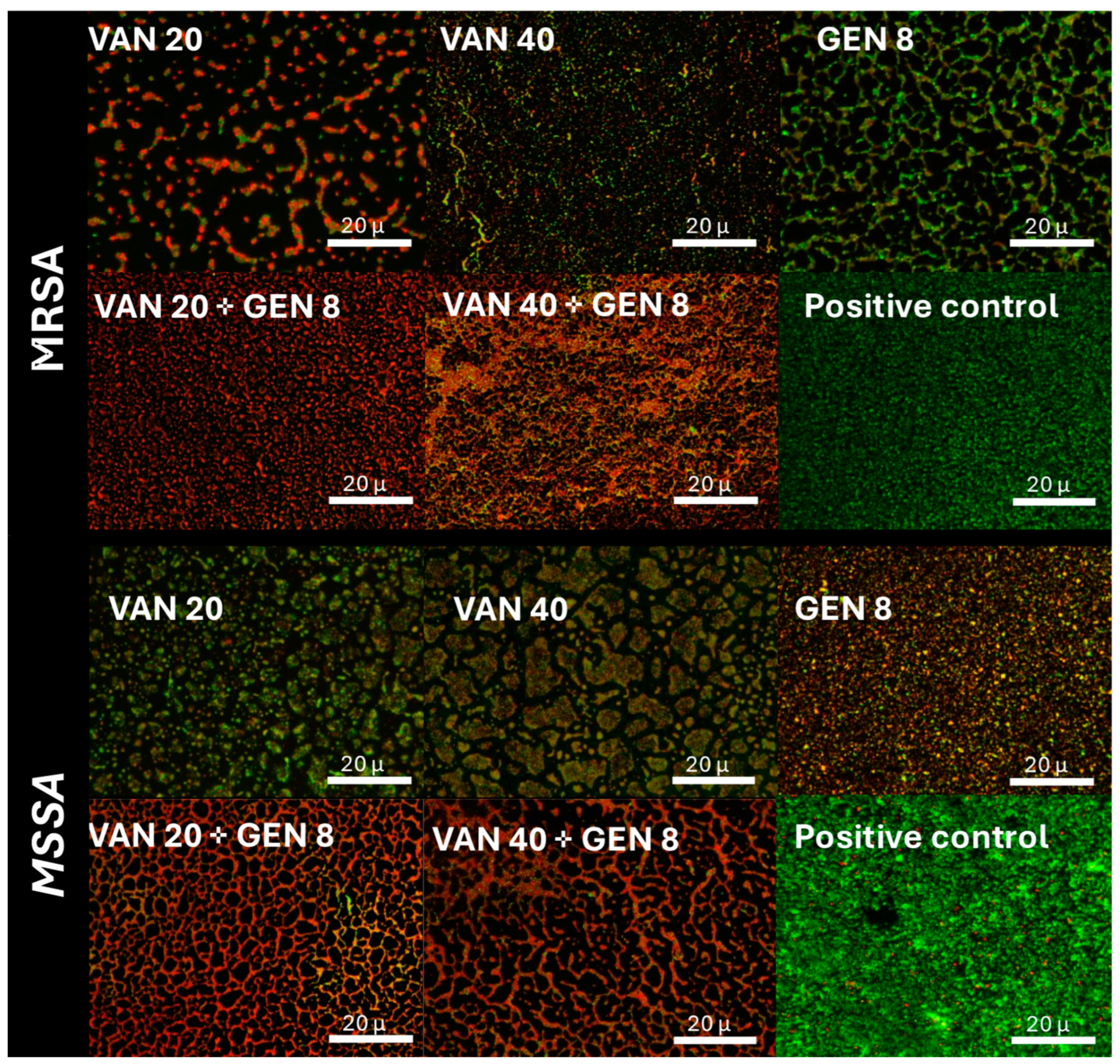
3.5. Fluorescence Microscopy Imaging of Biofilm Viability
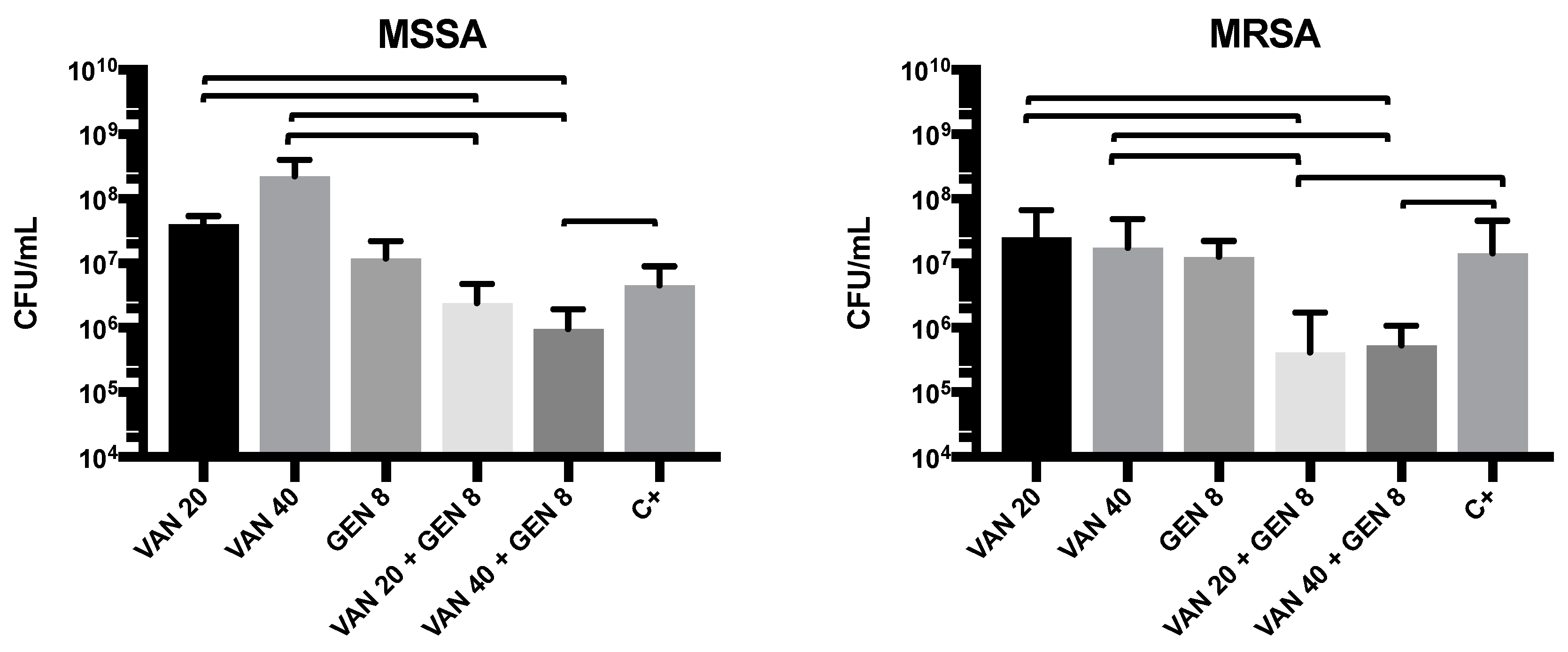
3.6. Evaluation of Antimicrobial Activity in the Dynamic Biofilm Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunelli, S.M.; Turenne, W.; Sibbel, S.; Hunt, A.; Pfaffle, A. Clinical and economic burden of bloodstream infections in critical care patients with central venous catheters. J. Crit. Care 2016, 35, 69–74. [Google Scholar] [CrossRef]
- Sohail, M.; Latif, Z. Molecular analysis, biofilm formation, and susceptibility of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in central venous catheters. Rev. Soc. Bras. Med. Trop. 2018, 51, 603–609. [Google Scholar] [CrossRef]
- Cento, V.; Carloni, S.; Sarti, R.; Bussini, L.; Asif, Z.; Morelli, P.; De Fazio, F.; Tordato, F.M.; Casana, M.; Mondatore, D.; et al. Epidemiology and Resistance Profiles of Bacteria Isolated From Blood Samples in Septic Patients at Emergency Department Admission: A 6-Year Single Centre Retrospective Analysis From Northern Italy. J. Glob. Antimicrob. Resist. 2025, 41, 202–210. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Restivo, E.; Peluso, E.; Bloise, N.; Bello, G.L.; Bruni, G.; Giannaccari, M.; Raiteri, R.; Fassina, L.; Visai, L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. J. Funct. Biomater. 2024, 15, 24. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Alazzam, A. Transforming medical device biofilm control with surface treatment using microfabrication techniques. PLoS ONE 2023, 18, e0292647. [Google Scholar] [CrossRef]
- Kuwada, N.; Fujii, Y.; Nakatani, T.; Ousaka, D.; Tsuji, T.; Imai, Y.; Kobayashi, Y.; Oozawa, S.; Kasahara, S.; Tanemoto, K. Diamond-like carbon coating to inner surface of polyurethane tube reduces Staphylococcus aureus bacterial adhesion and biofilm formation. J. Artif. Organs 2024, 27, 108–116. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Yee, R.; Yuan, Y.; Tarff, A.; Brayton, C.; Gour, N.; Feng, J.; Zhang, Y. Eradication of Staphylococcus aureus Biofilm Infection by Persister Drug Combination. Antibiotics 2022, 11, 1278. [Google Scholar] [CrossRef]
- He, X.; Yuan, F.; Lu, F.; Yin, Y.; Cao, J. Vancomycin-induced biofilm formation by methicillin-resistant Staphylococcus aureus is associated with the secretion of membrane vesicles. Microb. Pathog. 2017, 110, 225–231. [Google Scholar] [CrossRef]
- Chapman, J.E.; George, S.E.; Wolz, C.; Olson, M.E. Biofilms: A developmental niche for vancomycin-intermediate Staphylococcus aureus. Infect. Genet. Evol. 2024, 117, 105545. [Google Scholar] [CrossRef]
- Hess, D.J.; Henry-Stanley, M.J.; Wells, C.L. Gentamicin promotes Staphylococcus aureus biofilms on silk suture. J. Surg. Res. 2011, 170, 302–308. [Google Scholar] [CrossRef]
- Pedroni, M.A.; Ribeiro, V.S.T.; Cieslinski, J.; Lopes, A.P.A.; Kraft, L.; Suss, P.H.; Tuon, F.F. Different concentrations of vancomycin with gentamicin loaded PMMA to inhibit biofilm formation of Staphylococcus aureus and their implications. J. Orthop. Sci. 2024, 29, 334–340. [Google Scholar] [CrossRef]
- CLSI. Clinical Laboratory Standard Insitute—M07—A10—Methods for Dilution Antimicrobial Suceptibility Tests for Bacteria that Grow Aerobically. 2015. Available online: https://clsi.org/shop/standards/m07/ (accessed on 9 April 2025).
- Bakke, V.; Sporsem, H.; Von der Lippe, E.; Nordoy, I.; Lao, Y.; Nyrerod, H.C.; Sandvik, L.; Harvig, K.R.; Bugge, J.F.; Helset, E. Vancomycin levels are frequently subtherapeutic in critically ill patients: A prospective observational study. Acta Anaesthesiol. Scand. 2017, 61, 627–635. [Google Scholar] [CrossRef]
- Tuon, F.F.; Yamada, C.H.; Cieslinski, J.; Dos Santos Oliveira, D.; Ribeiro, V.S.T.; Gasparetto, J.; Telles, J.P. Cerebrospinal Fluid Penetration of Vancomycin During Continuous Infusion Therapy in Patients with Nosocomial Ventriculitis. Ther. Drug Monit. 2021, 43, 807–811. [Google Scholar] [CrossRef]
- Telles, J.P.; Morales, R., Jr.; Yamada, C.H.; Marins, T.A.; D’Amaro Juodinis, V.; Sztajnbok, J.; Silva, M., Jr.; Bassetti, B.R.; Albiero, J.; Tuon, F.F. Optimization of Antimicrobial Stewardship Programs Using Therapeutic Drug Monitoring and Pharmacokinetics-Pharmacodynamics Protocols: A Cost-Benefit Review. Ther. Drug Monit. 2023, 45, 200–208. [Google Scholar] [CrossRef]
- Tuon, F.F.; Romero, R.; Gasparetto, J.; Cieslinski, J. Vancomycin trough level and loading dose. Infect. Drug Resist. 2018, 11, 2393–2396. [Google Scholar] [CrossRef]
- Yamada, C.H.; Telles, J.P.; Oliveira, D.D.S.; Cieslinski, J.; Ribeiro, V.S.T.; Gasparetto, J.; Tuon, F.F. Comparison of intermittent versus continuous-infusion vancomycin for treating severe patients in intensive care units. Braz. J. Infect. Dis. 2020, 24, 356–359. [Google Scholar] [CrossRef]
- Peixoto, B.C.; Contrera, G.G.; Cieslinski, J.; Gasparetto, J.; Tuon, F.F. Acute kidney injury in patients using low dose (3 mg/kg/day) of gentamicin under therapeutic dose monitoring. J. Infect. 2018, 76, 496–498. [Google Scholar] [CrossRef]
- Toledo, P.V.; Tuon, F.F.; Arend, L.; Aranha Junior, A.A. Efficacy of tigecycline, polymyxin, gentamicin, meropenem and associations in experimental Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae non-lethal sepsis. Braz. J. Infect. Dis. 2014, 18, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Kruger, H.C.; Francio, J.; Silva, A.S.D.; Oliveira, G.S.N.; Brancher, J.A.; Dantas, L.R.; Oliveira, R.C.; Tuon, F.F.; Carneiro, E. Antimicrobial action, cytotoxicity, calcium ion release, and pH variation of a calcium hydroxide-based paste associated with Myracrodruon urundeuva Allemao extract. Aust. Endod. J. 2022, 48, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Chaiben, V.; Yamada, C.H.; Telles, J.P.; de Andrade, A.P.; Arend, L.; Ribeiro, V.S.T.; Dantas, L.R.; Suss, P.H.; Tuon, F.F. A carbapenem-resistant Acinetobacter baumannii outbreak associated with a polymyxin shortage during the COVID pandemic: An in vitro and biofilm analysis of synergy between meropenem, gentamicin and sulbactam. J. Antimicrob. Chemother. 2022, 77, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Molina-Manso, D.; del Prado, G.; Ortiz-Perez, A.; Manrubia-Cobo, M.; Gomez-Barrena, E.; Cordero-Ampuero, J.; Esteban, J. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int. J. Antimicrob. Agents 2013, 41, 521–523. [Google Scholar] [CrossRef]
- Kraft, L.; Ribeiro, V.S.T.; Goncalves, G.A.; Suss, P.H.; Tuon, F.F. Comparison of amphotericin B lipid complex, deoxycholate amphotericin B, fluconazole, and anidulafungin activity against Candida albicans biofilm isolated from breakthrough candidemia. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2023, 41, 596–603. [Google Scholar] [CrossRef]
- Permuy, C.; Ruiz-Azcarate, J.; Sampedro, M.; Jimenez, C.; Baquero-Artigao, F.; Calvo, C.; Mendez-Echevarria, A. Usefulness of daptomycin lock therapy in children with catheter-related bacteremia after failed vancomycin lock therapy. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 48. [Google Scholar] [CrossRef]
- Lebeaux, D.; Leflon-Guibout, V.; Ghigo, J.M.; Beloin, C. In vitro activity of gentamicin, vancomycin or amikacin combined with EDTA or l-arginine as lock therapy against a wide spectrum of biofilm-forming clinical strains isolated from catheter-related infections. J. Antimicrob. Chemother. 2015, 70, 1704–1712. [Google Scholar] [CrossRef]
- Labriola, L. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin. Dial. 2019, 32, 402–405. [Google Scholar] [CrossRef]
- del Pozo, J.L. Role of antibiotic lock therapy for the treatment of catheter-related bloodstream infections. Int. J. Artif. Organs 2009, 32, 678–688. [Google Scholar] [CrossRef]
- Adamu, Y.; Puig-Asensio, M.; Dabo, B.; Schweizer, M.L. Comparative effectiveness of daptomycin versus vancomycin among patients with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections: A systematic literature review and meta-analysis. PLoS ONE 2024, 19, e0293423. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Zhang, P.; Bai, H.; Sun, Y.; Duan, J.; Mu, H. Lysozyme Associated Liposomal Gentamicin Inhibits Bacterial Biofilm. Int. J. Mol. Sci. 2017, 18, 784. [Google Scholar] [CrossRef] [PubMed]
- Soni, J.F.; Ribeiro, V.S.T.; Cieslinski, J.; de Andrade, A.P.; Dantas, L.R.; Pereira, B.Z.; de Almeida, B.; Suss, P.H.; Tuon, F.F. Evaluation of silver nanoparticle-impregnated PMMA loaded with vancomycin or gentamicin against bacterial biofilm formation. Injury 2023, 54 (Suppl. 6), 110649. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Ghosh, M.; Roy, S.; Basak, S.; Bhattacharjee, S. Quercetin combined with ciprofloxacin and gentamicin inhibits biofilm formation and virulence in Staphylococcus aureus. Microb. Pathog. 2025, 200, 107297. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.S.C.; Leitao, M.M.; Fernandes, J.R.; Saavedra, M.J.; Pereira, C.; Simoes, M.; Borges, A. Photodynamic activation of phytochemical-antibiotic combinations for combatting Staphylococcus aureus from acute wound infections. J. Photochem. Photobiol. B 2024, 258, 112978. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Knap, K.; Kwiecien, K.; Ochonska, D.; Reczynska-Kolman, K.; Pamula, E.; Brzychczy-Wloch, M. Synergistic effect of antibiotics, alpha-linolenic acid and solvent type against Staphylococcus aureus biofilm formation. Pharmacol. Rep. 2024, 76, 1456–1469. [Google Scholar] [CrossRef]
- Bauer, J.; Siala, W.; Tulkens, P.M.; Van Bambeke, F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2013, 57, 2726–2737. [Google Scholar] [CrossRef]
- Franklin, M.J.; Chang, C.; Akiyama, T.; Bothner, B. New Technologies for Studying Biofilms. Microbiol. Spectr. 2015, 1–41. [Google Scholar] [CrossRef]
- Hosseini, M.; Shapouri Moghaddam, A.; Derakhshan, S.; Hashemipour, S.M.A.; Hadadi-Fishani, M.; Pirouzi, A.; Khaledi, A. Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated from Clinical Samples in Iran: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2020, 26, 1071–1080. [Google Scholar] [CrossRef]
- Brahma, U.; Sharma, P.; Murthy, S.; Sharma, S.; Chakraborty, S.; Appalaraju, S.N.; Bhandari, V. Decreased expression of femXAB genes and fnbp mediated biofilm pathways in OS-MRSA clinical isolates. Sci. Rep. 2019, 9, 16028. [Google Scholar] [CrossRef]
- Coenye, T. Biofilm antimicrobial susceptibility testing: Where are we and where could we be going? Clin. Microbiol. Rev. 2023, 36, e0002423. [Google Scholar] [CrossRef] [PubMed]
- Sabino, H.A.C.; Valera, F.C.P.; Santos, D.V.; Fantucci, M.Z.; Titoneli, C.C.; Martinez, R.; Anselmo-Lima, W.T.; Tamashiro, E. Biofilm and Planktonic Antibiotic Resistance in Patients with Acute Exacerbation of Chronic Rhinosinusitis. Front. Cell. Infect. Microbiol. 2021, 11, 813076. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Di Franco, S.; Passavanti, M.B.; Pace, M.C.; Simeon, V.; Chiodini, P.; Leone, S.; Fiore, M. Antimicrobial Lock Therapy in Clinical Practice: A Scoping Review. Microorganisms 2025, 13, 406. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Thorarinsdottir, H.R.; Kander, T.; Holmberg, A.; Petronis, S.; Klarin, B. Biofilm formation on three different endotracheal tubes: A prospective clinical trial. Crit. Care 2020, 24, 382. [Google Scholar] [CrossRef]
- Mathias, S.; Wiseman, O. Silicone vs. Polyurethane Stent: The Final Countdown. J. Clin. Med. 2022, 11, 2746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, N.H.; Suss, P.H.; Ortis, G.B.; Dantas, L.R.; Tuon, F.F. Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface. Microorganisms 2025, 13, 1119. https://doi.org/10.3390/microorganisms13051119
Borges NH, Suss PH, Ortis GB, Dantas LR, Tuon FF. Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface. Microorganisms. 2025; 13(5):1119. https://doi.org/10.3390/microorganisms13051119
Chicago/Turabian StyleBorges, Nicolas Henrique, Paula Hansen Suss, Gabriel Burato Ortis, Leticia Ramos Dantas, and Felipe Francisco Tuon. 2025. "Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface" Microorganisms 13, no. 5: 1119. https://doi.org/10.3390/microorganisms13051119
APA StyleBorges, N. H., Suss, P. H., Ortis, G. B., Dantas, L. R., & Tuon, F. F. (2025). Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface. Microorganisms, 13(5), 1119. https://doi.org/10.3390/microorganisms13051119





