Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings
Abstract
1. Introduction
2. Background of Shewanella
3. Microbiological Characteristics of Shewanella
4. Molecular Identification/Characterization of Shewanella
5. Heavy Metals’ Roles in Antibiotic Resistance Acceleration
6. Antibiotic Reservoirs in the Aquatic Environment
7. Shewanella in Human Infections
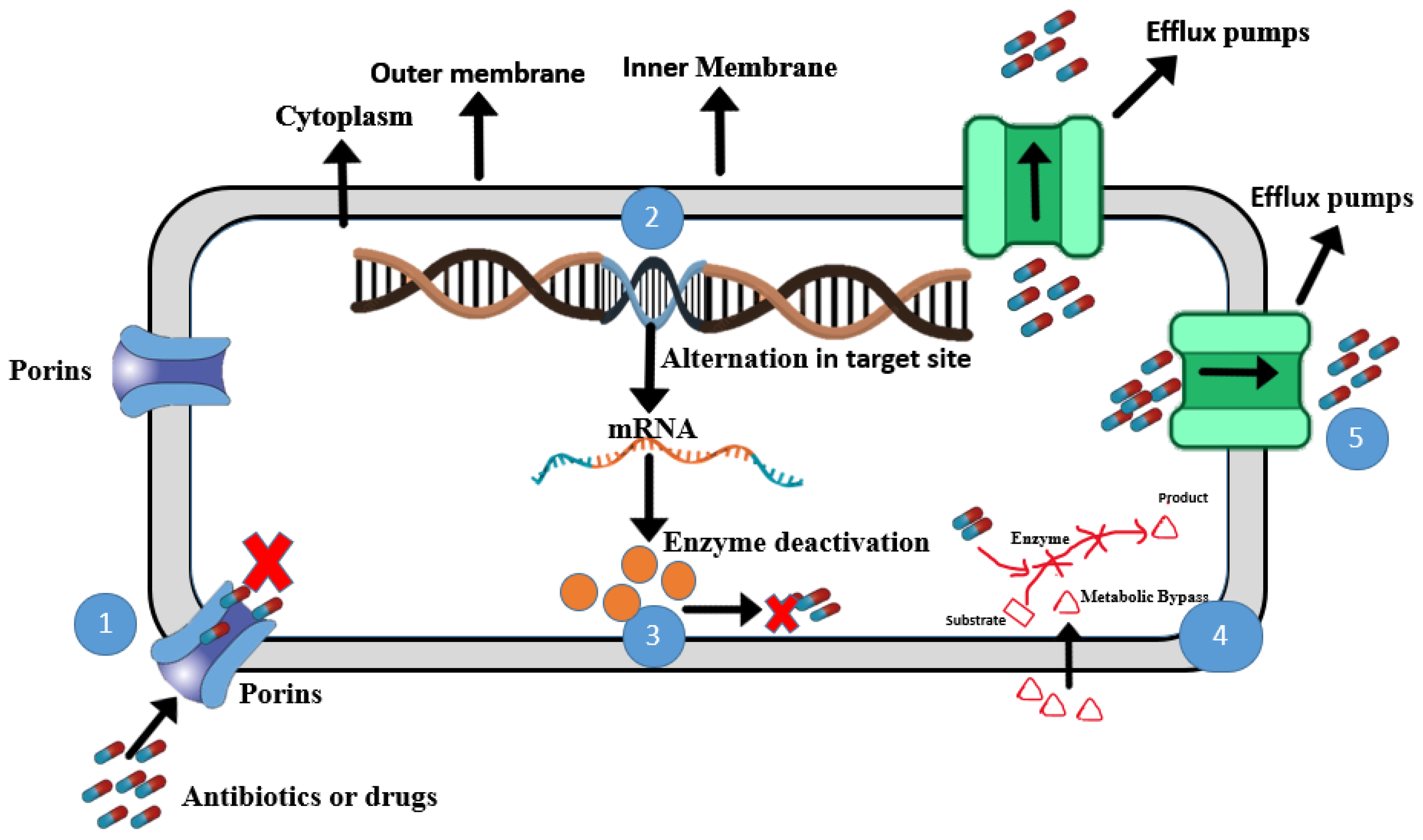
8. Mechanisms of Antibiotic Resistance
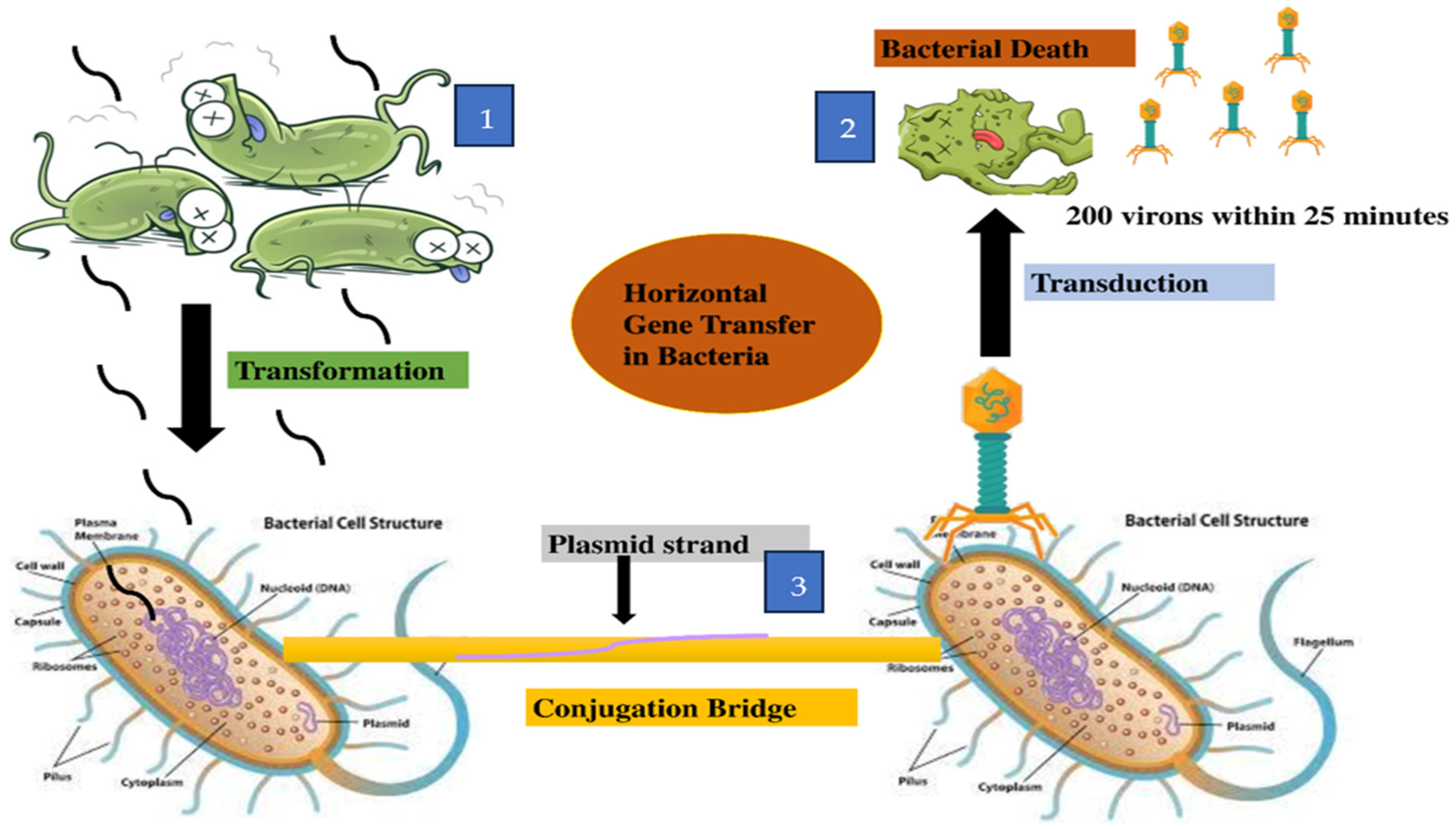
9. Transmission of Resistance Genes
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Fact. 2023, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Capasso, C.; Supuran, C.T. Carbonic anhydrase inhibitors as Novel antibacterials in the era of antibiotic resistance: Where are we now? Antibiotics 2023, 12, 142. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Yekani, M.; Azargun, R.; Sharifi, S.; Nabizadeh, E.; Nahand, J.S.; Ansari, N.K.; Soki, J. Collateral sensitivity: An evolutionary trade-off between antibiotic resistance mechanisms, attractive for dealing with drug-resistance crisis. Health Sci. Rep. 2023, 6, e1418. [Google Scholar] [CrossRef]
- Iu, H.T.V.; Fong, P.M.; Yam, H.C.B.; Gao, P.; Yan, B.; Lai, P.M.; Ng, K.H.K. Identification of a small molecule compound active against antibiotic-tolerant Staphylococcus aureus by boosting ATP synthesis. Int. J. Mol. Sci. 2023, 24, 6242. [Google Scholar] [CrossRef]
- Mahmoudi, L.; Mahdavinia, A.; Khajooi, S.; Zargari-Samadnejad, A.; Karimzadeh, I. Antibiotic use pattern of surgical site infection prophylaxis in surgical wards of a teaching hospital in Shiraz, Iran. Trends Pharm. Sci. 2023, 9, 87–92. [Google Scholar]
- Dyary, H.; Faraj, G.; Saeed, N. History, current situation, and future perspectives on antibiotics and antibiotic Resistance. In One Health Triad; Unique Scientific Publishers: Faisalabad, Pakistan, 2023; Volume 2, pp. 109–118. [Google Scholar]
- Ren, Z.; Luo, W. Metagenomic analysis reveals the diversity and distribution of antibiotic resistance genes in thermokarst lakes of the Yellow River Source Area. Environ. Pollut. 2022, 313, e120102. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef]
- Yousfi, K.; Bekal, S.; Usongo, V.; Touati, A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1353–1362. [Google Scholar] [CrossRef]
- Azizi, S.M.M.; Haffiez, N.; Zakaria, B.S.; Elbeshbishy, E.; Dhar, B.R. Nano-and microplastics as carriers for antibiotics and antibiotic resistance genes. In Curr. Dev. Biotechnol. Bioengin. 2023, 361–385. [Google Scholar]
- Johnson, T.; Richards, G.P.; Jacobs, J.; Townsend, H.; Almuhaideb, E.; Rosales, D.; Parveen, S. Prevalence and pathogenic potential of Shewanella species in oysters and seawater collected from the Chesapeake Bay and Maryland Coastal Bays. Front. Microbiol. 2025, 16, e1502443. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.C.; Hugie, C.N.; Kile, M.L.; Nawab-Daneshmand, T. Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: A review. Front. Environ. Sci. Eng. 2019, 13, 46. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Su, J.Q. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef]
- Lu, Z.; Na, G.; Gao, H.; Wang, L.; Bao, C.; Yao, Z. Fate of sulfonamide resistance genes in estuary environment and effect of anthropogenic activities. Sci. Total Environ. 2015, 527, 429–438. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Nunes, B. Effects of low levels of the antibiotic ciprofloxacin on the polychaete Hediste diversicolor: Biochemical and behavioural effects. Environ. Toxicol. Pharmacol. 2020, 80, e103505. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.; Xiao, Y.; Wang, D. Shewanella infection in humans: Epidemiology, clinical features and pathogenicity. Virulence 2022, 13, 1515–1532. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Shewanella: From the briny depths below to human pathogen. Crit. Rev. Microbiol. 2014, 40, 293–312. [Google Scholar] [CrossRef]
- Dominguez, H.; Vogel, B.F.; Gram, L.; Hoffmann, S.; Schaebel, S. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin. Infect. Dis. 1996, 22, 1036–1039. [Google Scholar] [CrossRef][Green Version]
- Holt, H.M.; Gahrn-Hansen, B.; Bruun, B. Shewanella algae and Shewanella putrefaciens: Clinical and microbiological characteristics. Clin. Microbiol. Infect. 2005, 11, 347–352. [Google Scholar]
- Satomi, M. The family Shewanellaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 597–625. [Google Scholar]
- MacDonell, M.; Colwell, R. Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella. Syst. Appl. Microbiol. 1985, 6, 171–182. [Google Scholar] [CrossRef]
- Nouioui, I.; Tarhriz, V.; Kim, H.M.; Montazersaheb, S.; Hejazi, M.A.; Jeon, C.O.; Hejazi, M.S. Shewanella azerbaijanica sp. nov. a novel aquatic species with high bioremediation abilities. Arch. Microbiol. 2022, 204, 496. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Meier-Kolthoff, J.P. Whole genome-based taxonomy of Shewanella and Parashewanella. Int. J. Syst. Evol. Microbiol. 2022, 72, 005438. [Google Scholar] [CrossRef]
- Ortega, R.C.M.H.; Tabugo, S.R.M.; Martinez, J.G.T.; Padasas, C.S.; Balcázar, J.L. Occurrence of Aeromonas Species in the Cutaneous Mucus of Barbour’s Seahorses (Hippocampus barbouri) as Revealed by High-Throughput Sequencing. Animals 2023, 13, 1241. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Shin, Y.; Jeon, H.; Choi, J.H.; Jeong, S.; So, J.S. Antibiotic resistance of Shewanella putrefaciens isolated from shellfish collected from the west sea in Korea. Mar. Pollut. Bull. 2013, 76, 85–88. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological changes in fish gut in response to prebiotics and probiotics treatment to improve their health status: A review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Sabreena, K.Z.; Bhat, S.A.; Kumar, V.; Ameen, F.; Ganai, B.A. Marine bacteria and omic approaches: A novel and potential repository for bioremediation assessment. J. Appl. Microbiol. 2022, 133, 2299–2313. [Google Scholar] [CrossRef]
- Tseng, S.Y.; Liu, P.Y.; Lee, Y.H.; Wu, Z.Y.; Huang, C.C.; Cheng, C.C.; Tung, K.C. The pathogenicity of Shewanella algae and ability to tolerate a wide range of temperatures and salinities. Can. J. Infect. Dis. Med. Microbiol. 2018, 1, e6976897. [Google Scholar]
- Wang, J.H.; Tseng, S.Y.; Tung, K.C. Genomic investigation of emerging zoonotic pathogen Shewanella xiamenensis. Tzu Chi Med. J. 2020, 32, 162–166. [Google Scholar]
- Poovorawan, K.; Chatsuwan, T.; Lakananurak, N.; Chansaenroj, J.; Komolmit, P.; Poovorawan, Y. Shewanella haliotis associated with severe soft tissue infection, Thailand, 2012. Emerg. Infect. Dis. 2013, 19, 1019. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Y.; Pang, F.; Liang, S.; Lu, X.; Kan, B.; Wang, D. Rare Shewanella spp. associated with pulmonary and bloodstream infections of cancer patients, China: A case report. BMC Infect. Dis. 2018, 18, 1–5. [Google Scholar] [CrossRef]
- Müller, S.; von Bonin, S.; Schneider, R.; Krüger, M.; Quick, S.; Schröttner, P. Shewanella putrefaciens, a rare human pathogen: A review from a clinical perspective. Front. Cell. Infect. Microbiol. 2023, 12, e1033639. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Brosnahan, C.; Jones, B.; Ross, A.; Phiri, B.; Pal, C.; Bestbier, M. Tail Fan Necrosis in New Zealand Red Rock Lobster, Jasus edwardsii. MPI Tech. Pap. 2021, 33, 1–29. [Google Scholar]
- Gomes, M.P. The convergence of antibiotic contamination, resistance, and climate dynamics in freshwater ecosystems. Water 2024, 16, 2606. [Google Scholar] [CrossRef]
- Islam, S.S.; Midya, S. Growth Regulatory Pattern of Zooplankton in Herbicide and Antibiotic Contaminated Aquatic Ecosystem: An overview. Watershed Ecol. Environ. 2023, 5, 153–160. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Chen, S.; Guan, X.; Zhong, Y.; Yang, Q. Occurrence, risk assessment, and in vitro and in vivo toxicity of antibiotics in surface water in China. Ecotoxicol. Environ. Saf. 2023, 255, e114817. [Google Scholar] [CrossRef]
- Liang, J.; She, J.; Fu, J.; Wang, J.; Ye, Y.; Yang, B.; Tao, H. Advances in natural products from the marine-sponge-associated microorganisms with antimicrobial activity in the last decade. Mar. Drugs 2023, 21, 236. [Google Scholar] [CrossRef]
- Samantha, A.; Vrielink, A. Lipid A phosphoethanolamine transferase: Regulation, structure and immune response. J. Mol. Biol. 2020, 432, 5184–5196. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Crane, E.J.; Burt, I.G.; Bushek, D. Shewanella and Photobacterium spp. in oysters and seawater from the Delaware Bay. Appl. Environ. Microbiol. 2008, 74, 3323–3327. [Google Scholar] [CrossRef]
- Yousfi, K.; Touati, A.; Bekal, S. Complete genome sequence of an extensively drug-resistant Shewanella xiamenensis strain isolated from Algerian hospital effluents. Genome Announc. 2016, 4, e01236-16. [Google Scholar] [CrossRef]
- Satomi, M.; Oikawa, H.; Yano, Y. Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int. J. Syst. Evol. Microbiol. 2003, 53, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.; Li, Y.; Fu, Q.; Lin, L.; Wu, S.; Lan, R. Establishment and application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for detection of Shewanella genus. Front. Microbiol. 2021, 12, 625821. [Google Scholar] [CrossRef]
- Mateos, G.; Bonilla, A.M.; de Francisco de Polanco, S.; Martínez, J.M.; Escudero, C.; Rodríguez, N.; Amils, R. Shewanella sp. T2. 3D-1.1 a novel microorganism sustaining the iron cycle in the deep subsurface of the Iberian Pyrite Belt. Microorganisms 2022, 10, 1585. [Google Scholar] [CrossRef]
- Gupta, S.; Graham, D.W.; Sreekrishnan, T.; Ahammad, S.Z. Effects of heavy metals pollution on the co-selection of metal and antibiotic resistance in urban rivers in UK and India. Environ. Pollut. 2022, 306, e119326. [Google Scholar] [CrossRef]
- Li, Z.; Junaid, M.; Chen, G.; Wang, J. Interactions and associated resistance development mechanisms between microplastics, antibiotics and heavy metals in the aquaculture environment. Rev. Aquacult. 2022, 14, 1028–1045. [Google Scholar] [CrossRef]
- Bosch, J.; Bezuidenhout, C.; Coertze, R.; Molale-Tom, L. Metal-and antibiotic-resistant heterotrophic plate count bacteria from a gold mine impacted river: The Mooi River system, South Africa. Environ. Sci. Pollut. Res. 2023, 30, 31605–31619. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; M’ikanatha, N.M.; Whitehouse, C.A.; Tate, H.; Ottesen, A.; Lorch, J.M.; Dudley, E.G. Low occurrence of multi-antimicrobial and heavy metal resistance in Salmonella enterica from wild birds in the United States. Environ. Microbiol. 2022, 24, 1380–1394. [Google Scholar] [CrossRef]
- Dao, T.D.; Takemura, T.; Kasuga, I.; Hirabayashi, A.; Nga, N.T.; Anh, P.H.Q.; Shibayama, K. Emergence of plasmid-mediated RND-type efflux pump gene cluster tmexCD-toprJ in Shewanella xiamenensis in a water environment. bioRxiv 2015. [Google Scholar]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Cheng, G. Bacterial multidrug efflux pumps at the frontline of antimicrobial resistance: An overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Athar, M.; Gervasoni, S.; Catte, A.; Basciu, A.; Malloci, G.; Ruggerone, P.; Vargiu, A.V. Tripartite efflux pumps of the RND superfamily: What did we learn from computational studies? Microbiology 2023, 169, e001307. [Google Scholar] [CrossRef]
- Lloyd, N.A.; Nazaret, S.; Barkay, T. Whole genome sequences to assess the link between antibiotic and metal resistance in three coastal marine bacteria isolated from the mummichog gastrointestinal tract. Mar. Pollut. Bull. 2018, 135, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Kumar, P.; Gaurav, A.; Kaushal, K.; Pandey, A.; Yadav, S.R.M.; Omar, B.J. Association of antibiotics and heavy metal arsenic to horizontal gene transfer from multidrug-resistant clinical strains to antibiotic-sensitive environmental strains. J. Hazard. Mater. 2023, 443, e130260. [Google Scholar] [CrossRef]
- Prasad, P.; Reshi, S.; Nataraj, G. Topic: Shewanella Species: Case Series on a Rare Emerging Pathogen. Indian J. Microbiol. Res. 2019, 6, 303–307. [Google Scholar]
- Madrid, N.Y.; Mejia, L.F.; Urrego, J.F.G. Left knee septic monoarthritis in a pediatric patient due to Shewanella putrefaciens: Case report and literature review. Clin. Microbiol. Antimicrob. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Zago, V.; Veschetti, L.; Patuzzo, C.; Malerba, G.; Lleo, M.M. Resistome, mobilome and virulome analysis of Shewanella algae and Vibrio spp. strains isolated in italian aquaculture centers. Microorganisms 2020, 8, 572. [Google Scholar] [CrossRef]
- Cimmino, T.; Olaitan, A.O.; Rolain, J.M. Whole genome sequence to decipher the resistome of Shewanella algae, a multidrug-resistant bacterium responsible for pneumonia, Marseille, France. Expert Rev. Anti Infect. Ther. 2016, 14, 269–275. [Google Scholar] [CrossRef]
- Paździor, E.; Pękala-Safińska, A.; Wasyl, D. Phenotypic diversity and potential virulence factors of the Shewanella putrefaciens group isolated from freshwater fish. J. Vet. Res. 2019, 63, 321. [Google Scholar] [CrossRef]
- Jousset, A.B.; Dabos, L.; Bonnin, R.A.; Girlich, D.; Potron, A.; Cabanel, N.; Naas, T. CTX-M-15-producing Shewanella species clinical isolate expressing OXA-535, a chromosome-encoded OXA-48 variant, putative progenitor of the plasmid-encoded OXA-436. Antimicrob. Agents Chemother. 2018, 62, e01879-17. [Google Scholar] [CrossRef]
- Kalathuru, S.; Singla, A.; Kumar, A.; Swami, A. Shewanella algae, an emerging pathogen, causing skin and soft tissue infections. Infect. Dis. Clin. Pract. 2023, 31, e1286. [Google Scholar] [CrossRef]
- Muñoz-Gallego, I.; Chaves, F.; Orellana, M.A. Epidemiological and clinical characteristics of Shewanella spp. infections in a tertiary hospital in Madrid. Infect. Dis. 2016, 48, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.W.S.; Shum, H.P.; To, K.K.W.; Sridhar, S. Emerging infections due to Shewanella spp.: A case series of 128 cases over 10 years. Front. Med. 2022, 9, e850938. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Martín-Pujol, O.; Artiles-Campelo, F.; Bolaños-Rivero, M.; Römling, U. Shewanella spp. infections in Gran Canaria, Spain: Retrospective analysis of 31 cases and a literature review. JMM Case Rep. 2017, 4, e005131. [Google Scholar] [CrossRef]
- Li, Y.; Qi, R.; Yang, H.; Zhang, X.L.; Wu, Y.; Huang, B.H.; Zhao, Q.; Gu, Y.F. Analysis of clinical characteristics of infections caused by Shewanella species. Indian J. Med. Microbiol. 2024, 49, e100574. [Google Scholar] [CrossRef]
- Luo, B.; Su, J.Y.; Wang, Y.N.; Guan, L.; Dong, K.S.; Niu, H.J.; Li, Y. Shewanella subflava sp. nov., a novel multi-resistant bacterium, isolated from the estuary of the Fenhe River into the Yellow River. Antonie Van Leeuwenhoek 2023, 116, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wahid, J. Study of the Distribution of Antimicrobial Resistance in Organic Crops and Their Environment. 2023. Available online: https://riunet.upv.es/handle/10251/197455 (accessed on 31 March 2025).
- Telke, A.A.; Rolain, J.M. Functional genomics to discover antibiotic resistance genes: The paradigm of resistance to colistin mediated by ethanolamine phosphotransferase in Shewanella algae MARS 14. Int. J. Antimicrob. Agents 2015, 46, 648–652. [Google Scholar] [CrossRef]
- Kühn, M.J.; Edelmann, D.B.; Thormann, K.M. Polar flagellar wrapping and lateral flagella jointly contribute to Shewanella putrefaciens environmental spreading. Environ. Microbiol. 2022, 24, 5911–5923. [Google Scholar] [CrossRef] [PubMed]
- Ghotbi, M.; Kelting, O.; Blümel, M.; Tasdemir, D. Gut and gill-associated microbiota of the flatfish European plaice (Pleuronectes platessa): Diversity, metabolome and bioactivity against human and aquaculture pathogens. Mar. Drugs. 2012, 20, 573. [Google Scholar] [CrossRef]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer membrane porins contribute to antimicrobial resistance in gram-negative bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef]
- Gao, T.; Ju, L.; Yin, J.; Gao, H. Positive regulation of the Shewanella oneidensis OmpS38, a major porin facilitating anaerobic respiration, by Crp and Fur. Sci. Rep. 2015, 5, e14263. [Google Scholar] [CrossRef]
- Huang, Y.T.; Liu, P.Y. Emergence of carbapenem resistance in persistent Shewanella algae bacteremia: The role of pdsS G547W mutation in adaptive subpopulation dynamics. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 102. [Google Scholar] [CrossRef]
- Poirel, L.; Rodriguez-Martinez, J.M.; Mammeri, H.; Liard, A.; Nordmann, P. Origin of plasmid-mediated quinolone resistance determinant QnrA. Antimicrob. Agents Chemother. 2005, 49, 3523–3525. [Google Scholar] [CrossRef]
- Lambert, P.A. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Cheng, J.F.; Chen, S.Y.; Chen, W.H.; Shi, Z.Y.; Lin, Y.H.; Tsai, C.A.; Lin, S.P.; Chen, Y.C.; Lin, Y.C.; et al. Detection of S83V GyrA mutation in quinolone-resistant Shewanella algae using comparative genomics. J. Microbiol. Immunol. Infect. 2021, 54, 658–664. [Google Scholar] [CrossRef]
- Azargun, R.; Gholizadeh, P.; Sadeghi, V.; Hosainzadegan, H.; Tarhriz, V.; Memar, M.Y.; Pormohammad, A.; Eyvazi, S. Molecular mechanisms associated with quinolone resistance in Enterobacteriaceae: Review and update. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Tacão, M.; Araujo, S.; Vendas, M.; Alves, A.; Henriques, I. Shewanella species as the origin of blaOXA-48 genes: Insights into gene diversity, associated phenotypes and possible transfer mechanisms. Int. J. Antimicrob. Agents 2018, 51, 340–348. [Google Scholar] [CrossRef]
- Poirel, L.; Héritier, C.; Nordmann, P. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 2004, 48, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Nordmann, P. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis. Antimicrob. Agents Chemother. 2011, 55, 4405–4407. [Google Scholar] [CrossRef]
- Yin, J.; Sun, L.; Dong, Y.; Chi, X.; Zhu, W.; Qi, S.H.; Gao, H. Expression of blaA underlies unexpected ampicillin-induced cell lysis of Shewanella oneidensis. PLoS ONE 2013, 8, e60460. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Wu, S.; Gao, H. Recent advances in the siderophore biology of Shewanella. Front. Microbiol. 2022, 13, e823758. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Li, Y.; Ding, J.; Qin, W. Effectively facilitating the degradation of chloramphenicol by the synergism of Shewanella oneidensis MR-1 and the metal-organic framework. J. Hazard. Mater. 2023, 454, e131545. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired antibiotic resistance genes: An overview Front. Microbiol. 2011, 2, e203. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Meadows, A.L.; Kirby, J.; Keasling, J.D. Anaerobic central metabolic pathways in Shewanella oneidensis MR-1 reinterpreted in the light of isotopic metabolite labeling. J. Bacteriol. 2007, 189, 894–901. [Google Scholar] [CrossRef]
- Xue, Y.; Xue, B.; Zhang, L. Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the development and lifespan of marine nematode litoditis marina. Int. J. Mol. Sci. 2024, 25, 9111. [Google Scholar] [CrossRef]
- Wang, H.; Xia, F.; Xia, Y.; Li, J.; Hu, Y.; Deng, Y.; Zou, M. Pangenome analysis of Shewanella xiamenensis revealed important genetic traits concerning genetic diversity, pathogenicity and antibiotic resistance. BMC Genom. 2024, 25, 1–14. [Google Scholar] [CrossRef]
- Milewska, K.; Węgrzyn, G.; Szalewska-Pałasz, A. Transformation of Shewanella baltica with ColE1-like and P1 plasmids and their maintenance during bacterial growth in cultures. Plasmid 2015, 81, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, M.; Haro-Moreno, J.M.; Gonzalez-Serrano, R.; Parras-Molto, M.; Rodriguez-Valera, F. Genome diversity of marine phages recovered from Mediterranean metagenomes: Size matters. PLoS Genet. 2017, 13, e1007018. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, K.; Wang, M.; Ma, K.; Ren, L.; Guo, R.; Ma, L.; Zhang, H.; Liu, Y.; Xiong, Y.; et al. Shewanella phage encoding a putative anti-CRISPR-like gene represents a novel potential viral family. Microbiol. Spectrum. 2024, 12, e0336723. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.; Li, Z.; Liu, Z.; Li, X.; Diao, B.; Kan, B.; Wang, D. Distribution and genetic characteristics of SXT/R391 integrative conjugative elements in Shewanella spp. from China. Front. Microbiol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Amirfard, K.D.; Moriyama, M.; Suzuki, S.; Sano, D. Effect of environmental factors on conjugative transfer of antibiotic resistance genes in aquatic settings, J. Appl. Microbiol. 2024, 135, lxae129. [Google Scholar]
- Zhao, J.Y.; Mu, X.D.; Zhu, Y.Q.; Xi, L.; Xiao, Z. Identification of an integron containing the quinolone resistance gene qnrA1 in Shewanella xiamenensis. FEMS Microbiol. Lett. 2015, 362, p.fnv146. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; So, J.S. Antibiotic and heavy metal resistance in Shewanella putrefaciens strains isolated from shellfishes collected from West Sea, Korea. Mar. Pollut. Bull. 2016, 112, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Tom-Petersen, A.; Nybroe, O. Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett. Appl. Microbiol. 2005, 40, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Akinbowale, O.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile Aeromonads and Pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents. 2007, 30, 177–182. [Google Scholar] [CrossRef]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Dragoi, E.N. A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2022, 312, e137099. [Google Scholar] [CrossRef]
| Shewanella Species | Genome Assemblies | No. of Chromo-Somes | No. of Plasmids | Genome Size (Mb) | GC (%) | No. of Genes (Avg) | No. of Proteins (Avg) | First Genome Release Date |
|---|---|---|---|---|---|---|---|---|
| Shewanella putrefaciens | 22 | 1 | 3 | 4.38–5.05 | 44.30–47.90 | 4055 | 3820 | 01/10/2014 |
| Shewanella algae | 230 | 1 | 1 | 4.60–5.20 | 52.50–53.2 | 4185 | 4450 | 04/01/2014 |
| Shewanella baltica | 58 | 1 | 4 | 3.26–5.55 | 45.99–47.50 | 4414 | 4587 | 02/20/2007 |
| Shewanella benthica | 4 | 1 | ND | 4.03–5.71 | 45.8–46 | 3431 | 3259 | 11/28/2007 |
| Shewanella woodyi | 4 | 1 | ND | 5.82–5.94 | 43.60–43.70 | 4874 | 5085 | 03/13/2008 |
| Shewanella halifaxensis | 2 | 1 | ND | 5.23–5.46 | 42.60–42.80 | 4674 | 4494 | 02/07/2008 |
| Shewanella oneidensis | 7 | 1 | 1 | 3.71–5.13 | 45.93–46.50 | 4438 | 4261 | 09/12/2002 |
| Shewanella colwelliana | 8 | ND | ND | 4.47–4.81 | 45.30–45.60 | 4135 | 4014 | 01/10/2014 |
| Shewanella vesiculosa | 12 | 1 | ND | 4.46–4.78 | 41–60–41.70 | 4068 | 3840 | 11/19/2018 |
| Shewanella chilikensis | 16 | 1 | ND | 4.39–4.91 | 52.20–52.50 | 3945 | 3845 | 12/12/2017 |
| Shewanella indica | 15 | 1 | ND | 4.38–4.99 | 52.20–52.60 | 4089 | 3897 | 09/11/2020 |
| Shewanella profunda | 1 | 1 | ND | 4.74 | 44.9 | 4080 | 4303 | 05/09/2022 |
| Shewanella glacialimarina | 1 | 1 | ND | 4.46 | 41.1 | 3763 | 3915 | 10/15/2021 |
| Shewanella algicola | 3 | ND | ND | 4.85–4.97 | 42.30 | 4267 | 4459 | 09/11/2020 |
| Shewanella saliphila | 2 | ND | ND | 4.62–4.63 | 42.50 | 3926 | 4086 | 09/11/2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, S.; Richards, G.P.; Parveen, S.; Williams, H.N. Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings. Microorganisms 2025, 13, 1115. https://doi.org/10.3390/microorganisms13051115
Sher S, Richards GP, Parveen S, Williams HN. Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings. Microorganisms. 2025; 13(5):1115. https://doi.org/10.3390/microorganisms13051115
Chicago/Turabian StyleSher, Shahid, Gary P. Richards, Salina Parveen, and Henry N. Williams. 2025. "Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings" Microorganisms 13, no. 5: 1115. https://doi.org/10.3390/microorganisms13051115
APA StyleSher, S., Richards, G. P., Parveen, S., & Williams, H. N. (2025). Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings. Microorganisms, 13(5), 1115. https://doi.org/10.3390/microorganisms13051115






