Abstract
Brevibacterium species are Gram-positive, non-sporulating, coryneform, aerobic rods that are catalase positive and exhibit a distinctive transition from diptheroid to coccoid morphology during culture. Infections by these species are seldom identified. Objective: This narrative review aims to present all the reported cases of Brevibacterium spp. infections in humans, focusing on data about epidemiology, antimicrobial resistance, antimicrobial treatment, and mortality. A narrative review based on a literature search of PubMed/MedLine and Scopus databases was performed. In total, 41 studies providing data on 42 patients with Brevibacterium spp. infections were included in the present analysis. The median age was 48 years, while 57.5% were male. The presence of a central venous catheter and malignancy, and end-stage renal disease on peritoneal dialysis were the main predisposing factors. Bacteremia was the most common type of infection, with peritoneal dialysis-associated infections being the second most common. B. casei was the most commonly identified species. Microbial identification required the use of advanced molecular techniques, such as 16s rRNA sequencing or matrix-assisted laser desorption/ionization time of flight mass spectrometry in most cases. Brevibacterium spp. was highly resistant to the combination of trimethoprim with sulfamethoxazole, clindamycin, and common beta-lactams. The most commonly used antimicrobials were vancomycin and aminoglycosides. The mortality was about 10%. Clinicians and laboratory personnel should consider this pathogen in the differential diagnosis in patients with malignancy or peritoneal dialysis-associated peritonitis. Vancomycin should be used for empirical treatment and while antimicrobial susceptibility testing results are pending.
1. Introduction
Brevibacterium species are Gram-positive, non-sporulating, coryneform, aerobic rods that are catalase positive and exhibit a distinctive transition from diptheroid to coccoid morphology during culture [1]. Initially considered as environmental contaminants, Brevibacterium spp. are now recognized as opportunistic pathogens in humans, particularly in immunocompromised patients or those with indwelling medical devices [2,3]. The concept of opportunistic infection was introduced many decades ago after the notion that some microorganisms are more likely to cause infections in specific hosts. Thus, an opportunistic infection can be defined as a serious infection by a microorganism with limited pathogenic capacity in common circumstances, but it can cause serious diseases in the presence of predisposing factors such as some diseases or specific treatments [4]. An opportunistic pathogen may be able to cause infection in the absence of predisposing factors, but usually, the infection is more severe when occurring in patients with the predisposing factors [4].
The genus Brevibacterium includes various species, such as B. casei, B. epidermidis, B. paucivorans, B. luteolum, and B. otitis, but B. casei appears to be the most frequent species isolated from clinical specimens [2,5]. Historically, Brevibacterium spp. were associated with dairy products and human skin microbiota, contributing to cheese ripening and foot odor [6,7]. However, since the first reported case of bacteremia related to Brevibacterium spp. in 1969 in a patient with post-operative meningitis and prolonged fever, these organisms have been involved in a range of infections, including bacteremia, endocarditis, brain abscesses, peritonitis, osteomyelitis, and pericarditis [8,9,10,11]. Infections tend to arise most often among patients with central venous catheters, prosthetic devices, or underlying diseases such as malignancies or immunosuppression (e.g., patients with HIV infection) [12,13,14].
Diagnosis of Brevibacterium may be challenging as Gram stain usually shows Gram-positive rods like diphtheroids. Biochemical testing could further differentiate between other microorganisms [15]. Advanced molecular techniques such as either matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) or 16s-RNA sequencing are frequently used for identifying or confirming adequate pathogen identification. Other methods of identification can be the API Coryne gallery or a combination of microscopy, culture, biochemical tests, and chemical composition analysis [13,14,16].
Given the scarce data that are seldom reported mainly through case reports, the present study aimed to comprehensively review all the available information of all the types of human infections caused by this species in the literature and to assess the clinical data, microbiology, treatment, and outcomes.
2. Materials and Methods
2.1. Search Strategy and the Inclusion and Exclusion Criteria
This review aims to present all the data on Brevibacterium species infections in humans that have been published in the literature. The primary aim of the study was to present the data on patients’ demographics, clinical characteristics, and mortality. The secondary aims were to present the data regarding the infection site, the clinical presentation, the microbiological characteristics regarding species, identification, and antimicrobial resistance, and the treatment provided for the infection. For this narrative review, the PubMed/Medline and Scopus databases were searched until 13 February 2025. Data were extracted using a predefined template. The following keywords were used for the search strategy: “Brevibacterium” AND “infection”. Studies providing original data, such as case series, case reports, and cohort studies providing information into the epidemiology and clinical outcomes of Brevibacterium spp. infections in humans were included. Studies not in the English language, reviews, and systematic reviews were excluded. Studies in animals, and articles without full-text access, were also excluded from the analysis. Additionally, cases of colonization by Brevibacterium species were excluded from the analysis. The references of all the included articles were examined to identify any studies potentially missed in the initial search.
2.2. Data Extraction and Definitions
The data extracted from each included study were the publication year, article type, country of origin, patient demographics (age, sex), relevant medical history, details of infection, and the key clinical characteristics, such as the specific infection site, complications, as well as microbiological characteristics, such as the identified pathogen, antibiotic susceptibilities, and finally, the treatment used and outcome (survival or mortality). The relationship between mortality and the initial infection was documented according to each study’s authors.
2.3. Statistical Analysis
Data are presented as numbers (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Continuous variables were compared using the Mann–Whitney U-test for non-normally distributed variables or the t-test for normally distributed variables. All the tests were two-tailed, and a p-value equal to or lower than 0.05 was considered significant.
3. Results
3.1. Included Studies’ Characteristics
A total of 325 articles were screened from the PubMed and Scopus databases. Eventually, after duplicate removal, record screening, and applying the snowball procedure, only 41 articles met the inclusion criteria and were selected for analysis [2,3,8,9,10,12,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. These studies presented data on 42 patients. A flow diagram of the selection process is illustrated in Figure 1. Among the included cases, 21 were diagnosed in Europe (50%), 12 in Asia (28.6%), and 9 in North and South America (21.4%). Among the 41 articles that were eventually included, 39 (95.1%) were case reports. Table 1 shows the characteristics of the included studies in the present review. The data sheet can be found in File S1.
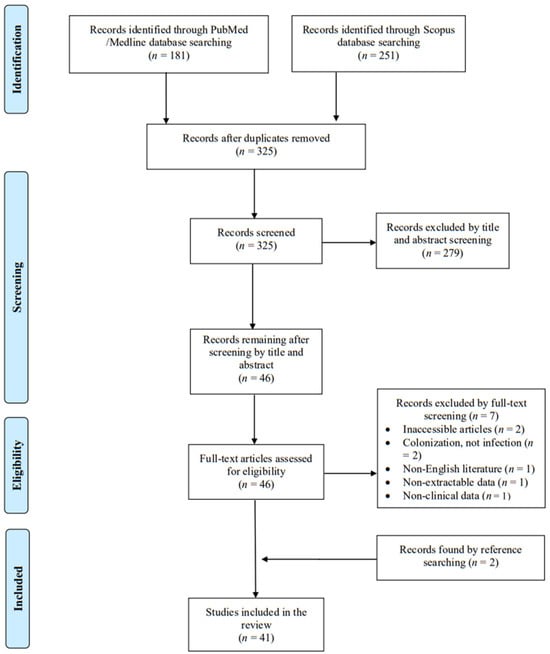
Figure 1.
Trial flow of this narrative review.

Table 1.
Characteristics of all included studies.
3.2. Epidemiology of Brevibacterium spp. Infections
The median age of patients with Brevibaterium spp. infections was 48 years, with a range of 0 to 94 years, while 57.5% (23 out of 40 patients with available data) were male. Regarding patients’ medical history and predisposing risk factors, 17 out of 41 (41.5%) had a central venous catheter, 10 out of 40 (25%) had an active malignancy which was hematologic in 6 patients (15% of all patients), 7 out of 40 (17.5%) had end-stage kidney disease on peritoneal dialysis, 6 out of 36 patients (16.7%) had had surgery in the preceding three months, 3 out of 40 patients (7.5%) were people living with the human immunodeficiency virus (PLWHIV), and 2 out of 41 patients (4.9%) had had organ transplantation. The demographic and clinical characteristics of patients with infections by Brevibacterium spp. are shown in Table 2.

Table 2.
Characteristics of patients with Brevibacterium species infection.
3.3. Microbiology and Antimicrobial Resistance of Brevibacterium spp. Infections
Brevibacterium spp. was isolated in the blood of twenty-four patients (59.5%), from peritoneal fluid in seven (16.7%), from pus or tissue cultures in five (11.9%), from vitreous fluid in two (4.8%), from cerebrospinal fluid in two (4.8%), and from pericardial fluid, or ventriculoperitoneal shunt valve culture in one (2.4%) each. B. casei was the identified species in twenty patients (47.6%), B. epidermidis and B. otitidis were identified in three (7.1%) each, and B. iadinum, B. paicivorans, B. luteolum, and B. sanguinis were identified in one patient (2.4%) each. In ten patients (23.8%), the species was not reported. In 14 patients (33.3%), identification was performed with matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS), and in 13 patients (31%), 16s-rRNA sequencing was used for pathogen identification. The API Coryne gallery was used for identification in 10 patients (23.8%), and a combination of microscopy, culture, biochemical test, and chemical composition analysis was used for identification in 1 patient (2.4%). The means of pathogen identification were not mentioned in eight patients (19%). The antimicrobial resistance of Brevibacterium spp. is shown in Table 3. In 2 out of 42 patients (4.8%), the infection was polymicrobial.

Table 3.
Antimicrobial resistance rates of Brevibacterium spp.
3.4. Clinical Presentation of Brevibacterium spp. Infections
The most common type of Brevibacterium spp. infections were those of the bloodstream in 24 patients (57.1%). Peritoneal dialysis-associated peritonitis was diagnosed in seven (4.8%), central nervous system infections in four (9.5%), osteoarticular infections in three (7.1%), infective endocarditis in three (7.1%), skin and soft tissue infections in two (4.8%), endophthalmitis in two (4.8%), and VP shunt-associated infections in one (2.4%). The symptoms’ duration ranged from one day to more than 60 days.
3.5. Treatment and Outcome of Brevibacterium Infections
The treatment of patients with Brevibacterium spp. infections is shown in detail in Table 1 and is also summarized in Table 2. Based on the available data, vancomycin was the most frequently administered antimicrobial used in twenty-three out of forty patients with available data (57.5%), followed by cephalosporins in nine (22.5%), aminoglycosides in eight (20%), quinolones in six (15%), teicoplanin in four (10%), carbapenem and aminopenicillins in three (7.5%) each, antipseudomonal penicillin, macrolides, and daptomycin in two (5%) each, and antistaphylococcal penicillin, linezolid, rifampicin, and tetracyclins in one (2.5%) each. Surgical interventions were applied in combination with antimicrobial treatment in 10 out of 41 patients (24.4%). The median treatment duration for survivors was 19.5 days. The overall mortality rate was estimated at 10.3% (4 out of 39 patients with available data), with the mortality directly associated with the Brevibacterium spp. infection being 7.7% (3 patients).
3.6. Bacteremia Due to Brevibacterium
Bacteremia was diagnosed in 24 patients (57.1%). Among them, 11 (50% among 22 with available data) were male and the median age was 46 years. Among these patients, 73.9% (17 out of 23 with available data) had a central venous catheter, 39.1% (9) had malingnancy that was hematological in 66.7% of them, and 27.3% (6 out of 22) had neutropenia. Fever was present in 91.7% (22 out of 24 patients) and sepsis was present in 34.8% (8 out of 23). Vancomycin and aminoglycosides were the most commonly used antimicrobials. The overall mortality was 13% (3 out of 23 patients).
3.7. Peritoneal Dialysis-Associated Peritonitis Due to Brevibacterium
Peritoneal dialysis-associated peritonitis was diagnosed in seven patients. The median age in this patient group was 63 years old, and four patients were male (57.1%). The diagnosis was made using peritoneal dialysis fluid in all patients. Fever was present in 57.1% (four out of seven patients) but no patient had sepsis. The median treatment duration was 24.5 days. The most commonly used antimicrobial agents were vancomycin cephalosporins, and aminoglycosides. No patient died due to this infection.
3.8. Characteristics of Patients with Brevibacterium spp. in Regard to Survival
Table 4 shows a comparison of the characteristics of patients with Brevibacterium spp. who lived with those who died. A statistical analysis by directly comparing patients who survived with those who died was not pursued due to the small number of patients, especially those who died. However, patients who died had a higher age.

Table 4.
Characteristics of patients with Brevibacterium species infection in regard to the outcome.
4. Discussion
The present narrative review summarizes the characteristics of infections by Brevibacterium spp. in humans by gathering all the published studies in the literature that provide the relevant clinical and microbiological data. The most common types of infections were bacteremia and peritoneal dialysis-associated peritonitis. Antimicrobial resistance to the combination of trimethoprim with sulfamethoxazole, and clindamycin was very common, while the resistance to first-line beta lactams was also common, while vancomycin, tetracyclines, and cabapenems were active in the vast majority of cases. Vancomycin, cephalosporins, and aminoglycosides were the most commonly used antimicrobials for treating these infections. The mortality from infections by Brevibacterium spp. was relatively low.
Several reports of infections by Brevibacterium spp. have been published in the last decades. Given the rarity of these bacteria as causes of human infections and the possibility for their misidentification when only common microbiological techniques relying on morphology and biochemical assays are used, more advanced techniques such as 16s rRNA sequencing and MALDI-TOF MS may be required for adequate identification in patients with such rare bacteria [49,50,51]. Indeed, in the present review, MALDI-TOF MS and 16s rRNA sequencing were used in more than half of the patients.
Among the patients’ medical history, the most commonly reported condition was the presence of a central venous catheter, the history of a malignancy, most commonly hematological, and the history of end-stage kidney disease on peritoneal dialysis. The first two conditions were associated with infections of the bloodstream. Indeed, among the different clinical presentations, bacteremia was the most common presentation, with peritoneal dialysis-associated peritonitis being the second more common. The presence of malignancy, either hematological or solid is a well-known risk factor for infection. These patients are usually treated with chemotherapy that leads to immunosuppression, making these patients more susceptible to infection, and severe complications, such as sepsis [52,53,54]. Patients with hematological malignancy are at a particularly high risk of infections, due to the neutropenia seen in patients treated with myeloablative chemotherapy and due to the underlying disease. Additionally, some of these people have hypogammaglobulinemia, while the mucosal damage due to the treatment provided and the common use of intravascular devices also add to the high infection risk [53,54]. The presence of these other risk factors could not have been evaluated via the present review since the information provided by the case reports included herein is brief; thus, the presence of mucosal damage and hypogammaglobulinemia could not have been recorded during data extraction. However, neutropenia was identified in many patients, underlining the high risk that these patients have, specifically for bacteremia when treated for the malignancy. In a study that did not provide adequate data to allow inclusion in the present review, Shweta et al. identified 48 isolates from 45 unique patients, with about 31% of those having malignancy and 20% being on chemotherapy at the time of diagnosis [55]. Moreover, about 15% had received stem cell or solid organ transplantation. These are confirmatory of the results of the present review.
Peritoneal dialysis was also a common risk factor among the patients with Brevibacterium spp. infection and was associated only with peritoneal dialysis-associated peritonitis by these pathogens. Most cases of peritoneal dialysis-associated peritonitis are caused by bacteria. The majority of cases are due to Gram-positive bacteria, and more specifically, staphylococci, streptococci, enterococci, and corynebacterial, while Gram-negative bacteria are the cause in up to 35%. In up to 25% of cases, the infection was polymicrobial [56,57,58,59]. However, several rare pathogens are identified nowadays and are reported in the literature given the better microbiological identification techniques that are available [60,61,62,63].
In the present review, beyond bacteremia, and peritoneal dialysis-associated peritonitis, some cases of infective endocarditis, central nervous system infection, skin and soft tissue infection, osteoarticular infection, prosthetic material in the central nervous system, and endophthalmitis were also identified. In the study by Shweta et al., 70% of the infections were bacteremias, while the other types were not mentioned [55]. However, the rates may vary depending on the type of hospital, while the fact that not all cases of Brevibacterium spp. may have been reported in the literature may have affected the types of infection depicted in the present review.
In the present study, the antimicrobial resistance to the combination of trimethoprim with sulfamethoxazole, clindamycin, and commonly used beta-lactams was higher than 50%. The antimicrobial resistance to carbapenems, tetracyclines, aminoglycosides, and vancoymycin was low. These results are similar to those published by Shweta et al., where the antimicrobial resistance to common beta-lactams and susceptibility to vancomycin were also shown [55]. Thus, vancomycin can be used for the empirical treatment of these infections while antimicrobial susceptibility results are pending. Indeed, in most of the patients included in the analysis of the present review, vancomycin was the most commonly used antimicrobial for treating these infections by Brevibacterium spp. Even though a narrative review with such a low number of included patients cannot provide strong recommendations, given the inability to draw firm conclusions by other means and the lack of international guidelines for the empirical treatment of these infections, it is reasonable to consider vancomycin, for empirical treatment when Brevibacterium spp. is identified and until the results of the antimicrobial susceptibility testing are available.
The mortality of Brevibacterium infections was about 10%. Importantly, three out of four patients who died had bacteremia. However, even though in this review, an attempt to compare patients who survived with those who died was performed, the small number of patients who died precluded statistical analysis. Thus, future studies should focus on prospectively evaluating the pathophysiology, as well as the clinical and microbiological characteristics of patients with Brevibacterium spp. infections to allow more adequate identification of those characteristics that could allow adequate pathogen identification, and the appropriate empirical treatment of patients with such infections.
This study has some notable limitations. First of all, the studies included in the present review may not be representative of the infections by Brevibacteriun spp. due to the problems associated with the identification of the pathogen and the reasonable possibility that not all cases of Brevibacterium spp. infections have been published. Additionally, some studies may have been missed during the screening process. Moreover, this review identified only a small number of studies, mostly case reports, that carry a specific risk of bias, providing data for only a small number of patients, thus limiting the credibility of the conclusions of the study. Finally, even though in the present review, we tried to evaluate the outcome of mortality, the heterogeneity in reporting the mortality in the case reports that provided data in the present study made it impossible to evaluate it at a specific timepoint, even though this outcome probably reflects the hospital mortality in most cases.
5. Conclusions
The present review provides important information about Brevibacterium spp. infections in humans. B. casei was the most commonly identified species, while the most common infections were those of the bloodstream and peritoneal dialysis-associated infections. MALDI-TOF MS or 16s rRNA sequencing can aid in microbial identification. Brevibacterium spp. had significant antimicrobial resistance to the combination of trimethoprim with sulfamethoxazole, clindamycin, and common beta lactams, while the antimicrobial resistance to vancomycin, carbapenems, tetracyclines, and aminoglycosides was very low. Brevibacterium spp. infections should be empirically treated with vancomycin until antimicrobial susceptibility tests are pending, especially in the case of bacteremia, that may carry a higher risk of mortality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13051097/s1, File S1: Datasheet used for data extraction.
Author Contributions
Conceptualization, P.I.; methodology, P.I., S.B. and A.G.T.; software, P.I.; validation, P.I.; formal analysis, P.I. and A.G.T.; investigation, A.V., T.P. and S.B.; resources, P.I.; data curation, P.I.; writing—original draft preparation, P.I., T.P. and A.V.; writing—review and editing, S.B. and A.G.T.; visualization, P.I.; supervision, P.I.; project administration, P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Funke, G.; Von Graevenitz, A.; Clarridge, J.E.; Bernard, K.A. Clinical Microbiology of Coryneform Bacteria. Clin. Microbiol. Rev. 1997, 10, 125–159. [Google Scholar] [CrossRef] [PubMed]
- Eidensohn, Y.; Wei, A.; Sirkin, M.; Dever, L.L. Brevibacteria Tibial Osteomyelitis. IDCases 2021, 23, e01046. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Suematsu, H.; Yamada, A.; Watanabe, H.; Nishiyama, N.; Sakanashi, D.; Kato, H.; Shiota, A.; Hagihara, M.; Koizumi, Y.; et al. Brevibacterium paucivorans Bacteremia: Case Report and Review of the Literature. BMC Infect. Dis. 2019, 19, 344. [Google Scholar] [CrossRef] [PubMed]
- Symmers, W.S. Opportunistic Infections. The Concept of “Opportunistic Infections”. Proc. R. Soc. Med. 1965, 58, 341–346. [Google Scholar] [CrossRef]
- Funke, G.; Carlotti, A. Differentiation of Brevibacterium spp. Encountered in Clinical Specimens. J. Clin. Microbiol. 1994, 32, 1729–1732. [Google Scholar] [CrossRef]
- Ritschard, J.S.; Schuppler, M. The Microbial Diversity on the Surface of Smear-Ripened Cheeses and Its Impact on Cheese Quality and Safety. Foods 2024, 13, 214. [Google Scholar] [CrossRef]
- Marcellino, O.S.B.S.N.; Benson, D.R. The Good, the Bad, and the Ugly: Tales of Mold-Ripened Cheese. Microbiol. Spectr. 2013, 1. [Google Scholar] [CrossRef]
- Cannon, J.P.; Spandoni, S.L.; Pesh-Iman, S.; Johnson, S. Pericardial Infection Caused by Brevibacterium casei. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2005, 11, 164–165. [Google Scholar] [CrossRef]
- Kumar, V.A.; Augustine, D.; Panikar, D.; Nandakumar, A.; Dinesh, K.R.; Karim, S.; Philip, R. Brevibacterium casei as a Cause of Brain Abscess in an Immunocompetent Patient. J. Clin. Microbiol. 2011, 49, 4374–4376. [Google Scholar] [CrossRef]
- McCaughey, C.; Damani, N.N. Central Venous Line Infection Caused by Brevibacterium epidermidis. J. Infect. 1991, 23, 211–212. [Google Scholar] [CrossRef]
- Fleurette, J.; Moulin, A.; Monnet, P.; Lapras, C. Postoperative meningitis and prolonged fever. Repeated isolation of Brevibacterium fermentans. Pathogenic hypotheses. Ann. Inst. Pasteur 1969, 116, 327–330. [Google Scholar]
- Brazzola, P.; Zbinden, R.; Rudin, C.; Schaad, U.B.; Heininger, U. Brevibacterium casei Sepsis in an 18-Year-Old Female with AIDS. J. Clin. Microbiol. 2000, 38, 3513–3514. [Google Scholar] [CrossRef] [PubMed]
- Beukinga, I.; Rodriguez-Villalobos, H.; Deplano, A.; Jacobs, F.; Struelens, M.J. Management of Long-Term Catheter-Related Brevibacterium Bacteraemia. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004, 10, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Ochi, F.; Tauchi, H.; Moritani, K.; Murakami, S.; Miyamoto, H.; Ueda, M.; Nagai, K.; Eguchi-Ishimae, M.; Eguchi, M. A Catheter-Related Bloodstream Infection by Brevibacterium casei in a Child with Acute Myeloid Leukemia: Case Report and Literature Review. Case Rep. Pediatr. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Pitcher, D.G.; Malnick, H. Identification of Brevibacterium from Clinical Sources. J. Clin. Pathol. 1984, 37, 1395–1398. [Google Scholar] [CrossRef]
- Dass, K.N.; Smith, M.A.; Gill, V.J.; Goldstein, S.A.; Lucey, D.R. Brevibacterium endocarditis: A First Report. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 35, e20–e21. [Google Scholar] [CrossRef]
- Neumeister, B.; Mandel, T.; Gruner, E.; Pfyffer, G.E. Brevibacterium Species as a Cause of Osteomyelitis in a Neonate. Infection 1993, 21, 177–178. [Google Scholar] [CrossRef]
- Lina, B.; Carlotti, A.; Lesaint, V.; Devaux, Y.; Freney, J.; Fleurette, J. Persistent Bacteremia Due to Brevibacterium Species in an Immunocompromised Patient. Clin. Infect. Dis. 1994, 18, 487–488. [Google Scholar] [CrossRef]
- Reinert, R.R.; Schnitzler, N.; Haase, G.; Lütticken, R.; Fabry, U.; Schaal, K.P.; Funke, G. Recurrent Bacteremia Due to Brevibacterium casei in an Immunocompromised Patient. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 1082–1085. [Google Scholar] [CrossRef]
- Kaukoranta-Tolvanen, S.S.E.; Sivonen, A.; Kostiala, A.A.I.; Hormila, P.; Vaara, M. Bacteremia Caused by Brevibacterium Species in an Immunocompromised Patient. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 801–804. [Google Scholar] [CrossRef]
- Antoniou, S.; Dimitriadis, A.; Polydorou, F.; Malaka, E. Brevibacterium iodinum Peritonitis Associated with Acute Urticaria in a Capd Patient. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 1997, 17, 614–615. [Google Scholar] [CrossRef]
- Castagnola, E.; Conte, M.; Venzano, P.; Garaventa, A.; Viscoli, C.; Barretta, M.A.; Pescetto, L.; Tasso, L.; Nantron, M.; Milanaccio, C.; et al. Broviac Catheter-Related Bacteraemias Due to Unusual Pathogens in Children with Cancer: Case Reports with Literature Review. J. Infect. 1997, 34, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Wauters, G.; Van Bosterhaut, B.; Avesani, V.; Cuvelier, R.; Charlier, J.; Janssens, M.; Delmée, M. Peritonitis Due to Brevibacterium otitidis in a Patient Undergoing Continuous Ambulatory Peritoneal Dialysis. J. Clin. Microbiol. 2000, 38, 4292–4293. [Google Scholar] [CrossRef]
- Öğünç, D.; Gültekin, M.; Öngüt, G.; Timurağaoğlu, A.; Hathi, D.; Mutlu, G.; Ündar, L.; Çolak, D. Bacteremia Caused by Brevibacterium Species in a Patient with Chronic Lymphocytic Leukemia. Haematologia 2002, 32, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Janda, W. Brevibacterium casei Bacteremia and Line Sepsis in a Patient with AIDS. J. Infect. 2003, 46, 61–64. [Google Scholar] [CrossRef]
- Ulrich, S.; Zbinden, R.; Pagano, M.; Fischler, M.; Speich, R. Central Venous Catheter Infection with Brevibacterium sp. in an Immunocompetent Woman: Case Report and Review of the Literature. Infection 2006, 34, 103–106. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Brevibacterium massiliense sp. Nov., Isolated from a Human Ankle Discharge. Int. J. Syst. Evol. Microbiol. 2009, 59, 1960–1964. [Google Scholar] [CrossRef]
- Manetos, C.M.; Pavlidis, A.N.; Kallistratos, M.S.; Tsoukas, A.S.; Chamodraka, E.S.; Levantakis, I.; Manolis, A.J. Native Aortic Valve Endocarditis Caused by Brevibacterium epidermidis in an Immunocompetent Patient. Am. J. Med. Sci. 2011, 342, 257–258. [Google Scholar] [CrossRef]
- Poesen, K.; Meeus, G.; Boudewijns, M.; Colaert, J.; Doubel, P. Relapsing Brevibacterium casei Peritonitis: Value of 16S rRNA Gene Sequencing in Accurate Species Identification. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2012, 32, 341–344. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, C.S.; Park, J.W.; Bae, E.H.; Ma, S.K.; Kim, S.W. Consecutive Episodes of Peritonitis in a Patient Undergoing Peritoneal Dialysis Caused by Unusual Organisms: Brevibacterium and Pantoea agglomerans. Kidney Res. Clin. Pract. 2012, 31, 121–123. [Google Scholar] [CrossRef][Green Version]
- Banu, A. Post-Traumatic Endophthalmitis Due to Brevibacterium Casei: A Case Report. Australas. Med. J. 2013, 6, 70–72. [Google Scholar] [CrossRef]
- Talento, A.F.; Malnick, H.; Cotter, M.; Brady, A.; McGowan, D.; Smyth, E.; Fitzpatrick, F. Brevibacterium otitidis: An Elusive Cause of Neurosurgical Infection. J. Med. Microbiol. 2013, 62, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Althaf, M.M.; Abdelsalam, M.S.; Alsunaid, M.S.; Hussein, M.H. Brevibacterium casei Isolated as a Cause of Relapsing Peritonitis. BMJ Case Rep. 2014, 2014, bcr2014203611. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.S.; Sen, S.; Karapinar, D.Y.; Aydemir, S.; Vardar, F. The First Reported Catheter-Related Brevibacterium casei Bloodstream Infection in a Child with Acute Leukemia and Review of the Literature. Braz. J. Infect. Dis. 2015, 19, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Vecten, M.; Gouriet, F.; Cano, A.; Raoult, D. Brevibacterium massiliense Bacteremia. IDCases 2017, 7, 25–26. [Google Scholar] [CrossRef]
- Piccinelli, G.; Morello, E.; Cancelli, V.; Turra, A.; Malagola, M.; Ravizzola, G.; Caccuri, F.; Russo, D.; Caruso, A.; De Francesco, M.A. Central Venous Catheter-Related Bloodstream Infection Caused by Brevibacterium casei in a Hematology Patient. Clin. Microbiol. Newsl. 2018, 40, 112–114. [Google Scholar] [CrossRef]
- Magi, B.; Migliorini, L.; Sansoni, A.; Cusi, M.G. Brevibacterium casei Bacteraemia in a Port-a-Cath Carrier Patient: A Case Report. Infez. Med. 2018, 26, 263–265. [Google Scholar]
- Joshi, S.; Misra, R.; Kirolikar, S.; Mushrif, S. Catheter-Related Brevibacterium casei Bloodstream Infection in a Child with Aplastic Anaemia. Indian J. Med. Microbiol. 2020, 38, 226–228. [Google Scholar] [CrossRef]
- Olate-Pérez, A.; Díaz-Céspedes, R.A.; Ruíz-del-Río, N.; Hernández-Pérez, D.; Duch-Samper, A. Brevibacterium casei Endophthalmitis after Intravitreal Dexamethasone Implant. Arch. Soc. Esp. Oftalmol. Engl. Ed. 2021, 96, 549–551. [Google Scholar] [CrossRef]
- Kimura, T.; Yokoyama, T.; Tanemoto, M. Complicated Peritoneal Dialysis-associated Peritonitis Caused by Brevibacterium. Ther. Apher. Dial. 2021, 25, 717–718. [Google Scholar] [CrossRef]
- Øvsthus, K.K.; Sjåvik, K.; Lier, T.; Klingenberg, C. Antibiotic Therapy of an Infant With a Brevibacterium casei Ventriculoperitoneal Shunt Infection. Pediatr. Infect. Dis. J. 2021, 40, e519–e520. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Hossain, A.; Barajas-Ochoa, A.; Jaker, M.A. Brevibacterium Bacteremia in the Setting of Pyogenic Liver Abscess: A Case Report with Accompanying Literature Review. Case Rep. Infect. Dis. 2021, 2021, 034874. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.E.; Tatem, L. Successful Treatment of Brevibacterium Bacteremia Solely with Antimicrobial Therapy. Cureus 2021, 13, e16004. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Alsaedi, A.; Baloush, S.; Samarin, K.; Althaqafi, A.; Al-Amri, A. Brevibacterium luteolum Bacteremia: A Case Report and Literature Review. IDCases 2022, 30, e01609. [Google Scholar] [CrossRef]
- Roy, S.; Singh Garcha, A.; Patel, S.; Rahman, E.U.; Adapa, S. Brevibacterium casei Induced Peritonitis in a Patient Undergoing Continuous Cycler Peritoneal Dialysis: Case Report and Literature Review. J. Community Hosp. Intern. Med. Perspect. 2022, 12, 64–67. [Google Scholar] [CrossRef]
- Okoli, M.L.; Ishiekwene, C.C.; Madhu, C.; Alosi, M. A Rare Case of Ventriculoperitoneal Shunt Co-Infection with Brevibacterium and Corynebacterium minutissimum Organisms. IDCases 2023, 34, e01920. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Ormanoğlu, G.; Kocayiğit, İ.; Yıldız, M.Ş.; Köroğlu, M. The First Case of İnfective Endocarditis Caused by Brevibacterium sanguinis; Review of the Literature. Mediterr. J. Infect. Microbes Antimicrob. 2023, 12, 20. [Google Scholar] [CrossRef]
- Nguyen, J.; Nand, P. Unveiling Brevibacterium Species Isolated in the Cerebrospinal Fluid: A Report of a Rare Case. Cureus 2024, 16, e61072. [Google Scholar] [CrossRef]
- Schröttner, P.; Gunzer, F.; Schüppel, J.; Rudolph, W.W. Identification of Rare Bacterial Pathogens by 16S rRNA Gene Sequencing and MALDI-TOF MS. J. Vis. Exp. JoVE 2016, 53176. [Google Scholar] [CrossRef]
- Seng, P.; Abat, C.; Rolain, J.M.; Colson, P.; Lagier, J.-C.; Gouriet, F.; Fournier, P.E.; Drancourt, M.; La Scola, B.; Raoult, D. Identification of Rare Pathogenic Bacteria in a Clinical Microbiology Laboratory: Impact of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2013, 51, 2182–2194. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.-M. Use of MALDI-TOF Mass Spectrometry for Identification of Bacteria That Are Difficult to Culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4, 3–50. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.N.; Blijlevens, N.M.A.; Mahfouz, T.H.; Anaissie, E.J. Infections in Patients with Hematological Cancer: Recent Developments. Hematology 2003, 2003, 438–472. [Google Scholar] [CrossRef]
- Chen, S.; Lin, K.; Li, Q.; Luo, X.; Xiao, M.; Chen, M.; Zhu, H.; Chen, Y.; Wu, X.; Zeng, Y.; et al. A Practical Update on the Epidemiology and Risk Factors for the Emergence and Mortality of Bloodstream Infections from Real-World Data of 3014 Hematological Malignancy Patients Receiving Chemotherapy. J. Cancer 2021, 12, 5494–5505. [Google Scholar] [CrossRef]
- Shweta, F.; Gurram, P.R.; O’Horo, J.C.; Khalil, S. Brevibacterium Species: An Emerging Opportunistic Cause of Bloodstream Infections. Mayo Clin. Proc. 2021, 96, 1093–1094. [Google Scholar] [CrossRef]
- Kim, D.K.; Yoo, T.-H.; Ryu, D.-R.; Xu, Z.-G.; Kim, H.J.; Choi, K.H.; Lee, H.Y.; Han, D.-S.; Kang, S.-W. Changes in Causative Organisms and Their Antimicrobial Susceptibilities in CAPD Peritonitis: A Single Center’s Experience over One Decade. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2004, 24, 424–432. [Google Scholar] [CrossRef]
- Oo, T.N.; Roberts, T.L.; Collins, A.J. A Comparison of Peritonitis Rates from the United States Renal Data System Database: CAPD versus Continuous Cycling Peritoneal Dialysis Patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2005, 45, 372–380. [Google Scholar] [CrossRef]
- Kan, G.W.; Thomas, M.A.B.; Heath, C.H. A 12-Month Review of Peritoneal Dialysis-Related Peritonitis in Western Australia: Is Empiric Vancomycin Still Indicated for Some Patients? Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2003, 23, 465–468. [Google Scholar] [CrossRef]
- Kavanagh, D.; Prescott, G.J.; Mactier, R.A. Peritoneal Dialysis-Associated Peritonitis in Scotland (1999–2002). Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2004, 19, 2584–2591. [Google Scholar] [CrossRef]
- Varughese, S.; Bargman, J. Actinomyces Neuii PD Peritonitis–Resolution of Infection without Catheter Removal. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2014, 34, 815–816. [Google Scholar] [CrossRef]
- Piedade, A.; Inácio, A.; Mendes, B.; Domingues, P.; Valério, P.; Parreira, L.; Assunção, J. Chryseobacterium indologenes Peritonitis in Automated Peritoneal Dialysis Patient; Rare but Real. J. Nephropathol. 2025, 14, e21538. [Google Scholar] [CrossRef]
- Chao, C.-T.; Lee, S.-Y.; Yang, W.-S.; Chen, H.-W.; Fang, C.-C.; Yen, C.-J.; Chiang, C.-K.; Hung, K.-Y.; Huang, J.-W. Peritoneal Dialysis Peritonitis by Anaerobic Pathogens: A Retrospective Case Series. BMC Nephrol. 2013, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Helvaci, O.; Hızel, K.; Guz, G.; Arinsoy, T.; Derici, U. A Very Rare Pathogen in Peritoneal Dialysis Peritonitis: Serratia liquefaciens. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2019, 30, 738–740. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).