Ferric Uptake Regulator Contributes to Pseudomonas donghuensis HYS-Induced Iron Metabolic Disruption in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Nematode Strains, and Culture Conditions
2.2. Nematode Lethality Assay
2.3. Construction of Gene-Knockout Strains of P. donghuensis HYS
2.4. Pharyngeal Pumping Rate and Defecation Interval Calculation
2.5. Nematode Gut Colonization Assay
2.6. Nematode Food Preference and Avoidance Assay
2.7. RNA Sequencing Sample Collection
2.8. RNA Extraction
2.9. RNA Sequencing Data Analysis
2.10. Data Processing
2.11. VFDB Prediction
3. Results
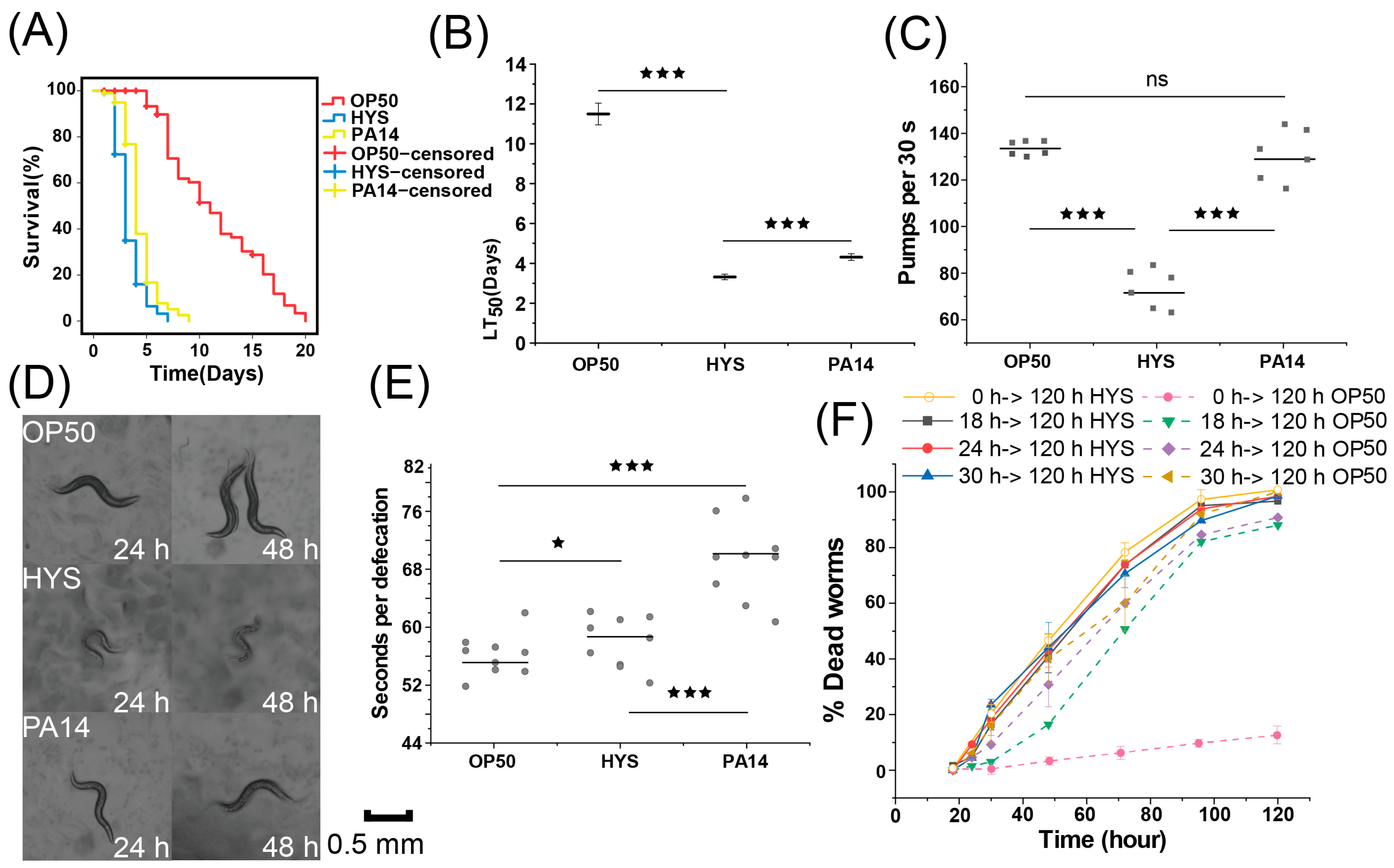
3.1. HYS Exhibits Stronger Toxicity Toward C. elegans than PA14
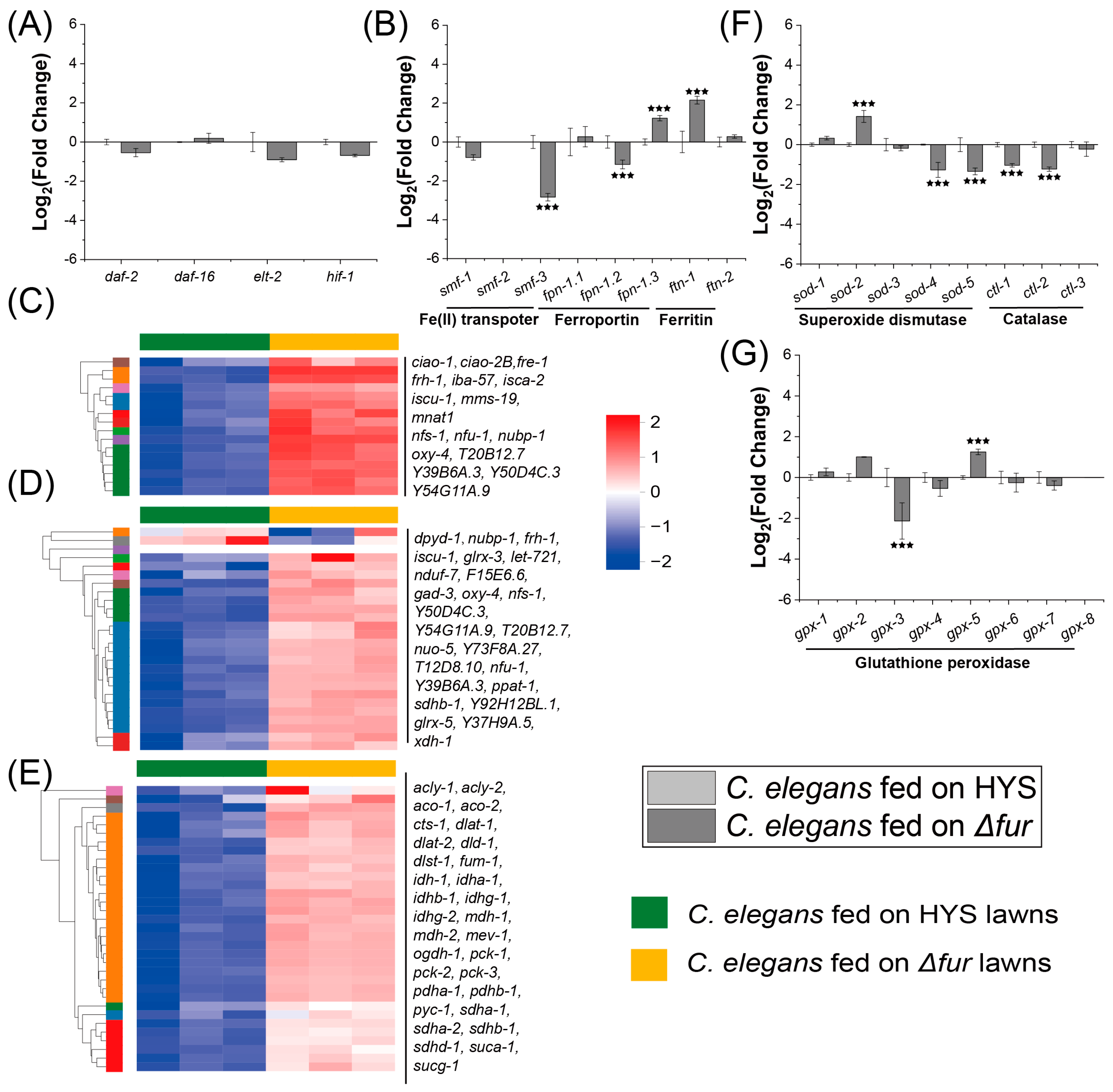
3.2. HYS Infestation Disrupts Iron Metabolism in C. elegans
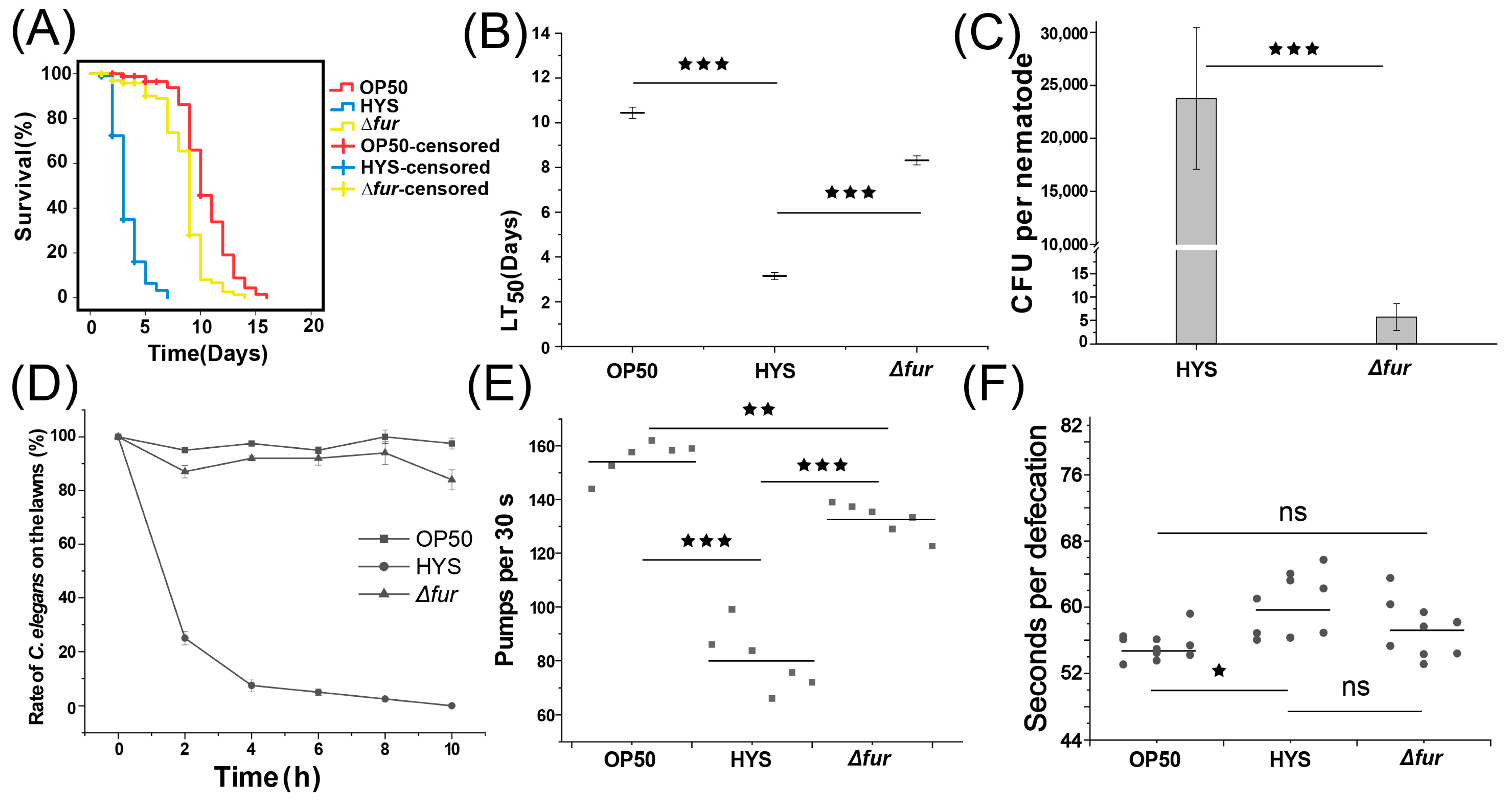
3.3. Fur Critically Influences HYS Virulence
3.4. Fur Is Critical in HYS-Mediated Disruption of Iron Metabolism in C. elegans
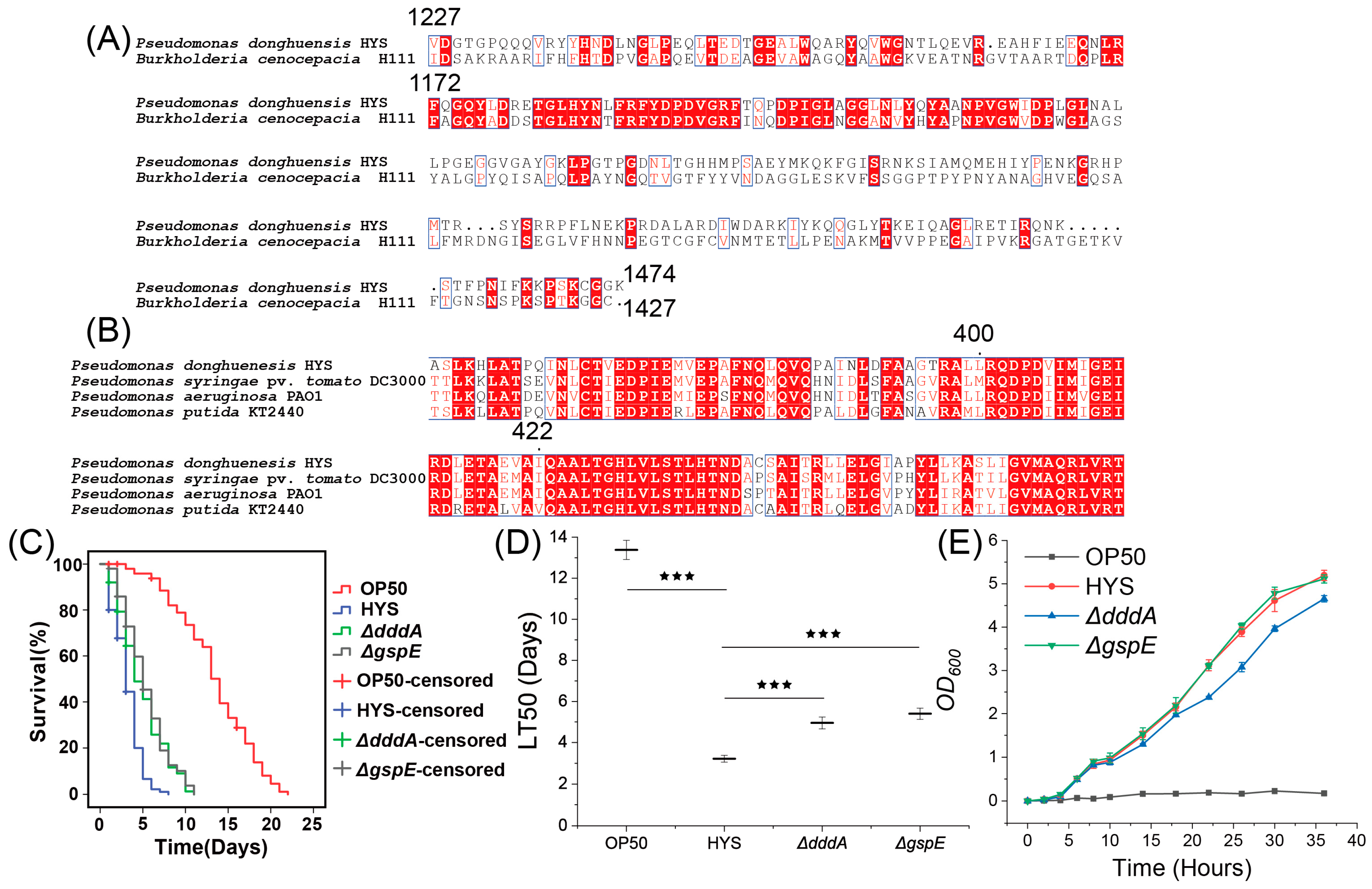
3.5. Screening of Fur-Regulated Virulence Factors in HYS
3.6. Validation of Fur-Regulated Virulence Factors
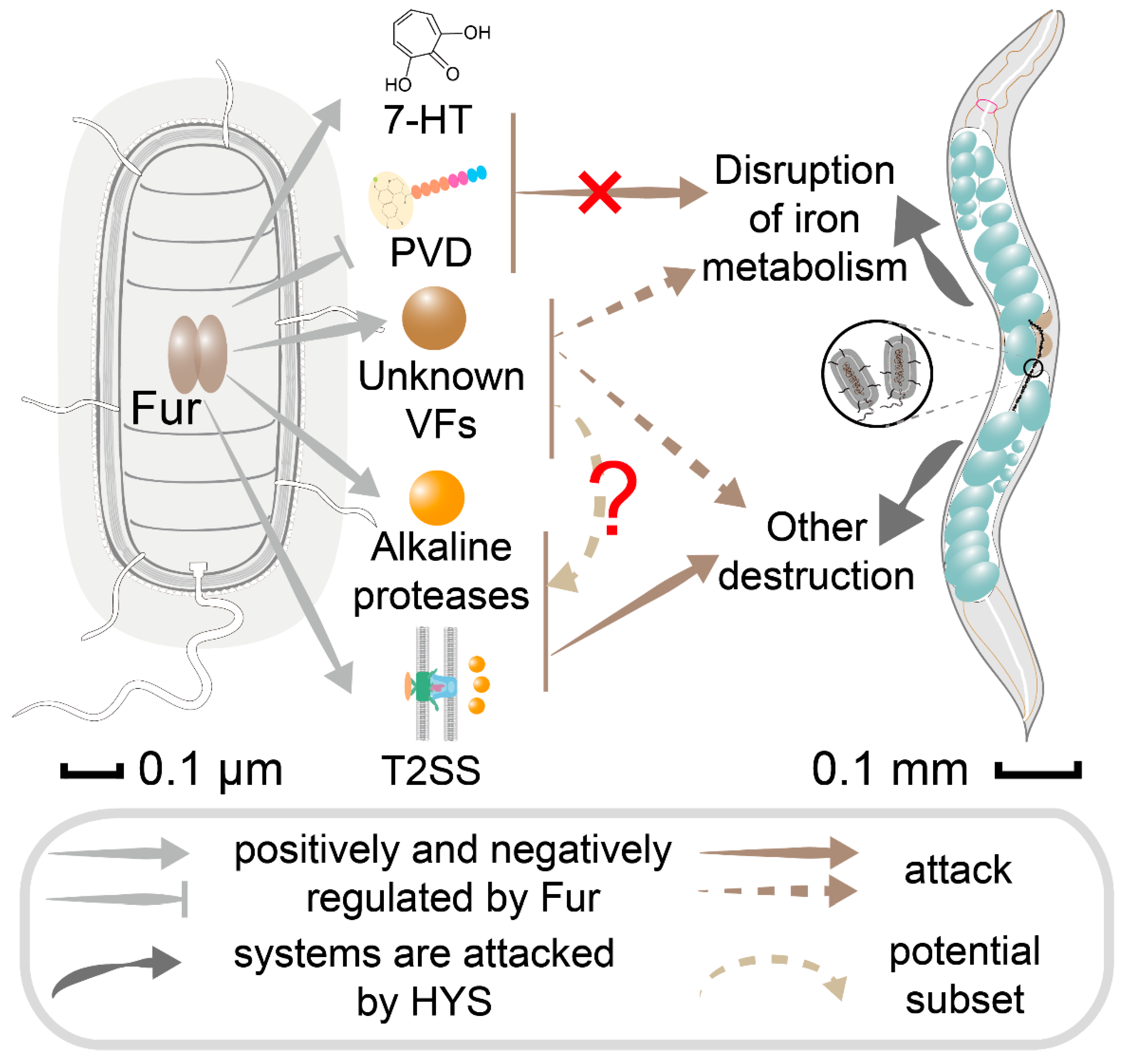
4. Discussion
4.1. HYS Exhibits Different Molecular Mechanism Underlying Its Enhanced and Irreversible Pathogenicity
4.2. HYS Infection Disrupts Iron Metabolism of C. elegans
4.3. Fur Plays Critical Positive Regulatory Role in HYS-Mediated Disruption of C. elegans Iron Metabolism
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen-Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Van Dingenen, J. Harder, Better, Faster, Stronger: Iron Strengthens Pathogenic Bacteria Too! Plant Cell 2021, 33, 1853–1854. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, Y.-C.; Vasquez-Rifo, A.; Jo, J.; Price-Whelan, A.; McDonald, S.T.; Brown, L.M.; Sieben, C.; Dietrich, L.E.P. Pseudomonas aeruginosa PA14 Produces R-Bodies, Extendable Protein Polymers with Roles in Host Colonization and Virulence. Nat. Commun. 2021, 12, 4613. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Yousefi, M.; Abdelgawad, H.A.; Abdelwarith, A.A.; Younis, E.M.; Sakr, E.; Khamis, T.; Ismail, S.H.; Abdel Rahman, A.N. Expression Profiling of Antimicrobial Peptides and Immune-Related Genes in Nile Tilapia Following Pseudomonas putida Infection and Nano-Titanium Dioxide Gel Exposure. Fish Shellfish Immunol. 2025, 156, 110037. [Google Scholar] [CrossRef]
- Bashir, A.; Tian, T.; Yu, X.; Meng, C.; Ali, M.; Li, L. Pyoverdine-Mediated Killing of Caenorhabditis elegans by Pseudomonas syringae MB03 and the Role of Iron in Its Pathogenicity. Int. J. Mol. Sci. 2020, 21, 2198. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa Infection: Lessons from a Versatile Opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Xin, X.-F.; He, S.Y. Pseudomonas syringae Pv. Tomato DC3000: A Model Pathogen for Probing Disease Susceptibility and Hormone Signaling in Plants. Annu. Rev. Phytopathol. 2013, 51, 473–498. [Google Scholar] [CrossRef]
- Jones, A.M.; Wildermuth, M.C. The Phytopathogen Pseudomonas syringae Pv. Tomato DC3000 Has Three High-Affinity Iron-Scavenging Systems Functional under Iron Limitation Conditions but Dispensable for Pathogenesis. J. Bacteriol. 2011, 193, 2767–2775. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron Metabolism in Pathogenic Bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef] [PubMed]
- Fillat, M.F. The Fur (Ferric Uptake Regulator) Superfamily: Diversity and Versatility of Key Transcriptional Regulators. Arch. Biochem. Biophys. 2014, 546, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.M.; Whitmire, J.M.; Merrell, D.S. This Is Not Your Mother’s Repressor: The Complex Role of Fur in Pathogenesis. Infect. Immun. 2009, 77, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, A.A.; Oglesby-Sherrouse, A.G. Regulation of Pseudomonas aeruginosa Virulence by Distinct Iron Sources. Genes 2016, 7, 126. [Google Scholar] [CrossRef]
- Taguchi, F.; Suzuki, T.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. The Siderophore Pyoverdine of Pseudomonas syringae Pv. Tabaci 6605 Is an Intrinsic Virulence Factor in Host Tobacco Infection. J. Bacteriol. 2010, 192, 117–126. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial Iron Homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Gao, J.; Xie, G.; Peng, F.; Xie, Z. Pseudomonas donghuensis Sp. Nov., Exhibiting High-Yields of Siderophore. Antonie Van Leeuwenhoek 2015, 107, 83–94. [Google Scholar] [CrossRef]
- Yu, X.; Chen, M.; Jiang, Z.; Hu, Y.; Xie, Z. The Two-Component Regulators GacS and GacA Positively Regulate a Nonfluorescent Siderophore through the Gac/Rsm Signaling Cascade in High-Siderophore-Yielding Pseudomonas sp. strain HYS. J. Bacteriol. 2014, 196, 3259–3270. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, M.; Yu, X.; Xie, Z. 7-Hydroxytropolone Produced and Utilized as an Iron-Scavenger by Pseudomonas donghuensis. Biometals 2016, 29, 817–826. [Google Scholar] [CrossRef]
- Xie, G.; Zeng, M.; You, J.; Xie, Z. Pseudomonas donghuensis HYS Virulence towards Caenorhabditis elegans Is Regulated by the Cbr/Crc System. Sci. Rep. 2019, 9, 8772. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, P.; Zhu, X.; Xie, Z. Pseudomonas donghuensis HYS gtrA/B/II Gene Cluster Contributes to Its Pathogenicity toward Caenorhabditis elegans. Int. J. Mol. Sci. 2021, 22, 10741. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa Used to Model Mammalian Bacterial Pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; You, J.; Xie, G.; Qin, Y.; Wu, T.; Xie, Z. Pseudomonas donghuensis HYS 7-Hydroxytropolone Contributes to Pathogenicity toward Caenorhabditis elegans and Is Influenced by Pantothenic Acid. Biochem. Biophys. Res. Commun. 2020, 533, 50–56. [Google Scholar] [CrossRef]
- Romero-Afrima, L.; Zelmanovich, V.; Abergel, Z.; Zuckerman, B.; Shaked, M.; Abergel, R.; Livshits, L.; Smith, Y.; Gross, E. Ferritin Is Regulated by a Neuro-Intestinal Axis in the Nematode Caenorhabditis elegans. Redox Biol. 2020, 28, 101359. [Google Scholar] [CrossRef]
- Anderson, C.P.; Leibold, E.A. Mechanisms of Iron Metabolism in Caenorhabditis elegans. Front. Pharmacol. 2014, 5, 113. [Google Scholar] [CrossRef]
- Ast, T.; Meisel, J.D.; Patra, S.; Wang, H.; Grange, R.M.H.; Kim, S.H.; Calvo, S.E.; Orefice, L.L.; Nagashima, F.; Ichinose, F.; et al. Hypoxia Rescues Frataxin Loss by Restoring Iron Sulfur Cluster Biogenesis. Cell 2019, 177, 1507–1521.e16. [Google Scholar] [CrossRef]
- Ye, H.; Rouault, T.A. Human Iron-Sulfur Cluster Assembly, Cellular Iron Homeostasis, and Disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef]
- Zhang, S.; Xin, W.; Anderson, G.J.; Li, R.; Gao, L.; Chen, S.; Zhao, J.; Liu, S. Double-Edge Sword Roles of Iron in Driving Energy Production versus Instigating Ferroptosis. Cell Death Dis. 2022, 13, 40. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Woolf, C.J.; Weiss, G.; Penninger, J.M. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front. Mol. Biosci. 2019, 6, 116. [Google Scholar] [CrossRef]
- Puntarulo, S. Iron, Oxidative Stress and Human Health. Mol. Asp. Med. 2005, 26, 299–312. [Google Scholar] [CrossRef]
- Singh, V.; Aballay, A. Regulation of DAF-16-Mediated Innate Immunity in Caenorhabditis elegans. J. Biol. Chem. 2009, 284, 35580–35587. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Roberts, B.R.; Hare, D.J.; de Jonge, M.D.; Birchall, I.E.; Jenkins, N.L.; Cherny, R.A.; Bush, A.I.; McColl, G. Direct in Vivo Imaging of Ferrous Iron Dyshomeostasis in Ageing Caenorhabditis elegans. Chem. Sci. 2015, 6, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.A.; Sanson-Iglesias, M.; Mukherjee, P.; Buchan, K.D.; Morrison, G.; Hohlt, A.E.; Flores, A.R. LiaR-Dependent Gene Expression Contributes to Antimicrobial Responses in Group A Streptococcus. Antimicrob. Agents Chemother. 2024, 68, e0049624. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Nitanai, H.; Hoshino, K.; Otani, T. Impact of Siderophore Production on Pseudomonas aeruginosa Infections in Immunosuppressed Mice. Infect. Immun. 2000, 68, 1834–1839. [Google Scholar] [CrossRef]
- de Moraes, M.H.; Hsu, F.; Huang, D.; Bosch, D.E.; Zeng, J.; Radey, M.C.; Simon, N.; Ledvina, H.E.; Frick, J.P.; Wiggins, P.A.; et al. An Interbacterial DNA Deaminase Toxin Directly Mutagenizes Surviving Target Populations. Elife 2021, 10, e62967. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Guo, B.; Liu, F. Old Role with New Feature: T2SS ATPase as a Cyclic-Di-GMP Receptor to Regulate Antibiotic Production. Appl. Environ. Microbiol. 2024, 90, e0041824. [Google Scholar] [CrossRef]
- Filloux, A. The Underlying Mechanisms of Type II Protein Secretion. Biochim. Biophys. Acta 2004, 1694, 163–179. [Google Scholar] [CrossRef]
- Mirza, Z.; Walhout, A.J.M.; Ambros, V. A Bacterial Pathogen Induces Developmental Slowing by High Reactive Oxygen Species and Mitochondrial Dysfunction in Caenorhabditis elegans. Cell Rep. 2023, 42, 113189. [Google Scholar] [CrossRef]
- Vaitkevicius, K.; Lindmark, B.; Ou, G.; Song, T.; Toma, C.; Iwanaga, M.; Zhu, J.; Andersson, A.; Hammarström, M.-L.; Tuck, S.; et al. A Vibrio cholerae Protease Needed for Killing of Caenorhabditis elegans Has a Role in Protection from Natural Predator Grazing. Proc. Natl. Acad. Sci. USA 2006, 103, 9280–9285. [Google Scholar] [CrossRef]
- Au, C.; Benedetto, A.; Anderson, J.; Labrousse, A.; Erikson, K.; Ewbank, J.J.; Aschner, M. SMF-1, SMF-2 and SMF-3 DMT1 Orthologues Regulate and Are Regulated Differentially by Manganese Levels in C. elegans. PLoS ONE 2009, 4, e7792. [Google Scholar] [CrossRef]
- Rajan, M.; Anderson, C.P.; Rindler, P.M.; Romney, S.J.; Ferreira Dos Santos, M.C.; Gertz, J.; Leibold, E.A. NHR-14 Loss of Function Couples Intestinal Iron Uptake with Innate Immunity in C. elegans through PQM-1 Signaling. Elife 2019, 8, e44674. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Hou, W.; Wang, Z.-Q.; Xu, X. Biogenesis of Iron-Sulfur Clusters and Their Role in DNA Metabolism. Front. Cell Dev. Biol. 2021, 9, 735678. [Google Scholar] [CrossRef] [PubMed]
- Vallières, C.; Benoit, O.; Guittet, O.; Huang, M.-E.; Lepoivre, M.; Golinelli-Cohen, M.-P.; Vernis, L. Iron-Sulfur Protein Odyssey: Exploring Their Cluster Functional Versatility and Challenging Identification. Metallomics 2024, 16, mfae025. [Google Scholar] [CrossRef] [PubMed]
- Terzi, E.M.; Sviderskiy, V.O.; Alvarez, S.W.; Whiten, G.C.; Possemato, R. Iron-Sulfur Cluster Deficiency Can Be Sensed by IRP2 and Regulates Iron Homeostasis and Sensitivity to Ferroptosis Independent of IRP1 and FBXL5. Sci. Adv. 2021, 7, eabg4302. [Google Scholar] [CrossRef]
- Ploumi, C.; Kyriakakis, E.; Tavernarakis, N. Coupling of Autophagy and the Mitochondrial Intrinsic Apoptosis Pathway Modulates Proteostasis and Ageing in Caenorhabditis elegans. Cell Death Dis. 2023, 14, 110. [Google Scholar] [CrossRef]
- Gancz, H.; Censini, S.; Merrell, D.S. Iron and pH Homeostasis Intersect at the Level of Fur Regulation in the Gastric Pathogen Helicobacter pylori. Infect. Immun. 2006, 74, 602–614. [Google Scholar] [CrossRef]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa Adapts Its Iron Uptake Strategies in Function of the Type of Infections. Front. Cell Infect. Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, A.; Llamas, M.A.; Marcos-Torres, F.J. Transcriptional Regulators Controlling Virulence in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2023, 24, 11895. [Google Scholar] [CrossRef]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef]
- Spiga, L.; Fansler, R.T.; Perera, Y.R.; Shealy, N.G.; Munneke, M.J.; David, H.E.; Torres, T.P.; Lemoff, A.; Ran, X.; Richardson, K.L.; et al. Iron Acquisition by a Commensal Bacterium Modifies Host Nutritional Immunity during Salmonella Infection. Cell Host Microbe 2023, 31, 1639–1654.e10. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef] [PubMed]
- Laarman, A.J.; Bardoel, B.W.; Ruyken, M.; Fernie, J.; Milder, F.J.; van Strijp, J.A.G.; Rooijakkers, S.H.M. Pseudomonas aeruginosa Alkaline Protease Blocks Complement Activation via the Classical and Lectin Pathways. J. Immunol. 2012, 188, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Krekhno, Z.; Woodward, S.E.; Serapio-Palacios, A.; Peña-Díaz, J.; Moon, K.M.; Foster, L.J.; Finlay, B.B. Citrobacter rodentium Possesses a Functional Type II Secretion System Necessary for Successful Host Infection. Gut Microbes 2024, 16, 2308049. [Google Scholar] [CrossRef] [PubMed]


| Gene ID | Gene Name | Fold Change | Gene Description |
|---|---|---|---|
| UW3_RS0123895 | hasE | 4.676 | Alkaline protease secretion protein |
| UW3_RS0123890 | hasF | 4.507 | Alkaline protease secretion protein |
| UW3_RS0115515 | dppF | 3.647 | Di/tripeptide transport ATP-binding protein |
| UW3_RS0104960 | gspE | 2.924 | General secretion pathway protein E |
| UW3_RS0105380 | dddA | 2.907 | Double-stranded DNA deaminase toxin A |
| UW3_RS0117820 | comEA | 2.666 | Uncharacterized protein |
| UW3_RS0116245 | ddpF | 2.583 | Probable peptide ABC transporter ATP-binding protein |
| UW3_RS27610 | hasD | 2.094 | Alkaline protease secretion ATP-binding protein |
| UW3_RS0105455 | impL | 2.043 | Type VI secretion system component |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Shen, L.; Lin, Y.; Huang, S.; Xie, Z. Ferric Uptake Regulator Contributes to Pseudomonas donghuensis HYS-Induced Iron Metabolic Disruption in Caenorhabditis elegans. Microorganisms 2025, 13, 1081. https://doi.org/10.3390/microorganisms13051081
Gao D, Shen L, Lin Y, Huang S, Xie Z. Ferric Uptake Regulator Contributes to Pseudomonas donghuensis HYS-Induced Iron Metabolic Disruption in Caenorhabditis elegans. Microorganisms. 2025; 13(5):1081. https://doi.org/10.3390/microorganisms13051081
Chicago/Turabian StyleGao, Donghao, Liwen Shen, Yelong Lin, Shuo Huang, and Zhixiong Xie. 2025. "Ferric Uptake Regulator Contributes to Pseudomonas donghuensis HYS-Induced Iron Metabolic Disruption in Caenorhabditis elegans" Microorganisms 13, no. 5: 1081. https://doi.org/10.3390/microorganisms13051081
APA StyleGao, D., Shen, L., Lin, Y., Huang, S., & Xie, Z. (2025). Ferric Uptake Regulator Contributes to Pseudomonas donghuensis HYS-Induced Iron Metabolic Disruption in Caenorhabditis elegans. Microorganisms, 13(5), 1081. https://doi.org/10.3390/microorganisms13051081






