Probiotic Administration Contributes to the Improvement in Intestinal Dysregulation Induced by Allergic Contact Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Study Design
2.3. Induction of Allergic Contact Dermatitis
2.4. Probiotic Cultivation and Administration
2.5. Evaluation of Inflammatory Infiltration and Morphology of Skin and Intestine
2.6. Determination of Cytokine Levels in Colon
2.7. Gene Expression Analysis
2.8. Sequencing Processing
2.9. Comparative Analyses
2.10. Quantification of Short-Chain Fatty Acids (SCFAs)
2.11. Statistical Methodology
3. Results
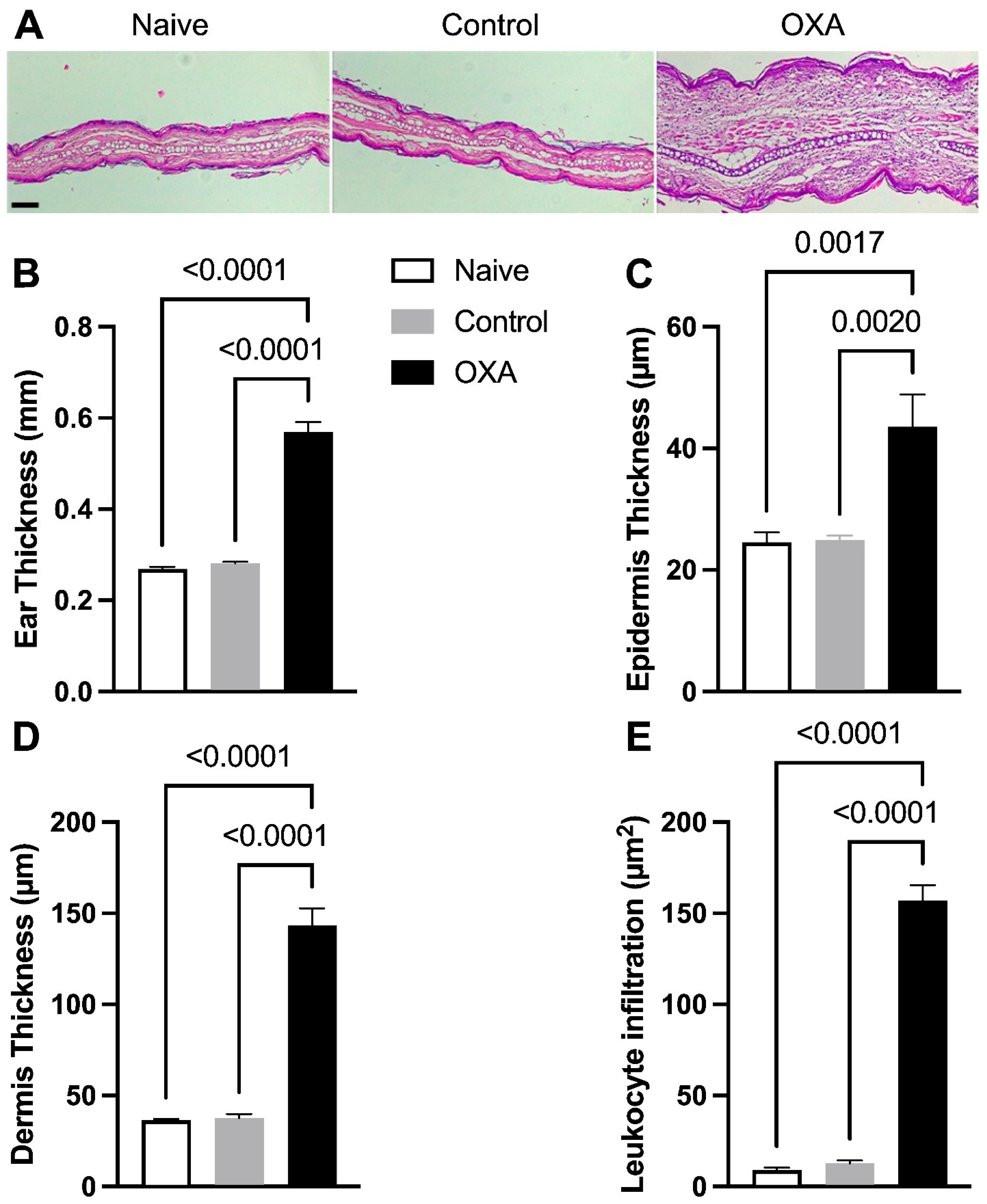
3.1. Oxazolone-Induced DCA Increases Skin Thickness and Inflammatory Cell Infiltration
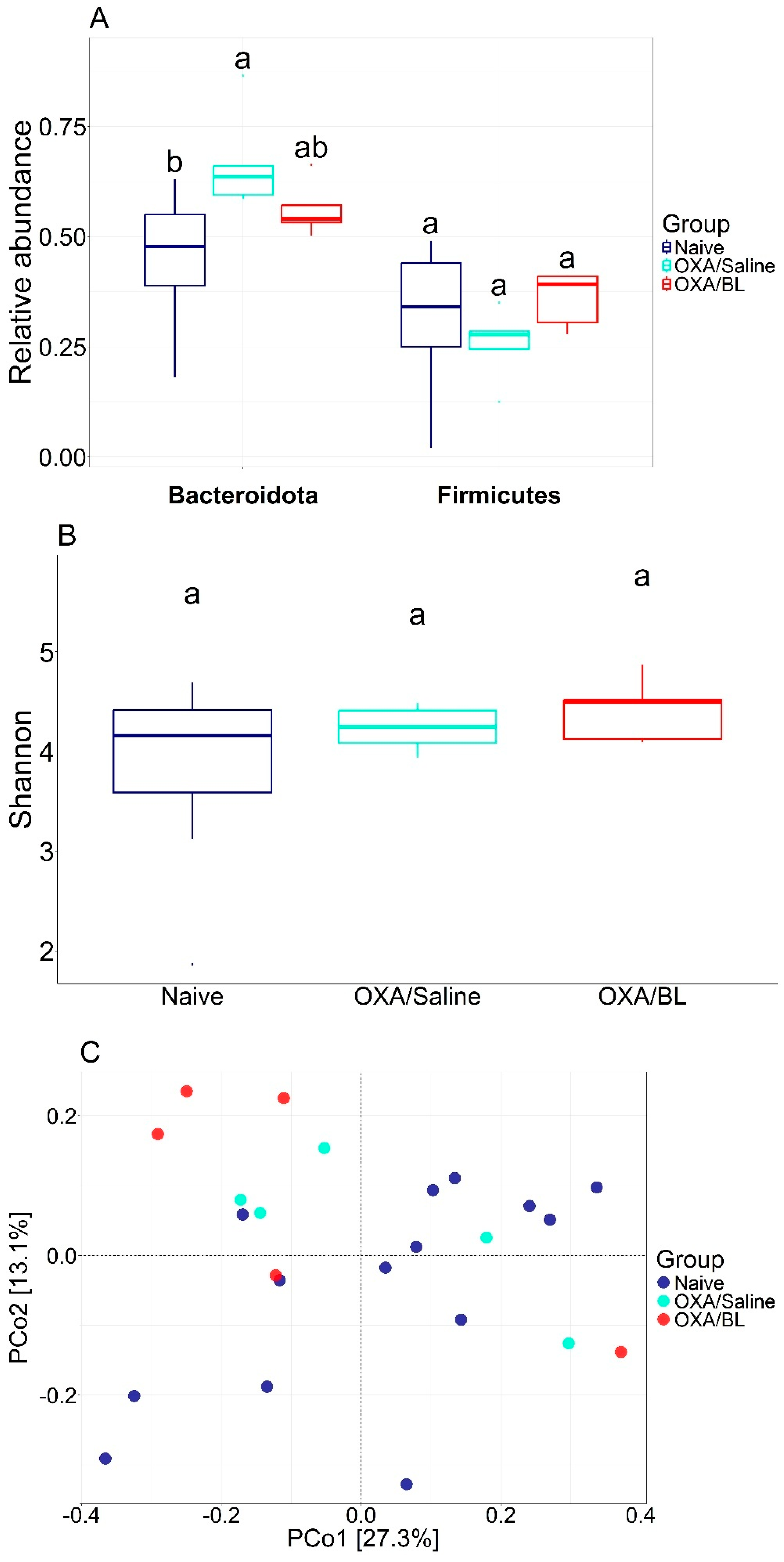
3.2. Effects of Dermatitis and Probiotic Treatment on Gut Microbiota Composition
3.3. The Induction of Dermatitis Does Not Affect the Amount of SCFAs in the Feces, but Probiotic Treatment Increases the Production of Acetate in the Feces
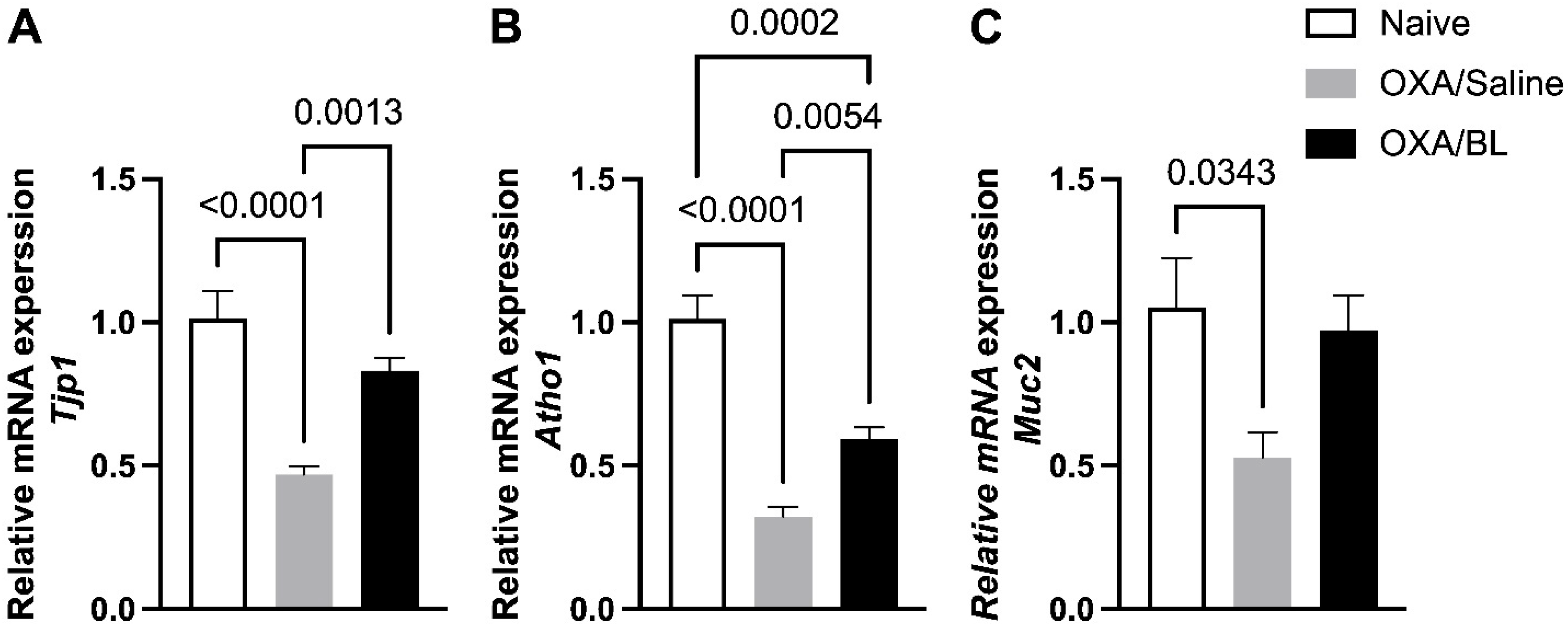
3.4. Dermatitis-Induced Intestinal Barrier Gene Disruption Reversed by Probiotic Treatment in Mice
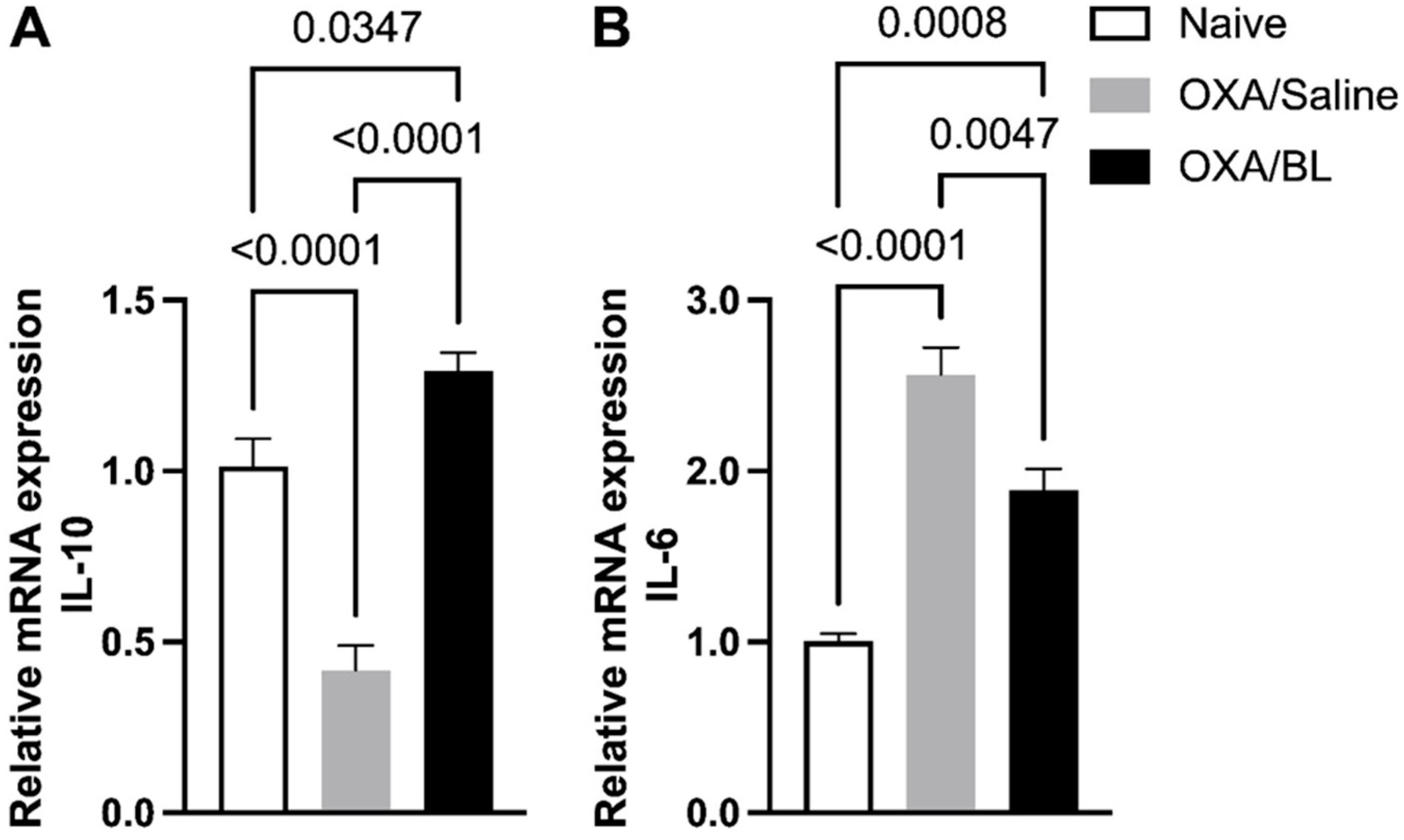
3.5. Dermatitis-Induced Intestinal Il-10 and Il-6 Gene Alteration Reversed by Probiotic Treatment in Mice
3.6. Impact of Intestinal Mucus Production Following Skin Injury and Probiotic Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Ramessur, R.; Dand, N.; Langan, S.M.; Saklatvala, J.; Fritzsche, M.-C.; Holland, S.; Arents, B.W.M.; McAteer, H.; Proctor, A.; McMahon, D.; et al. Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: An interdisciplinary perspective. Br. J. Dermatol. 2024, 191, 14–23. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated With Gut Barrier Dysfunction in Healthy Relatives of Patients With Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e1310. [Google Scholar] [CrossRef]
- Umborowati, M.A.; Damayanti, D.; Anggraeni, S.; Endaryanto, A.; Surono, I.S.; Effendy, I.; Prakoeswa, C.R.S. The role of probiotics in the treatment of adult atopic dermatitis: A meta-analysis of randomized controlled trials. J. Health Popul. Nutr. 2022, 41, 37. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Bonita, L.; Karim, A.; Herwanto, N.; Umborowati, M.A.; Setyaningrum, T.; Hidayati, A.N.; Surono, I.S. Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: A randomized controlled trial. J. Dermatol. Treat. 2022, 33, 1491–1498. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Herwanto, N.; Prameswari, R.; Astari, L.; Sawitri, S.; Hidayati, A.N.; Indramaya, D.M.; Kusumowidagdo, E.R.; Surono, I.S. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 2017, 8, 833–840. [Google Scholar] [CrossRef]
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Ribeiro, W.R.; Queiroz, A.G.; Mendes, E.; Casaro, M.B.; Nascimento, C.M.; Coelho, L.; Martins, F.S.; Leite-Silva, V.R.; Ferreira, C.M. Preventive oral supplementation with Bifidobacterium longum 5(1A) alleviates oxazolone-induced allergic contact dermatitis-like skin inflammation in mice. Benef. Microbes 2021, 12, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Hong, R.; Choi, E.Y.; Yu, K.; Kim, N.; Hyeon, J.Y.; Cho, K.K.; Choi, I.S.; Yun, C.H. A Probiotic Mixture Regulates T Cell Balance and Reduces Atopic Dermatitis Symptoms in Mice. Front. Microbiol. 2018, 9, 2414. [Google Scholar] [CrossRef] [PubMed]
- Wrześniewska, M.; Wołoszczak, J.; Świrkosz, G.; Szyller, H.; Gomułka, K. The Role of the Microbiota in the Pathogenesis and Treatment of Atopic Dermatitis—A Literature Review. Int. J. Mol. Sci. 2024, 25, 6539. [Google Scholar] [CrossRef]
- Zhang, X.E.; Zheng, P.; Ye, S.Z.; Ma, X.; Liu, E.; Pang, Y.B.; He, Q.Y.; Zhang, Y.X.; Li, W.Q.; Zeng, J.H.; et al. Microbiome: Role in Inflammatory Skin Diseases. J. Inflamm. Res. 2024, 17, 1057–1082. [Google Scholar] [CrossRef]
- Scheinman, P.L.; Vocanson, M.; Thyssen, J.P.; Johansen, J.D.; Nixon, R.L.; Dear, K.; Botto, N.C.; Morot, J.; Goldminz, A.M. Contact dermatitis. Nat. Rev. Dis. Primers 2021, 7, 38. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- McFadden, J.P.; White, J.M.; Basketter, D.A.; Kimber, I. Does hapten exposure predispose to atopic disease? The hapten-atopy hypothesis. Trends Immunol. 2009, 30, 67–74. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L. R/Bioconductor Framework for Microbiome Data Science. Available online: https://microbiome.github.io/index.html#services (accessed on 12 January 2025).
- Cao, Y.; Dong, Q.; Wang, D.; Zhang, P.; Liu, Y.; Niu, C. microbiomeMarker: An R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics 2022, 38, 4027–4029. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Lahti, L. Microbiome data science. J. Biosci. 2019, 44, 115. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Ribeiro, W.R.; Vinolo, M.A.R.; Calixto, L.A.; Ferreira, C.M. Use of Gas Chromatography to Quantify Short Chain Fatty Acids in the Serum, Colonic Luminal Content and Feces of mice. Bio-protocol 2018, 8, e3089. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef]
- Pereira, M.S.; Redanz, S.; Kriegel, M.A. Skin Deep: The Role of the Microbiota in Cutaneous Autoimmunity. J. Investig. Dermatol. 2022, 142, 834–840. [Google Scholar] [CrossRef]
- Liu, X.C.; Du, T.T.; Gao, X.; Zhao, W.J.; Wang, Z.L.; He, Y.; Bao, L.; Li, L.Q. Gut microbiota and short-chain fatty acids may be new biomarkers for predicting neonatal necrotizing enterocolitis: A pilot study. Front. Microbiol. 2022, 13, 969656. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Shen, Z.; Xue, Y.; Zhang, B.; Yu, D.; Liu, T.; Luo, D.; Xing, G.; Tang, J.; et al. Increase Dietary Fiber Intake Ameliorates Cecal Morphology and Drives Cecal Species-Specific of Short-Chain Fatty Acids in White Pekin Ducks. Front. Microbiol. 2022, 13, 853797. [Google Scholar] [CrossRef]
- Volland, J.-M. Small cells with big secrets. Nat. Rev. Microbiol. 2023, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Katase, M.; Tsumura, K. Dietary soyasaponin attenuates 2,4-dinitrofluorobenzene-induced contact hypersensitivity via gut microbiota in mice. Clin. Exp. Immunol. 2019, 195, 86–95. [Google Scholar] [CrossRef]
- Wang, M.; Karlsson, C.; Olsson, C.; Adlerberth, I.; Wold, A.E.; Strachan, D.P.; Martricardi, P.M.; Aberg, N.; Perkin, M.R.; Tripodi, S.; et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, Á.; Huertas, Á.J.; Larramendi, C.H.; García-Abujeta, J.L.; Bartra, J.; Lavín, J.R.; Andreu, C.; Pagán, J.A.; López-Matas, M.A.; Fernández-Caldas, E.; et al. Usefulness of manufactured tomato extracts in the diagnosis of tomato sensitization: Comparison with the prick-prick method. Clin. Mol. Allergy 2008, 6, 1. [Google Scholar] [CrossRef]
- Sjögren, Y.M.; Jenmalm, M.C.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2009, 39, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.Y.; Lee, B.W.; Aw, M.; Shek, L.P.; Yap, G.C.; Chua, K.Y.; Liu, W.T. Comparative analysis of fecal microbiota in infants with and without eczema. PLoS ONE 2010, 5, e9964. [Google Scholar] [CrossRef]
- Yap, G.C.; Loo, E.X.L.; Aw, M.; Lu, Q.; Shek, L.P.-C.; Lee, B.W. Molecular analysis of infant fecal microbiota in an Asian at-risk cohort–correlates with infant and childhood eczema. BMC Res. Notes 2014, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Gueimonde, M.; Hernández-Barranco Ana, M.; Ruas-Madiedo, P.; de los Reyes-Gavilán Clara, G. Exopolysaccharides Produced by Intestinal Bifidobacterium Strains Act as Fermentable Substrates for Human Intestinal Bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef]
- Zaplana, T.; Miele, S.; Tolonen, A.C. Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1324396. [Google Scholar] [CrossRef] [PubMed]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Vlachou, A.; Verbrugghe, K.; De Vuyst, L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 2006, 72, 7835–7841. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-H.; Hong, S.-J. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy Asthma Immunol. Res. 2020, 12, 137–148. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, microbiota, and the mucus layer: The guardians of our health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef]
- Alyssa, G.; Brenton, P.; Melinda, A.E. Bifidobacterium and the intestinal mucus layer. Microbiome Res. Rep. 2023, 2, 36. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, Q.; Evivie, S.E.; Yue, Y.; Yang, H.; Lv, X.; Liu, F.; Li, B.; Huo, G. Bifidobacterium longum subsp. longum K5 alleviates inflammatory response and prevents intestinal barrier injury induced by LPS in vitro based on comparative genomics. J. Funct. Foods 2022, 92, 105030. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.Z.; Saito, T.; Kitazawa, H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 2013, 8, e59259. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef]
- Iemoli, E.; Trabattoni, D.; Parisotto, S.; Borgonovo, L.; Toscano, M.; Rizzardini, G.; Clerici, M.; Ricci, E.; Fusi, A.; De Vecchi, E.; et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J. Clin. Gastroenterol. 2012, 46, S33–S40. [Google Scholar] [CrossRef]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Chen, W. Correction to: Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur. J. Nutr. 2021, 60, 4659. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hamajima, Y.; Komori, M.; Yokota, M.; Suzuki, M.; Lin, J. The role of atoh1 in mucous cell metaplasia. Int. J. Otolaryngol. 2012, 2012, 438609. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Gao, Q.; Zhang, J.Z.; Lu, Y.T.; Xing, D.M.; Qin, Y.Q.; Fang, J. Prolyl hydroxylase 3 controls the intestine goblet cell generation through stabilizing ATOH1. Cell Death Differ. 2020, 27, 2131–2142. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, M.; Bhullar, K.; Montero, M.; Ma, C.; Huang, T.; Velcich, A.; Xia, L.; Vallance, B.A. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 2013, 81, 3672–3683. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Kiecka, A.; Macura, B.; Szczepanik, M. Modulation of allergic contact dermatitis via gut microbiota modified by diet, vitamins, probiotics, prebiotics, and antibiotics. Pharmacol. Rep. PR 2023, 75, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Valacchi, G. Role of microbiota in the GUT-SKIN AXIS responses to outdoor stressors. Free Radic. Biol. Med. 2024, 225, 894–909. [Google Scholar] [CrossRef] [PubMed]



| Naïve | OXA/Saline | OXA/BL | ||||||
|---|---|---|---|---|---|---|---|---|
| Taxon | IndVal Stat | p Value | Taxon | IndVal Stat | p Value | Taxon | IndVal Stat | p Value |
| p__Firmicutes|g__[Eubacterium] brachy group|ASV233 | 0.914 | 0.018 | p__Bacteroidota|f__Muribaculaceae|ASV83 | 0.934 | 0.004 | p__Firmicutes|o__Clostridia UCG-014|ASV200 | 0.989 | 0.001 |
| p__Firmicutes|f__Erysipelotrichaceae|ASV160 | 0.879 | 0.033 | p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV99 | 0.916 | 0.010 | p__Firmicutes|f__Lachnospiraceae|ASV344 | 0.956 | 0.001 |
| p__Firmicutes|f__Ruminococcaceae|ASV214 | 0.833 | 0.042 | p__Bacteroidota|f__Muribaculaceae|ASV33 | 0.916 | 0.012 | p__Bacteroidota|f__Muribaculaceae|ASV83 | 0.934 | 0.004 |
| p__Firmicutes|f__Lactobacillaceae|ASV283 | 0.779 | 0.029 | p__Bacteroidota|f__Muribaculaceae|ASV97 | 0.914 | 0.001 | p__Bacteroidota|f__Muribaculaceae|ASV33 | 0.916 | 0.012 |
| p__Firmicutes|g__[Eubacterium] siraeum group|ASV113 | 0.903 | 0.031 | p__Firmicutes|g__[Eubacterium] brachy group|ASV233 | 0.914 | 0.018 | |||
| p__Bacteroidota|f__Muribaculaceae|ASV91 | 0.901 | 0.002 | p__Firmicutes|o__Clostridia UCG-014|ASV184 | 0.900 | 0.006 | |||
| p__Firmicutes|o__Clostridia UCG-014|ASV184 | 0.900 | 0.006 | p__Firmicutes|g__Tuzzerella|ASV435 | 0.897 | 0.004 | |||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV54 | 0.878 | 0.002 | p__Bacteroidota|f__Muribaculaceae|ASV108 | 0.894 | 0.002 | |||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV138 | 0.858 | 0.045 | p__Firmicutes|f__Erysipelotrichaceae|ASV160 | 0.879 | 0.033 | |||
| p__Patescibacteria|g__Candidatus Saccharimonas|ASV251 | 0.830 | 0.019 | p__Firmicutes|f__Lachnospiraceae|ASV164 | 0.861 | 0.004 | |||
| p__Firmicutes|g__Marvinbryantia|ASV298 | 0.793 | 0.011 | p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV138 | 0.858 | 0.045 | |||
| p__Firmicutes|f__Lachnospiraceae|ASV325 | 0.758 | 0.031 | p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV116 | 0.840 | 0.028 | |||
| p__Firmicutes|o__Clostridia UCG-014|ASV156 | 0.756 | 0.027 | p__Firmicutes|f__Ruminococcaceae|ASV214 | 0.833 | 0.042 | |||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV217 | 0.744 | 0.012 | p__Patescibacteria|g__Candidatus Saccharimonas|ASV251 | 0.830 | 0.019 | |||
| p__Firmicutes|g__Lachnospiraceae UCG-010|ASV542 | 0.715 | 0.014 | p__Bacteroidota|f__Muribaculaceae|ASV249 | 0.829 | 0.004 | |||
| p__Bacteroidota|g__Bacteroides|ASV237 | 0.695 | 0.031 | p__Firmicutes|g__Oscillibacter|ASV336 | 0.819 | 0.005 | |||
| p__Firmicutes|f__Ruminococcaceae|ASV335 | 0.691 | 0.036 | p__Firmicutes|f__Oscillospiraceae|ASV294 | 0.815 | 0.013 | |||
| p__Bacteroidota|f__Muribaculaceae|ASV252 | 0.792 | 0.009 | ||||||
| p__Firmicutes|f__Ruminococcaceae|ASV293 | 0.788 | 0.016 | ||||||
| p__Firmicutes|f__Lachnospiraceae|ASV235 | 0.781 | 0.017 | ||||||
| p__Bacteroidota|f__Muribaculaceae|ASV189 | 0.779 | 0.019 | ||||||
| p__Firmicutes|f__Lachnospiraceae|ASV255 | 0.775 | 0.008 | ||||||
| p__Firmicutes|f__Lachnospiraceae|ASV449 | 0.775 | 0.013 | ||||||
| p__Firmicutes|g__Lachnoclostridium|ASV459 | 0.775 | 0.013 | ||||||
| p__Actinobacteriota|g__Enterorhabdus|ASV340 | 0.770 | 0.017 | ||||||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV241 | 0.761 | 0.026 | ||||||
| p__Firmicutes|o__Clostridia UCG-014|ASV156 | 0.756 | 0.027 | ||||||
| p__Bacteroidota|f__Muribaculaceae|ASV285 | 0.731 | 0.015 | ||||||
| p__Firmicutes|g__Lachnospiraceae FCS020 group|ASV354 | 0.729 | 0.022 | ||||||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV179 | 0.727 | 0.011 | ||||||
| p__Firmicutes|f__Lachnospiraceae|ASV359 | 0.710 | 0.019 | ||||||
| p__Firmicutes|g__Lachnoclostridium|ASV300 | 0.709 | 0.016 | ||||||
| p__Firmicutes|g__Lachnospiraceae NK4A136 group|ASV229 | 0.707 | 0.02 | ||||||
| p__Firmicutes|g__GCA-900066575|ASV564 | 0.706 | 0.032 | ||||||
| p__Firmicutes|f__Lachnospiraceae|ASV410 | 0.699 | 0.034 | ||||||
| p__Firmicutes|f__Christensenellaceae|ASV496 | 0.697 | 0.031 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, E.; Umana, E.R.P.; Di Pace Soares Penna, D.; de Oliveira, F.A.; Lemos, L.N.; Ribeiro, W.R.; Casaro, M.B.; Lazarini, M.; Oliveira, V.M.; Ferreira, C.M. Probiotic Administration Contributes to the Improvement in Intestinal Dysregulation Induced by Allergic Contact Dermatitis. Microorganisms 2025, 13, 1082. https://doi.org/10.3390/microorganisms13051082
Mendes E, Umana ERP, Di Pace Soares Penna D, de Oliveira FA, Lemos LN, Ribeiro WR, Casaro MB, Lazarini M, Oliveira VM, Ferreira CM. Probiotic Administration Contributes to the Improvement in Intestinal Dysregulation Induced by Allergic Contact Dermatitis. Microorganisms. 2025; 13(5):1082. https://doi.org/10.3390/microorganisms13051082
Chicago/Turabian StyleMendes, Eduardo, Evelyn Roxana Perez Umana, Daniel Di Pace Soares Penna, Fernando Augusto de Oliveira, Leandro Nascimento Lemos, Willian Rodrigues Ribeiro, Mateus Barbosa Casaro, Mariana Lazarini, Valéria Maia Oliveira, and Caroline Marcantonio Ferreira. 2025. "Probiotic Administration Contributes to the Improvement in Intestinal Dysregulation Induced by Allergic Contact Dermatitis" Microorganisms 13, no. 5: 1082. https://doi.org/10.3390/microorganisms13051082
APA StyleMendes, E., Umana, E. R. P., Di Pace Soares Penna, D., de Oliveira, F. A., Lemos, L. N., Ribeiro, W. R., Casaro, M. B., Lazarini, M., Oliveira, V. M., & Ferreira, C. M. (2025). Probiotic Administration Contributes to the Improvement in Intestinal Dysregulation Induced by Allergic Contact Dermatitis. Microorganisms, 13(5), 1082. https://doi.org/10.3390/microorganisms13051082







