Dysregulated Adaptive Immune Responses to SARS-CoV-2 in Immunocompromised Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sampling
2.2. Activation-Induced Marker (AIM) Assay
2.3. Flow Cytometry
2.4. Spike and ACE2 Inhibition Assay
2.5. Meso Scale
2.6. Polymerase Chain Reaction (PCR)
2.7. Viral Cultures for Cytopathic Effect (CPE) Assessment
2.8. Memory B Cell FluoroSpot Assay
2.9. Statistical Analysis
3. Results
3.1. Patient Characteristics and Outcome
3.2. Immune Cell Composition in Immunosuppressed Patients During COVID-19
3.3. SARS-CoV-2-Specific IgG Levels Differ Across Immunocompromised Patient Groups over Time
3.4. Spike-Specific Memory Antibody-Secreting Cells Are Diminished in Immunocompromised Patients
3.5. Immunocompromised Patients Display Robust Yet Variable CD4+ T Cell Responses
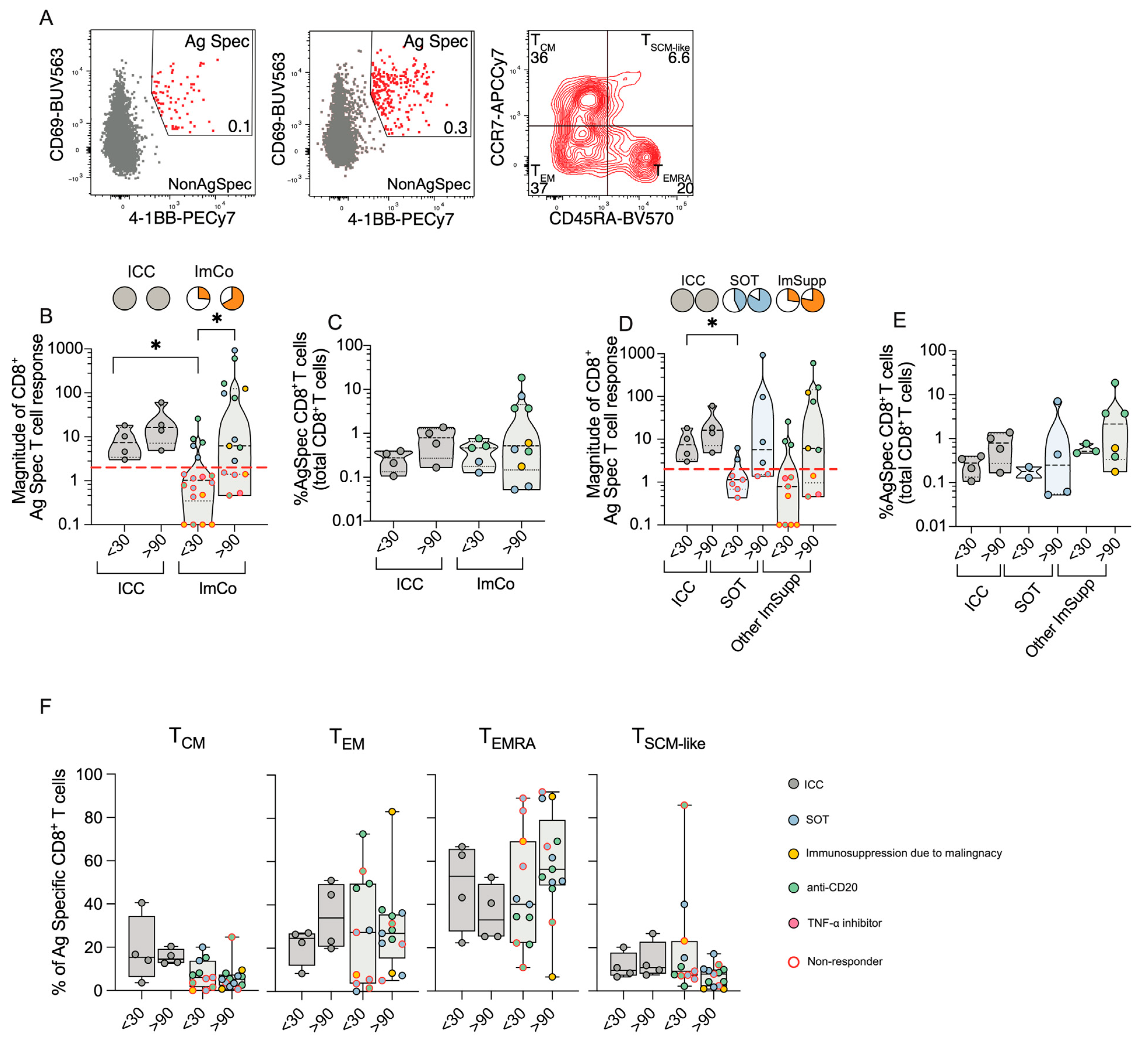
3.6. CD8+ T Cell Responses Reveal Compensatory Immunity in Immunocompromised Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on Immunocompromised Populations during the Omicron Era: Insights from the Observational Population-Based INFORM Study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef] [PubMed]
- Bertini, C.D.; Khawaja, F.; Sheshadri, A. Coronavirus Disease-2019 in the Immunocompromised Host. Clin. Chest Med. 2023, 44, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Aguilar-Company, J.; Ferrante, D.; Hanbury, G.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Plaja, A.; Cucurull, M.; et al. Outcomes of the SARS-CoV-2 Omicron (B.1.1.529) Variant Outbreak among Vaccinated and Unvaccinated Patients with Cancer in Europe: Results from the Retrospective, Multicentre, OnCovid Registry Study. Lancet Oncol. 2022, 23, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease Severity Dictates SARS-CoV-2-Specific Neutralizing Antibody Responses in COVID-19. Signal Transduct. Target. Ther. 2020, 5, 180. [Google Scholar] [CrossRef]
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 Months after Infection. iScience 2022, 25, 103743. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Vasileiou, I.V.; Sakellariou, K.S.; Spyrou, N.I.; Tsakris, A. Persistence of T-Cell Immunity Responses against SARS-CoV-2 for over 12 Months Post COVID-19 Infection in Unvaccinated Individuals with No Detectable IgG Antibodies. Vaccines 2023, 11, 1764. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-Specific T Cell Immunity Is Maintained at 6 Months Following Primary Infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The Effectiveness of COVID-19 Vaccines in Reducing the Incidence, Hospitalization, and Mortality from COVID-19: A Systematic Review and Meta-Analysis. Front. Public. Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and Efficacy of the mRNA BNT162b2 Vaccine against SARS-CoV-2 in Five Groups of Immunocompromised Patients and Healthy Controls in a Prospective Open-Label Clinical Trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Dib, M.; Le Corre, N.; Ortiz, C.; García, D.; Ferrés, M.; Martinez-Valdebenito, C.; Ruiz-Tagle, C.; Ojeda, M.J.; Espinoza, M.A.; Jara, A.; et al. SARS-CoV-2 Vaccine Booster in Solid Organ Transplant Recipients Previously Immunised with Inactivated versus mRNA Vaccines: A Prospective Cohort Study. Lancet Reg. Health Am. 2022, 16, 100371. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, A.; Luna, A.; Micic, D. A Systematic Review and Meta-Analysis of Serologic Response Following Coronavirus Disease 2019 (COVID-19) Vaccination in Solid Organ Transplant Recipients. Viruses 2022, 14, 1822. [Google Scholar] [CrossRef] [PubMed]
- Napuri, N.I.; Curcio, D.; Swerdlow, D.L.; Srivastava, A. Immune Response to COVID-19 and mRNA Vaccination in Immunocompromised Individuals: A Narrative Review. Infect. Dis. Ther. 2022, 11, 1391–1414. [Google Scholar] [CrossRef] [PubMed]
- Bytyci, J.; Ying, Y.; Lee, L.Y.W. Immunocompromised Individuals Are at Increased Risk of COVID-19 Breakthrough Infection, Hospitalization, and Death in the Post-Vaccination Era: A Systematic Review. Immun. Inflamm. Dis. 2024, 12, e1259. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Madelon, N.; Lauper, K.; Breville, G.; Sabater Royo, I.; Goldstein, R.; Andrey, D.O.; Grifoni, A.; Sette, A.; Kaiser, L.; Siegrist, C.A.; et al. Robust T-Cell Responses in Anti-CD20-Treated Patients Following COVID-19 Vaccination: A Prospective Cohort Study. Clin. Infect. Dis. 2022, 75, e1037–e1045. [Google Scholar] [CrossRef]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 Vaccination in Rituximab-Treated Patients: B Cells Promote Humoral Immune Responses in the Presence of T-Cell-Mediated Immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Wullimann, D.; Niessl, J.; Rivera-Ballesteros, O.; Chen, P.; Lange, J.; Cuapio, A.; Blennow, O.; Hansson, L.; et al. Immunodeficiency Syndromes Differentially Impact the Functional Profile of SARS-CoV-2-Specific T Cells Elicited by mRNA Vaccination. Immunity 2022, 55, 1732–1746.e5. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T Cells Contribute to Survival in Patients with COVID-19 and Hematologic Cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; I de Silva, T.; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-Specific Immune Responses and Clinical Outcomes after COVID-19 Vaccination in Patients with Immune-Suppressive Disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef]
- Lyudovyk, O.; Kim, J.Y.; Qualls, D.; Hwee, M.A.; Lin, Y.-H.; Boutemine, S.R.; Elhanati, Y.; Solovyov, A.; Douglas, M.; Chen, E.; et al. Impaired Humoral Immunity Is Associated with Prolonged COVID-19 despite Robust CD8 T Cell Responses. Cancer Cell 2022, 40, 738–753.e5. [Google Scholar] [CrossRef] [PubMed]
- Intensivvårdade Fall av COVID-19 Under Tidsperioder Med Olika Dominerande Virusvarianter [Intensive Care Cases of COVID-19 During Periods with Different Dominant Virus Variants]; Specialavsnitt tillhörande vecka 6-7 2023; Swedish Public Health Agency: Solna, Sweden, 2023; pp. 1–9.
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Euro Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.T.; Varnaitė, R.; Christ, W.; Chen, P.; Muvva, J.R.; Maleki, K.T.; García, M.; Dzidic, M.; Folkesson, E.; Skagerberg, M.; et al. SARS-CoV-2-Specific Humoral and Cellular Immunity Persists through 9 Months Irrespective of COVID-19 Severity at Hospitalisation. Clin. Transl. Immunol. 2021, 10, e1306. [Google Scholar] [CrossRef]
- Stadler, E.; Burgess, M.T.; Schlub, T.E.; Khan, S.R.; Chai, K.L.; McQuilten, Z.K.; Wood, E.M.; Polizzotto, M.N.; Kent, S.J.; Cromer, D.; et al. Monoclonal Antibody Levels and Protection from COVID-19. Nat. Commun. 2023, 14, 4545. [Google Scholar] [CrossRef]
- Huygens, S.; Preijers, T.; Swaneveld, F.H.; Kleine Budde, I.; GeurtsvanKessel, C.H.; Koch, B.C.P.; Rijnders, B.J.A. Dosing of Convalescent Plasma and Hyperimmune Anti-SARS-CoV-2 Immunoglobulins: A Phase I/II Dose-Finding Study. Clin. Pharmacokinet. 2024, 63, 497–509. [Google Scholar] [CrossRef]
- Plasencia-Rodríguez, C.; Martínez-Feito, A.; Hernández, M.; Del Pino-Molina, L.; Novella-Navarro, M.; Serrano, Y.; González-Muñoz, M.; Peiteado, D.; Bonilla, G.; Monjo, I.; et al. Immune Response after SARS-CoV-2 Vaccination in Patients with Inflammatory Immune-Mediated Diseases Receiving Immunosuppressive Treatment. Allergy Asthma Clin. Immunol. 2023, 19, 71. [Google Scholar] [CrossRef]
- Scharf, L.; Axelsson, H.; Emmanouilidi, A.; Mathew, N.R.; Sheward, D.J.; Leach, S.; Isakson, P.; Smirnov, I.V.; Marklund, E.; Miron, N.; et al. Longitudinal Single-Cell Analysis of SARS-CoV-2–Reactive B Cells Uncovers Persistence of Early-Formed, Antigen-Specific Clones. JCI Insight 2023, 8, e165299. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef]
- Saade, C.; Bruel, T.; Vrignaud, L.-L.; Killian, M.; Drouillard, A.; Barateau, V.; Espi, M.; Mariano, N.; Mignon, C.; Bruyère, L.; et al. BA.1 Breakthrough Infection Elicits Distinct Antibody and Memory B Cell Responses in Vaccinated-Only versus Hybrid Immunity Individuals. iScience 2025, 28, 111962. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, J.-W.; Kim, J.Y.; Kang, S.-W.; Jang, C.-Y.; Chang, E.; Yang, J.-S.; Kim, K.-C.; Jang, H.-C.; Kim, D.S.; et al. The Association Between Antibody Responses and Prolonged Viable Severe Acute Respiratory Syndrome Coronavirus 2 Shedding in Immunocompromised Patients: A Prospective Cohort Study. J. Infect. Dis. 2024, 229, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Vergouwe, M.; Biemond, J.J.; van der Straten, K.; van Pul, L.; Kerster, G.; Claireaux, M.; Burger, J.A.; van Dort, K.A.; Kootstra, N.A.; Jonges, M.; et al. A Robust Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Specific T- and B-Cell Response Is Associated With Early Viral Clearance in SARS-CoV-2 Omicron-Infected Immunocompromised Individuals. J. Infect. Dis. 2024, 230, e860–e871. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Di Lauria, N.; Maggi, L.; Salvati, L.; Vanni, A.; Capone, M.; Lamacchia, G.; Mantengoli, E.; Spinicci, M.; Zammarchi, L.; et al. First-Dose mRNA Vaccination Is Sufficient to Reactivate Immunological Memory to SARS-CoV-2 in Subjects Who Have Recovered from COVID-19. J. Clin. Investig. 2021, 131, e149150. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Favà, A.; Donadeu, L.; Jouve, T.; Gonzalez-Costello, J.; Lladó, L.; Santana, C.; Toapanta, N.; Lopez, M.; Pernin, V.; Facundo, C.; et al. A Comprehensive Assessment of Long-Term SARS-CoV-2–Specific Adaptive Immune Memory in Convalescent COVID-19 Solid Organ Transplant Recipients. Kidney Int. 2022, 101, 1027–1038. [Google Scholar] [CrossRef]
- Kim, P.S.; Dimcheff, D.E.; Siler, A.; Schildhouse, R.J.; Chensue, S.W. Effect of Monoclonal Antibody Therapy on the Endogenous SARS-CoV-2 Antibody Response. Clin. Immunol. 2022, 236, 108959. [Google Scholar] [CrossRef]
- Zhang, L.; Poorbaugh, J.; Dougan, M.; Chen, P.; Gottlieb, R.L.; Huhn, G.; Beasley, S.; Daniels, M.; Ngoc Vy Trinh, T.; Crisp, M.; et al. Endogenous Antibody Responses to SARS-CoV-2 in Patients With Mild or Moderate COVID-19 Who Received Bamlanivimab Alone or Bamlanivimab and Etesevimab Together. Front. Immunol. 2021, 12, 790469. [Google Scholar] [CrossRef]
- Isa, F.; Gonzalez Ortiz, A.M.; Meyer, J.; Hamilton, J.D.; Olenchock, B.A.; Brackin, T.; Ganguly, S.; Forleo-Neto, E.; Faria, L.; Heirman, I.; et al. Effect of Timing of Casirivimab and Imdevimab Administration Relative to mRNA-1273 COVID-19 Vaccination on Vaccine-Induced SARS-CoV-2 Neutralising Antibody Responses: A Prospective, Open-Label, Phase 2, Randomised Controlled Trial. Lancet Infect. Dis. 2025, 25, 52–67. [Google Scholar] [CrossRef]
- Fendler, A.; Au, L.; Shepherd, S.T.C.; Byrne, F.; Cerrone, M.; Boos, L.A.; Rzeniewicz, K.; Gordon, W.; Shum, B.; Gerard, C.L.; et al. Functional Antibody and T Cell Immunity Following SARS-CoV-2 Infection, Including by Variants of Concern, in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-Specific T Cells Cross-Recognize the Omicron Variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, H.M.; Blixt, L.; Wullimann, D.; Wu, J.; Gao, Y.; Healy, K.; Muschiol, S.; Bogdanovic, G.; Åberg, M.; Kjellander, C.; et al. Systemic and Mucosal Adaptive Immunity to SARS-CoV-2 during the Omicron Wave in Patients with Chronic Lymphocytic Leukemia. Haematologica 2023, 109, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jawad, S.; Baù, L.; Alaguthurai, T.; del Molino del Barrio, I.; Laing, A.G.; Hayday, T.S.; Monin, L.; Muñoz-Ruiz, M.; McDonald, L.; Francos Quijorna, I.; et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell 2021, 39, 257–275.e6. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; Surie, D.; Chappell, J.D.; Zhu, Y.; Martin, E.T.; Kwon, J.H.; Frosch, A.E.; Mohamed, A.; Gilbert, J.; Bendall, E.E.; et al. SARS-CoV-2 Shedding and Evolution in Patients Who Were Immunocompromised during the Omicron Period: A Multicentre, Prospective Analysis. Lancet Microbe 2024, 5, e235–e246. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-Specific T Cell Memory Is Sustained in COVID-19 Convalescent Patients for 10 Months with Successful Development of Stem Cell-like Memory T Cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]



| ICC (n = 4) | ImCo (n = 19) | |
|---|---|---|
| Age (years), median (range) | 65.5 (49–77) | 62 (35–78) |
| Females, n (%) | 2 (50) | 12 (63) |
| COVID-19 vaccination before SARS-CoV-2 infection, n (%) | 1 (25) | 9 (47) |
| Vaccination before SARS-CoV-2 infection, number of doses, median (range) | 1 (1) | 2 (1–3) |
| Days from vaccination (last dose) until symptom debut, median (range) | 1 (1) | 19 (1–103) |
| Patients who received Pfizer-BioNTech™ COVID-19 adenovirus-vectored Vaccine n (%) | N/A | 9 (47) |
| Patients who received Vaxzevria™ (Oxford-AstraZeneca) COVID-19 mRNA Vaccine n (%) | 1 (25) | N/A |
| Symptom onset to diagnostic PCR sampling, d, median (range) | 3 (2–7) | 6 (0–17) |
| Heart transplant n (%) | N/A | 2 (11) |
| Kidney transplant n (%) | N/A | 5 (26) |
| Multiple sclerosis n (%) | N/A | 1 (5) |
| Rheumatoid arthritis n (%) | N/A | 3 (16) |
| Kidney failure with nephrosclerosis n (%) | N/A | 1 (5) |
| Granulomatosis with polyangiitis n (%) | N/A | 2 (11) |
| Myeloma n (%) | N/A | 2 (11) |
| Marginal zone lymphoma n (%) | N/A | 1 (5) |
| Myelodysplastic syndrome with multilineage dysplasia n (%) | N/A | 1 (5) |
| Chronic lymphocytic leukemia n (%) | N/A | 2 (11) |
| Rituximab, n (%) | N/A | 7 (37) |
| Days since last RTX dose at symptom debut, median (range) | N/A | 69 (4–134) |
| TNF-α inhibitor n (%) | N/A | 1 (5) |
| Daratumumab n (%) | N/A | 2 (11) |
| Tacrolimus n (%) | N/A | 7 (37) |
| Everolimus n (%) | N/A | 1 (5) |
| Steroids n (%) | N/A | 9 (11) |
| Steroids (mg/day) median (range) | N/A | 5 (3–10) |
| Mycophenol or methotrexate n (%) | N/A | 8 (42) |
| Small molecules n (%) | N/A | 3 (16) |
| Other immunosuppressants n (%) | N/A | 1 (5) |
| Years since hemopathy diagnosis at symptom debut, median (range) | N/A | 7 (5–25) |
| Years since solid organ transplantation at symptom debut | N/A | 5 (<1–16) |
| Days since last RTX dose at symptom debut, median (range) | N/A | 69 (4–134) |
| ICC (n = 4) | ImCo (n = 19) | |
|---|---|---|
| Day of sampling <30 days after symptom debut Median (range) | 19 (15–27) | 15 (4–22) |
| Day of sampling >90 days after symptom debut Median (range) | 169 (158–176) | 182 (99–265) |
| Days of hospitalization Median (range) | 13 (10–22) | 10 (1–38) |
| Patients in ICU n (%) | 2 (50) | 2 (11) |
| Mild disease with need of assistance n (%) | N/A | 3 (16) |
| Moderate disease; no oxygen therapy n (%) | N/A | 10 (53) |
| Moderate disease; oxygen by mask n (%) | 3 (75) | 6 (32) |
| Severe disease; oxygen by non-invasive ventilation n (%) | 1 (25) | N/A |
| Diabetes mellitus n (%) | 2 (50) | 7 (37) |
| Hypertonia n (%) | 4 (100) | 14 (74) |
| Heart failure n (%) | N/A | 3 (16) |
| Lung disease (asthma, chronic bronchitis, chronic obstructive pulmonary disease) n (%) | N/A | 8 (42) |
| Kidney insufficiency n (%) | N/A | 10 (53) |
| Other * | 1 (25) | 7 (37) |
| BMI >30 n (%) | 2 (50) | 5 (26) |
| Smoker n (%) | N/A | 4 (21) |
| Positive virus culture from patient airway material n (%) | 2 (50) | 8 (42) |
| of positive samples in culture, positive for CPE n (%) | 0 | 2 (11) |
| Monoclonal antibody (mAb) therapy n (%) | 1 (25) | 8 (42) |
| mAb therapy received how many days before <30 day sampling median (range) | 8 (8) | 3 (0–8) |
| mAb therapy received how many days before >90 day sampling median (range) | 167 (167) | 162 (89–177) |
| Convalescent plasma (cPlasma) treatment n (%) | 0 | 8 (42) |
| cPlasma therapy received how many days before <30 day sampling median (range) | N/A | 4 (0–10) |
| cPlasma therapy received how many days before >90 day sampling median (range) | N/A | 180 (126–229) |
| Remdesivir treatment n (%) | 1 (25) | 11 (58) |
| Remdesivir received how many days before <30 day sampling median (range) | 6 (6) | 6 (3–24) |
| Remdesivir received how many days before >90 day sampling median (range) | 168 (168) | 181 (126–245) |
| Corticosteroids treatment n (%) | 4 (100) | 13 (68) |
| Corticosteroids received how many days before <30 day sampling median (range) | 9 (8–17) | 12 (4–37) |
| Corticosteroids received how many days before >90 day sampling median (range) | 160 (145–168) | 180 (89–245) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayola Danés, N.; Brownlie, D.; Folkman, R.; Nordlander, A.; Blom, K.; Varnaite, R.; Niessl, J.; Karlsson Lindsjö, O.; Söderholm, S.; Akber, M.; et al. Dysregulated Adaptive Immune Responses to SARS-CoV-2 in Immunocompromised Individuals. Microorganisms 2025, 13, 1077. https://doi.org/10.3390/microorganisms13051077
Mayola Danés N, Brownlie D, Folkman R, Nordlander A, Blom K, Varnaite R, Niessl J, Karlsson Lindsjö O, Söderholm S, Akber M, et al. Dysregulated Adaptive Immune Responses to SARS-CoV-2 in Immunocompromised Individuals. Microorganisms. 2025; 13(5):1077. https://doi.org/10.3390/microorganisms13051077
Chicago/Turabian StyleMayola Danés, Núria, Demi Brownlie, Rebecca Folkman, Anna Nordlander, Kim Blom, Renata Varnaite, Julia Niessl, Oskar Karlsson Lindsjö, Sandra Söderholm, Mira Akber, and et al. 2025. "Dysregulated Adaptive Immune Responses to SARS-CoV-2 in Immunocompromised Individuals" Microorganisms 13, no. 5: 1077. https://doi.org/10.3390/microorganisms13051077
APA StyleMayola Danés, N., Brownlie, D., Folkman, R., Nordlander, A., Blom, K., Varnaite, R., Niessl, J., Karlsson Lindsjö, O., Söderholm, S., Akber, M., Chen, P., Buggert, M., Bråve, A., Klingström, J., Nowak, P., Marquardt, N., Sondén, K., Blennow, O., & Gredmark-Russ, S. (2025). Dysregulated Adaptive Immune Responses to SARS-CoV-2 in Immunocompromised Individuals. Microorganisms, 13(5), 1077. https://doi.org/10.3390/microorganisms13051077







