Comparative Effectiveness of Antiviral Agents and Monoclonal Antibodies for Early SARS-CoV-2 Therapy in Immunocompromised Patients: A Multicenter Retrospective Cohort Study (March 2021–March 2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Early Anti-SARS-CoV-2 Therapies
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Potential Risk Factors Associated with Positive SARS-CoV-2 NPS on D7 and D30, COVID-19 Hospitalization, and Death
3.3. Last Administration of Immunosuppressive Drug as Potential Predictor of Positive SARS-CoV-2 NPS on Days 7 and 30
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Jassat, W. Decreased severity of disease during the first global omicron variant COVID-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Patel, M.M.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; McNeal, T.; et al. Effectiveness of a Third Dose of Pfizer-BioNTech and Moderna Vaccines in Preventing COVID-19 Hospitalization Among Immunocompetent and Immunocompromised Adults—United States, August–December 2021. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Malahe, S.R.K.; Hoek, R.A.S.; Dalm, V.A.S.H.; Broers, A.E.C.; den Hoed, C.M.; Manintveld, O.C.; Baan, C.C.; van Deuzen, C.M.; Papageorgiou, G.; Bax, H.I.; et al. Clinical Characteristics and Outcomes of Immunocompromised Patients with Coronavirus Disease 2019 Caused by the Omicron Variant: A Prospective, Observational Study. Clin. Infect. Dis. 2022, 76, e172–e178. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M.L.; Lapham, J. Prevalence of Immunosuppression Among US Adults. JAMA 2024, 331, 880. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, V.; Rotundo, S.; Marascio, N.; De Marco, C.; Lionello, R.; Veneziano, C.; Berardelli, L.; Quirino, A.; Olivadese, V.; Serapide, F.; et al. Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: A retrospective study. BMC Infect. Dis. 2022, 22, 793. [Google Scholar] [CrossRef]
- D’Abramo, A.; Vita, S.; Beccacece, A.; Navarra, A.; Pisapia, R.; Fusco, F.M.; Matusali, G.; Girardi, E.; Maggi, F.; Goletti, D.; et al. B-cell-depleted patients with persistent SARS-CoV-2 infection: Combination therapy or monotherapy? A real-world experience. Front. Med. 2024, 11, 1344267. [Google Scholar] [CrossRef] [PubMed]
- D’Abramo, A.; Vita, S.; Nicastri, E. The unmet need for COVID-19 treatment in immunocompromised patients. BMC Infect. Dis. 2022, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Vita, S.; Giombini, E.; De Marco, P.; Rueca, M.; Gruber, C.E.M.; Beccacece, A.; Scorzolini, L.; Mazzotta, V.; Pinnetti, C.; Caputi, P.; et al. Antivirals and monoclonal antibody combination therapy in haematological patients in the omicron era. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024043. [Google Scholar] [CrossRef] [PubMed]
- Orth, H.M.; Flasshove, C.; Berger, M.; Hattenhauer, T.; Biederbick, K.D.; Mispelbaum, R.; Klein, U.; Stemler, J.; Fisahn, M.; Doleschall, A.D.; et al. Early combination therapy of COVID-19 in high-risk patients. Infection 2023, 52, 877–889. [Google Scholar] [CrossRef]
- ESCMID Consensus Document for Management of COVID-19 in Immunocompromised Patients. Available online: https://www.escmid.org/guidelines-journals/guidelines/guidelines-in-development/escmid-consensus-document-for-management-of-covid-19-in-immunocompromised-patients/ (accessed on 25 February 2025).
- COVID-19: Guidance for People Whose Immune System Means They Are at Higher Risk. 2004. Available online: https://www.gov.uk/government/publications/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk (accessed on 25 February 2025).
- Determina AIFA Nella GU N.142 Del 16.06.2021. Available online: https://www.gazzettaufficiale.it/eli/gu/2021/06/16/142/sg/pdf (accessed on 18 January 2025).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A.G. Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Singson, J.R.C.; Kirley, P.D.; Pham, H.; Rothrock, G.; Armistead, I.; Meek, J.; Anderson, E.J.; Reeg, L.; Lynfield, R.; Ropp, S.; et al. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19—COVID-NET, 10 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.O.; Brennan-Rieder, D.; Ngo, B.; Kory, P.; Confalonieri, M.; Shapiro, L.; Iglesias, J.; Dube, M.; Nanda, N.; In, G.K.; et al. The Importance of Understanding the Stages of COVID-19 in Treatment and Trials. Aids Rev. 2021, 23, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.J.; Kang, J.; Kang, H.K.; Park, S.H.; Koo, H.-K.; Park, H.K.; Lee, S.-S.; Song, J.E.; Kwak, Y.G.; Kang, J. Impact of prior vaccination on clinical outcomes of patients with COVID-19. Emerg. Microbes Infect. 2022, 11, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Del Borgo, C.; Garattini, S.; Bortignon, C.; Carraro, A.; Di Trento, D.; Gasperin, A.; Grimaldi, A.; De Maria, S.G.; Corazza, S.; Tieghi, T.; et al. Effectiveness Tolerability Prescribing Choice of Antiviral Molecules Molnupiravir Remdesivir Nirmatrelvir/r: AReal-World Comparison in the First Ten Months of Use. Viruses 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.; Brengel-Pesce, K.; Gaymard, A.; Trabaud, M.-A.; Destras, G.; Oriol, G.; Cheynet, V.; Debombourg, M.; Mokdad, B.; Billaud, G.; et al. Dynamics of viral shedding during ancestral or Omicron BA.1 SARS-CoV-2 infection and enhancement of pre-existing immunity during breakthrough infections. Emerg. Microbes Infect. 2022, 11, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; Surie, D.; Chappell, J.D.; Zhu, Y.; Martin, E.T.; Kwon, J.H.; Frosch, A.E.; Mohamed, A.; Gilbert, J.; Bendall, E.E.; et al. SARS-CoV-2 shedding and evolution in patients who were immunocompromised during the omicron period: A multicentre, prospective analysis. Lancet Microbe 2024, 5, e235–e246. [Google Scholar] [CrossRef] [PubMed]

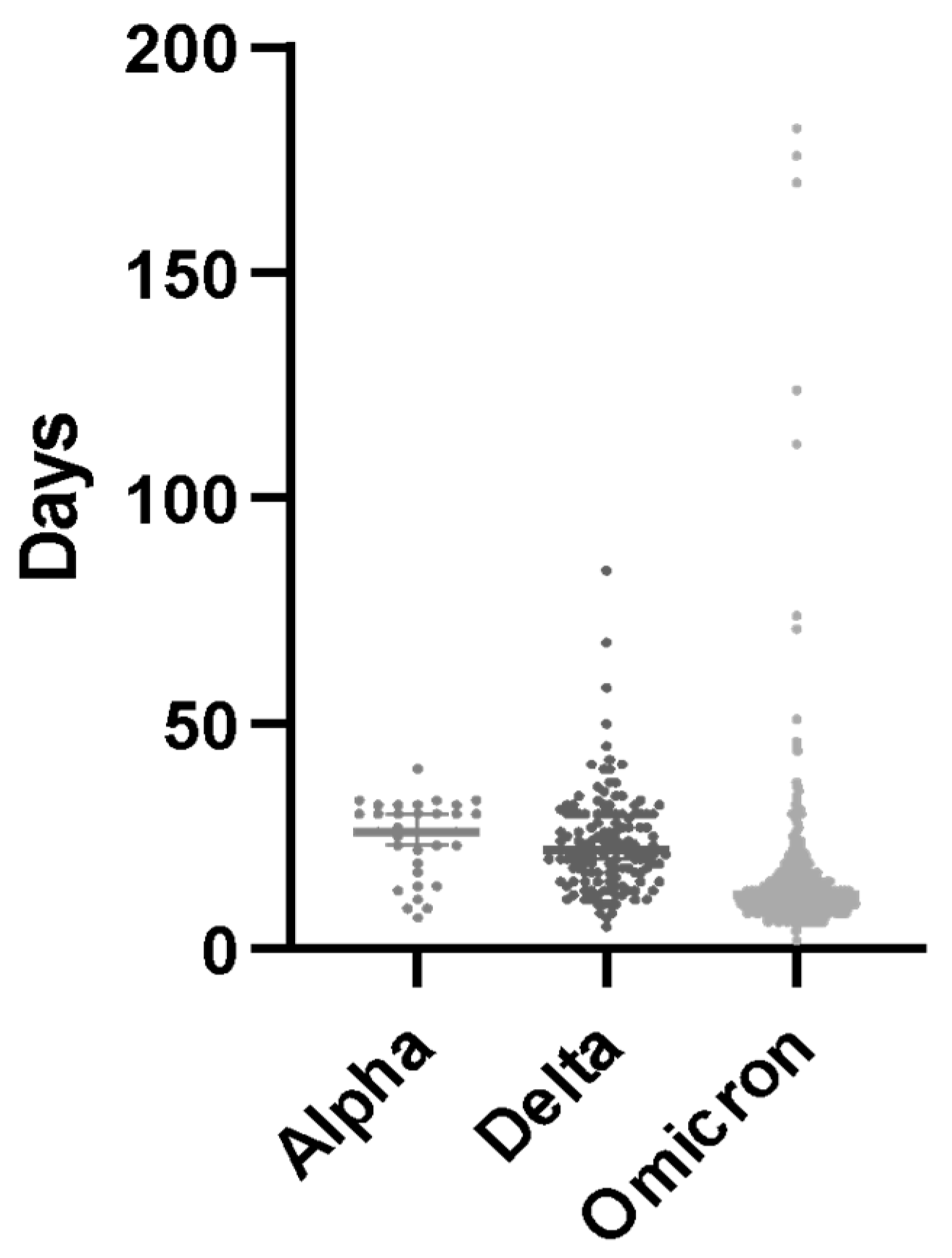
| Whole (n° 1472) | MoAbs (n° 688) | AVAs (n° 783) | p-Value | |
| Age, median (IQR) | 58 (48–69) | 57 (46–67) | 61 (51–72) | p < 0.01 |
| Variant of concern, no. (%) | alpha, 76 (5%) delta, 283 (19%) omicron, 1113 (75%) | alpha, 70 (10%) delta, 282 (41%) omicron, 336 (49%) | alpha, 6 (1%) delta, 1 (0.1%) omicron, 776 (99%) | |
| Treatment, no. (%) | 1472 | 688 (46%) | 783 (54%) | |
| Bamlanivimab 161 (23%) | Remdesevir 249 (31%) | |||
| Bamlanivimab/Etesevimab 65 (9%) | Nirmatrelvir/r 271 (34%) | |||
| Casirivimab/imdevimab 131 (19%) | Molnupiravir 263 (33%) | |||
| Sotrovimab 331 (48%) | ||||
| Male (% whole population) | 665 (45%) | 301 (44%) | 364 (47%) | n.s. |
| BMI (IQR) | 24.83 (22.49–27.75) | 24.30 (22.36–27.41) | 25.39 (22.54–27.82) | <0.01 |
| TTI, median days (IQR) | 3 (2–4) | 4 (2–5) | 3 (2–4) | <0.001 |
| TID median days (IQR) | 7 (7–9) | 7 (7–29) | 7 (7–7) | <0.001 |
| Cause of IC status | ||||
| Hematological disorders, no. (%) | 394 (26.7%) | 161 (23.4%) | 233 (29.7%) | <0.01 |
| Oncological disorders, no. (%) | 561 (38.1%) | 223 (32.4%) | 338 (43.2%) | <0.01 |
| Primary or secondary immunodeficiency, no. (%) | 517 (35%) | 304 (44.1%) | 212 (27%) | <0.01 |
| Comorbidities | ||||
| Diabetes, no. (%) | 124 (8.4%) | 56 (8.1%) | 68 (8.6%) | n.s. |
| Obesity, no. (%) | 158 (10.7%) | 65 (9.4%) | 93 (11.8%) | <0.01 |
| Hypertension, no. (%) | 154 (10.5%) | 90 (13%) | 64 (8.1%) | <0.01 |
| Chronic lung disorders, no. (%) | 188 (12.8%) | 80 (11.6%) | 108 (13.7%) | n.s. |
| Cardiovascular disorders, no. (%) | 402 (27.3%) | 144 (20.9%) | 258 (32.9%) | <0.01 |
| Cerebrovascular disorders, no. (%) | 342 (23.2%) | 113 (16.4%) | 229 (29.2%) | <0.01 |
| Renal failure, no. (%) | 103 (7%) | 65 (9.4%) | 38 (4.8%) | <0.01 |
| MASS, median | 3 (3-3) | 3 (3-4) | 3 (3-3) | n.s. |
| COVID-19 vaccination | ||||
| None, no. (%) | 149 (10.1%) | 105 (15.2%) | 43 (5.4%) | <0.05 |
| I dose, no. (%) | 39 (2.6%) | 29 (4.2%) | 10 (1.2%) | <0.05 |
| II doses, no. (%) | 298 (22.2%) | 236 (34%) | 61 (7.7%) | <0.05 |
| III doses or more, no. (%) | 886 (60.1%) | 266 (38.6%) | 599 (76.5%) | <0.05 |
| Clinical outcome | ||||
| TNT, median days (IQR) | 13 (10–19) | 17 (12–26) | 11 (9–14) | <0.05 |
| Admission to hospital, no. (%) | 31 (2.1%) | 20 (2.9%) | 11 (1.6%) | <0.05 |
| Death, n° (%) | 5 (0.34%) | 4 (0.58%) | 1 (0.15%) (1) | <0.05 |
| Clinical Features | MoAbs | AVAs |
| Positive NPS on D7 OR (95% CI), p-value | 3 (1.72–5.23), p <0.01 | 0.33 (0.19–0.58), p < 0.01 |
| Positive NPS on D30 OR (95% CI), p-value | 6 (3.7–10.5), p < 0.01 | 0.16 (0.09–0.27), p < 0.01 |
| Hospital admission OR (95% CI), p-value | 1.78 (0.98–3.23), p = 0.07 | 0.56 (0.3–1.02), p = 0.07 |
| All-cause death for all ORs (95% CI), p-value | 1.1 (0.41–2.95), p > 0.05 | 0.96 (0.34–2.42), p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vita, S.; Maffongelli, G.; Bartoli, T.A.; Benvenuto, D.; Marocco, R.; Rosati, S.; Mazzotta, V.; Del Borgo, C.; Mastrorosa, I.; De Marco, P.; et al. Comparative Effectiveness of Antiviral Agents and Monoclonal Antibodies for Early SARS-CoV-2 Therapy in Immunocompromised Patients: A Multicenter Retrospective Cohort Study (March 2021–March 2022). Microorganisms 2025, 13, 1076. https://doi.org/10.3390/microorganisms13051076
Vita S, Maffongelli G, Bartoli TA, Benvenuto D, Marocco R, Rosati S, Mazzotta V, Del Borgo C, Mastrorosa I, De Marco P, et al. Comparative Effectiveness of Antiviral Agents and Monoclonal Antibodies for Early SARS-CoV-2 Therapy in Immunocompromised Patients: A Multicenter Retrospective Cohort Study (March 2021–March 2022). Microorganisms. 2025; 13(5):1076. https://doi.org/10.3390/microorganisms13051076
Chicago/Turabian StyleVita, Serena, Gaetano Maffongelli, Tommaso Ascoli Bartoli, Domenico Benvenuto, Raffaella Marocco, Silvia Rosati, Valentina Mazzotta, Cosmo Del Borgo, Ilaria Mastrorosa, Patrizia De Marco, and et al. 2025. "Comparative Effectiveness of Antiviral Agents and Monoclonal Antibodies for Early SARS-CoV-2 Therapy in Immunocompromised Patients: A Multicenter Retrospective Cohort Study (March 2021–March 2022)" Microorganisms 13, no. 5: 1076. https://doi.org/10.3390/microorganisms13051076
APA StyleVita, S., Maffongelli, G., Bartoli, T. A., Benvenuto, D., Marocco, R., Rosati, S., Mazzotta, V., Del Borgo, C., Mastrorosa, I., De Marco, P., D’Abramo, A., Maggi, F., Antinori, A., Lichtner, M., Nicastri, E., & Group, C. (2025). Comparative Effectiveness of Antiviral Agents and Monoclonal Antibodies for Early SARS-CoV-2 Therapy in Immunocompromised Patients: A Multicenter Retrospective Cohort Study (March 2021–March 2022). Microorganisms, 13(5), 1076. https://doi.org/10.3390/microorganisms13051076










