The Relationship Between Gut Microbiota and Recurrent Spontaneous Abortion
Abstract
1. Gut Microbiota Composition and Function
2. Definition of Recurrent Spontaneous Abortion and Possible Mechanisms
3. Differences in Gut Microbiota and Metabolites Between Recurrent Miscarriages and Normal Pregnancies
4. Possible Mechanisms of Influence of Intestinal Microbiota on Recurrent Miscarriages
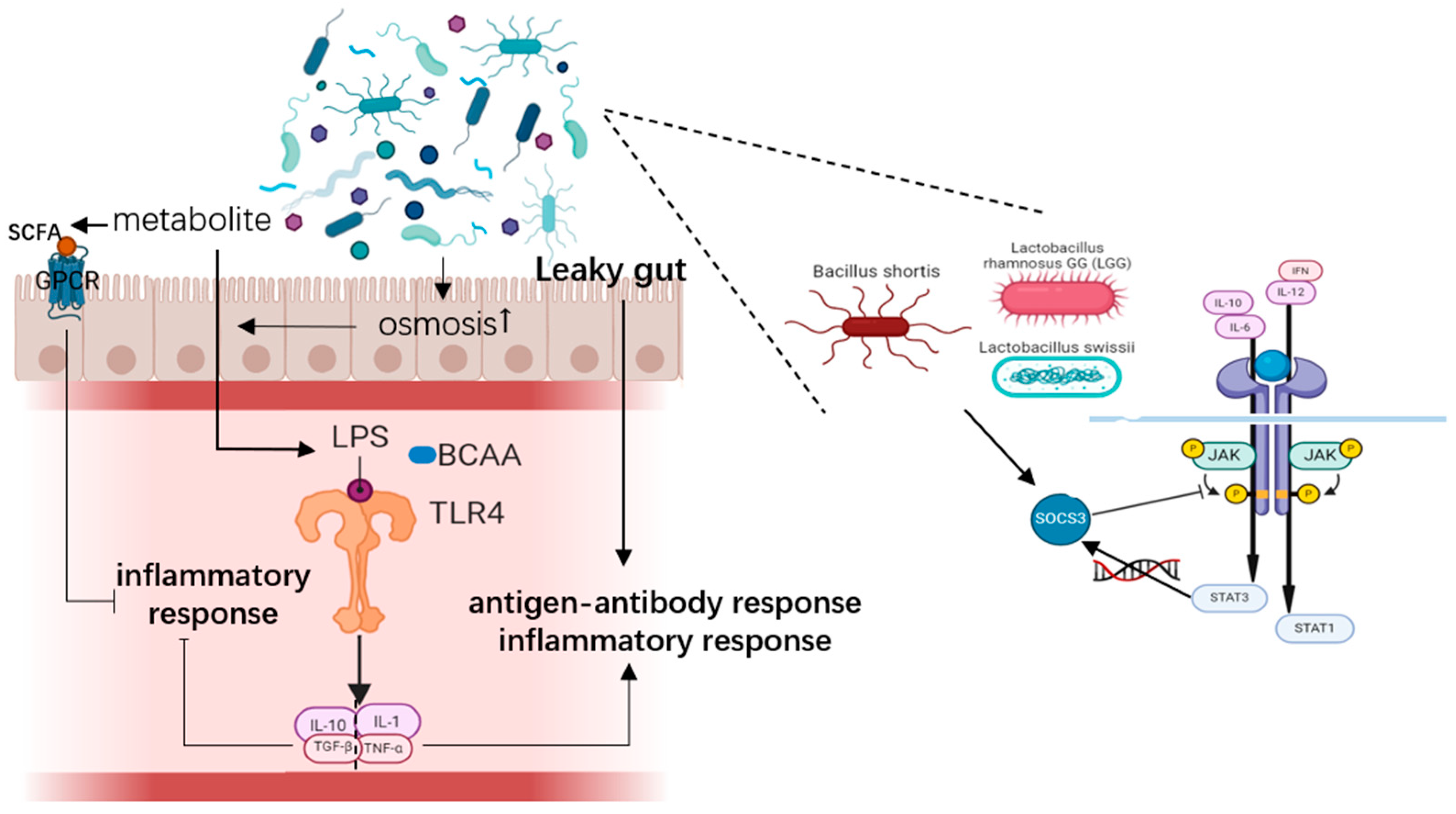
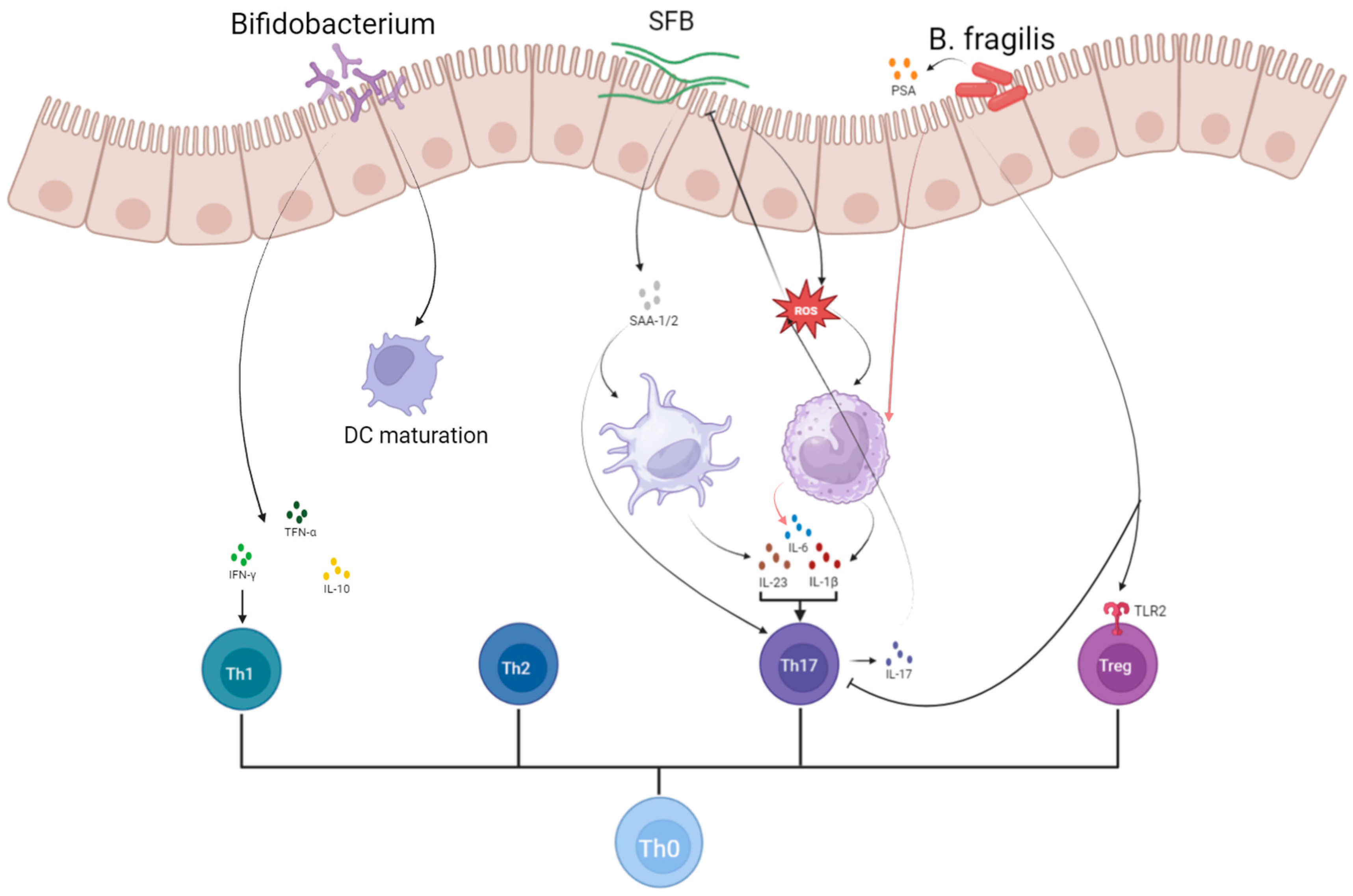
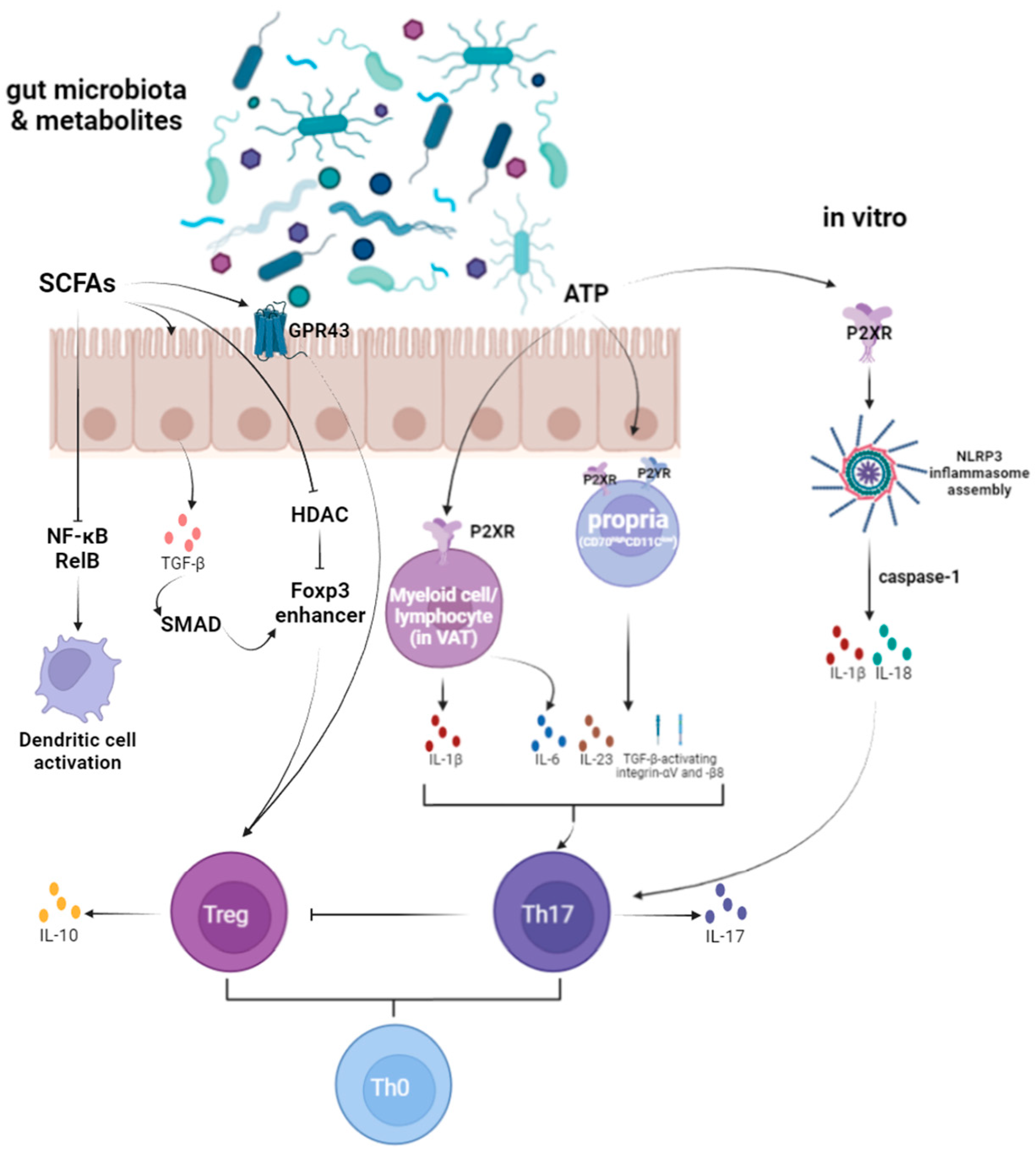
4.1. Immune Response
4.1.1. Inflammatory Response
4.1.2. T Cell Homeostasis
4.1.3. Macrophages Polarization and Trophocyte Invasion
4.1.4. Antigen Presenting Cells (APCs) System Response
4.1.5. Autoimmune
4.2. Damage to the Maternal–Fetal Interface
5. Potential Treatment Strategies
5.1. Treatment with Probiotics
5.2. High-Fibre Diet Therapy
5.3. Fecal Microbial Transplantation Treatment
5.4. Lipid Emulsion Treatments
5.5. Traditional Chinese Medicine (TCM) Treatments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RSA | Recurrent Spontaneous Abortion |
| IL | Interleukin |
| IFN-γ | Interferon Gamma |
| TNF-α | Tumor Necrosis Factor Alpha |
| SCFA | Short-Chain Fatty Acids |
| TCM | Traditional Chinese Medicine |
| NK | Natural Killer Cells |
| Th | T Helper Cells |
| Treg | Regulatory T Cells |
| Breg | Regulatory B Cells |
| DC | Dendritic Cells |
| APC | Antigen-Presenting Cells |
| LPS | Lipopolysaccharide |
| PRR | Pattern Recognition Receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear Factor Kappa B |
| TLR | Toll-Like Receptor |
| SOCS | Suppressor of Cytokine Signaling |
| ATP | Adenosine Triphosphate |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| FMT | Fecal Microbial Transplantation |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-Oxide |
| FMO3 | Flavin Monooxygenase 3 |
| HDAC | Histone Deacetylase |
| BCAA | Branched-Chain Amino Acids |
| DD | D-Dimer |
| APTT | Activated Partial Prothrombin Time |
| PT | Prothrombin Time |
| FIB | Fibrinogen |
| TEG | Thromboelastogram |
| MA | Maximum Thrombotic Amplitude |
| PAI-1 | Plasminogen Activator Inhibitor 1 |
| T-PA | Tissue Plasminogen Activator |
| DCA | Deoxycholic Acid |
| FFAR2 | Free Fatty Acid Receptor 2 |
| GPCR | G Protein-Coupled Receptors |
| TPH1 | Tryptophan Hydroxylase 1 |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three Letter Acronym |
| LD | Linear Dichroism |
| URSA | Unexplained Recurrent Spontaneous Abortion |
References
- Wang, S.; Chen, L.; Feng, X. Research progress on the correlation between gut microbiota and recurrent spontaneous abortion of kidney deficiency and blood stasis type. Shaanxi J. Tradit. Chin. Med. 2022, 43, 261–264+268. [Google Scholar]
- Yang, X.; Zhang, Y.; Liu, Y.; Zhao, R. Exploring the treatment of female reproductive disorders from the perspective of spleen and stomach theory based on gut microbiota. Chin. J. Integr. Tradit. West. Med. 2023, 43, 345–350. [Google Scholar]
- Li, Z.; Zheng, Y.; Zhang, M.; Wu, K.; Zhang, L.; Yao, Y.; Zheng, C. Gut microbiota-derived metabolites associate with circulating immune cell subsets in unexplained recurrent spontaneous abortion. Heliyon 2024, 10, e24571. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Qian, X.; Chen, Q. Research progress on the correlation between gut microbiota and pregnancy complications. Int. J. Reprod. Health/Fam. Plan. 2023, 42, 409–413. [Google Scholar]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Chen, P.; Chen, X.; Pan, L.; Han, L.; Zhu, T. Alteration of the Gut Microbiota in Missed Abortion. Indian J. Microbiol. 2023, 63, 106–119. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Kwok, L.-Y.; Sun, Z. Gut microbiome-targeted therapies for Alzheimer’s disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Kuziel, G.A.; Rakoff-Nahoum, S. The gut microbiome. Curr. Biol. 2022, 32, R257–R264. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, F. Microbial transmission, colonisation and succession: From pregnancy to infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Jabbar, K.S.; Ruohtula, T.; Honkanen, J.; Avila-Pacheco, J.; Siljander, H.; Stražar, M.; Oikarinen, S.; Hyöty, H.; Ilonen, J.; et al. Mobile genetic elements from the maternal microbiome shape infant gut microbial assembly and metabolism. Cell 2022, 185, 4921–4936.e15. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal gut microbiota in the health of mothers and offspring: From the perspective of immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef]
- Granito, A.; Zauli, D.; Muratori, P.; Muratori, L.; Grassi, A.; Bortolotti, R.; Petrolini, N.; Veronesi, L.; Gionchetti, P.; Bianchi, F.B.; et al. Anti-Saccharomyces cerevisiae and perinuclear anti-neutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment. Pharmacol. Ther. 2005, 21, 881–887. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Muratori, P.; Guidi, M.; Lenzi, M.; Bianchi, F.B.; Volta, U. Anti-saccharomyces cerevisiae antibodies (ASCA) in coeliac disease. Gut 2006, 55, 296. [Google Scholar]
- Granito, A.; Muratori, P.; Muratori, L. Editorial: Gut microbiota profile in patients with autoimmune hepatitis—A clue for adjunctive probiotic therapy? Aliment. Pharmacol. Ther. 2020, 52, 392–394. [Google Scholar] [CrossRef]
- Xia, M.; Zhu, M.; Huang, Y. Analysis of the correlation between endometrial microbiota, gut microbiota, and recurrent miscarriage. Chin. J. Matern. Child Health Res. 2021, 32, 1519–1523. [Google Scholar]
- Sun, Y.; Du, H.; Li, R.; Hai, J.; Feng, X.; Yu, C.; Wang, S. Guidelines for integrated traditional Chinese and Western medicine in the diagnosis and treatment of recurrent miscarriage. China J. Chin. Mater. Medica. 2024, 49, 2544–2556. [Google Scholar] [CrossRef]
- Guan, D.; Sun, W.; Gao, M.; Chen, Z.; Ma, X. Immunologic insights in recurrent spontaneous abortion: Molecular mechanisms and therapeutic interventions. Biomed. Pharmacother. 2024, 177, 117082. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Feng, L.; Zhang, J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zou, L.; Ye, Q.; Li, D.; Wu, H.; He, L. Gut Microbiota Composition and Functional Prediction in Recurrent Spontaneous Abortion. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Yao, H.; Chen, J.; Wang, Y.; Li, Y.; Jiang, Q. Assessing causal relationships between gut microbiota and abortion: Evidence from two sample Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1415730. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; He, R. Research Progress on Vaginal Microecology Based on Metagenomics. Chin. J. Microecol. 2018, 30, 114–120. [Google Scholar]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, K.K.-W.; Ko, E.Y.-L.; Chen, X.; Huang, J.; Tsui, S.K.-W.; Li, T.C.; Chim, S.S.-C. Systematic Comparison of Bacterial Colonization of Endometrial Tissue and Fluid Samples in Recurrent Miscarriage Patients: Implications for Future Endometrial Microbiome Studies. Clin. Chem. 2018, 64, 1743–1752. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Brogin Moreli, J.; Cirino Ruocco, A.M.; Vernini, J.M.; Rudge, M.V.C.; Calderon, I.M.P. Interleukin 10 and Tumor Necrosis Factor-Alpha in Pregnancy: Aspects of Interest in Clinical Obstetrics. ISRN Obstet. Gynecol. 2012, 2012, 230742. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989, 46, 111–147. [Google Scholar] [CrossRef]
- Agarwal, R.; Loganath, A.; Roy, A.C.; Wong, Y.C.; Ng, S.C. Effect of T-helper 1 cytokines on secretion of T-helper 2 cytokines by term trophoblast cells in culture. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2000, 14, 305–310. [Google Scholar] [CrossRef]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632–15649. [Google Scholar] [CrossRef]
- Tersigni, C.; D’Ippolito, S.; Di Nicuolo, F.; Marana, R.; Valenza, V.; Masciullo, V.; Scaldaferri, F.; Malatacca, F.; de Waure, C.; Gasbarrini, A.; et al. Recurrent pregnancy loss is associated to leaky gut: A novel pathogenic model of endometrium inflammation? J. Transl. Med. 2018, 16, 102. [Google Scholar] [CrossRef]
- Lehmann, S.; Hiller, J.; van Bergenhenegouwen, J.; Knippels, L.M.J.; Garssen, J.; Traidl-Hoffmann, C. In Vitro Evidence for Immune-Modulatory Properties of Non-Digestible Oligosaccharides: Direct Effect on Human Monocyte Derived Dendritic Cells. PLoS ONE 2015, 10, e0132304. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- La, X.; Wang, W.; Zhang, M.; Liang, L. Definition and Multiple Factors of Recurrent Spontaneous Abortion. In Environment and Female Reproductive Health; Zhang, H., Yan, J., Eds.; Springer: Singapore, 2021; pp. 231–257. ISBN 978-981-334-187-6. [Google Scholar]
- Sczesnak, A.; Segata, N.; Qin, X.; Gevers, D.; Petrosino, J.F.; Huttenhower, C.; Littman, D.R.; Ivanov, I.I. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host Microbe 2011, 10, 260–272. [Google Scholar] [CrossRef]
- Bradley, C.P.; Teng, F.; Felix, K.M.; Sano, T.; Naskar, D.; Block, K.E.; Huang, H.; Knox, K.S.; Littman, D.R.; Wu, H.-J.J. Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell Host Microbe 2017, 22, 697–704.e4. [Google Scholar] [CrossRef]
- Goto, Y.; Panea, C.; Nakato, G.; Cebula, A.; Lee, C.; Diez, M.G.; Laufer, T.M.; Ignatowicz, L.; Ivanov, I.I. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 2014, 40, 594–607. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Ravindran, R.; Loebbermann, J.; Nakaya, H.I.; Khan, N.; Ma, H.; Gama, L.; Machiah, D.K.; Lawson, B.; Hakimpour, P.; Wang, Y.-C.; et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 2016, 531, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Huang, W.; Hall, J.A.; Yang, Y.; Chen, A.; Gavzy, S.J.; Lee, J.-Y.; Ziel, J.W.; Miraldi, E.R.; Domingos, A.I.; et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2016, 164, 324. [Google Scholar] [CrossRef]
- Martinez, G.J. MINK1: The missing link between ROS and its inhibition of Th17 cells. J. Exp. Med. 2017, 214, 1205–1206. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Ngo, V.L.; Geem, D.; Harusato, A.; Hirota, S.A.; Parkos, C.A.; Lukacs, N.W.; Nusrat, A.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N.; et al. IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria. Mucosal Immunol. 2017, 10, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Stappers, M.H.T.; Janssen, N.A.F.; Oosting, M.; Plantinga, T.S.; Arvis, P.; Mouton, J.W.; Joosten, L.A.B.; Netea, M.G.; Gyssens, I.C. A role for TLR1, TLR2 and NOD2 in cytokine induction by Bacteroides fragilis. Cytokine 2012, 60, 861–869. [Google Scholar] [CrossRef]
- Chu, H.; Mazmanian, S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013, 14, 668–675. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Jiang, F.; Meng, D.; Weng, M.; Zhu, W.; Wu, W.; Kasper, D.; Walker, W.A. The symbiotic bacterial surface factor polysaccharide A on Bacteroides fragilis inhibits IL-1β-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLoS ONE 2017, 12, e0172738. [Google Scholar] [CrossRef]
- Tang, B.; Tang, L.; Li, S.; Liu, S.; He, J.; Li, P.; Wang, S.; Yang, M.; Zhang, L.; Lei, Y.; et al. Gut microbiota alters host bile acid metabolism to contribute to intrahepatic cholestasis of pregnancy. Nat. Commun. 2023, 14, 1305. [Google Scholar] [CrossRef]
- Cheng, H.; Guan, X.; Chen, D.; Ma, W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms 2019, 7, 583. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Gut Microbiome and Female Health. Biology 2022, 11, 1683. [Google Scholar] [CrossRef]
- Tersigni, C.; Barbaro, G.; Castellani, R.; Onori, M.; Granieri, C.; Scambia, G.; Di Simone, N. Oral administration of Bifidobacterium longum ES1 reduces endometrial inflammation in women with recurrent pregnancy loss. Am. J. Reprod. Immunol. 2024, 91, e13804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, Y.; Yang, Y.; Ren, L.; Li, J.; Wang, Y.; Shen, X.; He, F. Maternal gestational Bifidobacterium bifidum TMC3115 treatment shapes construction of offspring gut microbiota and development of immune system and induces immune tolerance to food allergen. Front. Cell. Infect. Microbiol. 2022, 12, 1045109. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Matys, V.; Marra, S.; De Canditiis, D.; Olivero, F.; Carraro, C.; Giugliano, A.; Zicari, A.M.; Piccioni, M.G. Effect of Supplementation with a Specific Probiotic (Bifidobacterium bifidum PRL2010) in Pregnancy for the Prevention of Atopic Dermatitis in Children: Preliminary Results of a Randomized Trial. Nutrients 2025, 17, 673. [Google Scholar] [CrossRef] [PubMed]
- ATP Drives Lamina Propria TH17 Cell Differentiation. Available online: https://www.nature.com/articles/nature07240 (accessed on 27 March 2024).
- Pandolfi, J.B.; Ferraro, A.A.; Sananez, I.; Gancedo, M.C.; Baz, P.; Billordo, L.A.; Fainboim, L.; Arruvito, L. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J. Immunol. 2016, 196, 3287–3296. [Google Scholar] [CrossRef]
- Couillin, I.; Gombault, A.; Baron, L. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front. Immunol. 2013, 3, 414. [Google Scholar] [CrossRef]
- Pathogen-Induced Human TH17 Cells Produce IFN-γ or IL-10 and Are Regulated by IL-1β. Available online: https://www.nature.com/articles/nature10957 (accessed on 27 March 2024).
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Gut Microbes Promote Colonic Serotonin Production Through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. Available online: https://faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fj.14-259598 (accessed on 25 August 2024).
- Li, N.; Ghia, J.-E.; Wang, H.; McClemens, J.; Cote, F.; Suehiro, Y.; Mallet, J.; Khan, W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 2011, 178, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, P.M.; Monteiro, C.; Dias, A.S.O.; Kasahara, T.M.; Ferreira, T.B.; Hygino, J.; Wing, A.C.; Andrade, R.M.; Rueda, F.; Sales, M.C.; et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T-cell subsets in multiple sclerosis patients. Eur. J. Immunol. 2018, 48, 1376–1388. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Chen, X.; Song, Q.L.; Ji, R.; Wang, J.Y.; Cao, M.L.; Guo, D.Y.; Zhang, Y.; Yang, J. JPT2 Affects Trophoblast Functions and Macrophage Polarization and Metabolism, and Acts as a Potential Therapeutic Target for Recurrent Spontaneous Abortion. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024, 11, e2306359. [Google Scholar] [CrossRef]
- Fan, X.; Mai, C.; Zuo, L.; Huang, J.; Xie, C.; Jiang, Z.; Li, R.; Yao, X.; Fan, X.; Wu, Q.; et al. Herbal formula BaWeiBaiDuSan alleviates polymicrobial sepsis-induced liver injury via increasing the gut microbiota Lactobacillus johnsonii and regulating macrophage anti-inflammatory activity in mice. Acta Pharm. Sin. B 2023, 13, 1164–1179. [Google Scholar] [CrossRef]
- Hu, X.-H.; Tang, M.-X.; Mor, G.; Liao, A.-H. Tim-3: Expression on immune cells and roles at the maternal-fetal interface. J. Reprod. Immunol. 2016, 118, 92–99. [Google Scholar] [CrossRef]
- Qian, Z.-D.; Huang, L.-L.; Zhu, X.-M. An immunohistochemical study of CD83- and CD1a-positive dendritic cells in the decidua of women with recurrent spontaneous abortion. Eur. J. Med. Res. 2015, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Hill, G.R. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood 2019, 134, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Zhang, B.; Duan, R.; Bai, X.; Wang, H.; Bai, J.; Song, W. Research Progress on the Etiology of Recurrent Spontaneous Abortion. Chin. J. Matern. Child Health Care 2023, 14, 72–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, C.; Chen, X.; Zhao, J.; Li, D. Correlation analysis of Immune Factors and Recurrent Miscarriage. J. Reprod. Med. 2023, 32, 1915–1921. [Google Scholar]
- Yao, H.; Ji, Y.; Zhou, Y. Analysis of blood coagulation indexes, thromboelastogram and autoantibodies in patients with recurrent pregnancy loss. Pak. J. Med. Sci. 2022, 38, 2005–2010. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertens. Dallas Tex 1979 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Hazen, S.L.; Brown, J.M. Eggs as a dietary source for gut microbial production of trimethylamine-N-oxide. Am. J. Clin. Nutr. 2014, 100, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Tumor Necrosis Factor Inhibitors as Therapeutic Agents for Recurrent Spontaneous Abortion. Available online: https://www.spandidos-publications.com/mmr/24/6/847 (accessed on 4 March 2024).
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef]
- Brugère, J.-F.; Borrel, G.; Gaci, N.; Tottey, W.; O’Toole, P.W.; Malpuech-Brugère, C. Archaebiotics: Proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 2014, 5, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.L.; Vasconcelos, A.G.; Nunes, A.K.S.; de Oliveira, W.H.; de Sousa Barbosa, K.P.; Peixoto, C.A. Effects of Sildenafil Citrate and Heparin Treatments on Placental Cell Morphology in a Murine Model of Pregnancy Loss. Cells Tissues Organs 2016, 201, 193–202. [Google Scholar] [CrossRef]
- Rätsep, M.T.; Carmeliet, P.; Adams, M.A.; Croy, B.A. Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta 2014, 35, 772–775. [Google Scholar] [CrossRef]
- Alipour, R.; Sereshki, N.; Rafiee, M.; Ahmadipanah, V.; Pashoutan Sarvar, D.; Rahimian, K.; Wilkinson, D. The effect of probiotic bacteria on toll-like receptor-2 and -4 expression by spermatozoa in couples with unexplained recurrent spontaneous abortion. Biochem. Biophys. Rep. 2022, 33, 101390. [Google Scholar] [CrossRef]
- Fernández, L.; Castro, I.; Arroyo, R.; Alba, C.; Beltrán, D.; Rodríguez, J.M. Immunomodulation of the Vaginal Ecosystem by Ligilactobacillus salivarius CECT 30632 Improves Pregnancy Rates among Women with Infertility of Unknown Origin or Habitual Abortions. Nutrients 2023, 15, 362. [Google Scholar] [CrossRef]
- Rafiee, M.; Sereshki, N.; Alipour, R.; Ahmadipanah, V.; Pashoutan Sarvar, D.; Wilkinson, D. The effect of probiotics on immunogenicity of spermatozoa in couples suffering from recurrent spontaneous abortion. BMC Immunol. 2022, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of Dietary Fiber in the Recovery of the Human Gut Microbiome and its Metabolome. Cell Host Microbe 2021, 29, 394–407.e5. [Google Scholar] [CrossRef]
- Wrønding, T.; Vomstein, K.; Bosma, E.F.; Mortensen, B.; Westh, H.; Heintz, J.E.; Mollerup, S.; Petersen, A.M.; Ensign, L.M.; DeLong, K.; et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: A proof of concept case study. eClinicalMedicine 2023, 61, 102070. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, J.; Chen, L.; Wang, Z.; Liu, M.; Liu, Y.; Chen, X.; Zhu, L.; Chen, H.; Zhang, J. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2016, 294, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhao, J.; Sun, Y.; Wang, Y.; Wei, A. The efficacy of modified Bushen Huoxue Decoction in the treatment of early unexplained recurrent miscarriage with kidney deficiency and blood stasis syndrome and its influence on intestinal flora. Chin. J. Exp. Formula Sci. 2020, 26, 102–108. [Google Scholar] [CrossRef]
| Gut Microbiota | ||||||||
| Author | Year | Group | Nation | Specimen Type | Composition: Phylum/Genus/Species | Mechanism | Conclusion | Reference |
| Xia Meiyan et al. | 2021 | Abortion group: RSA patients (n = 63); control group: healthy abortive pregnant women (n = 60); normal group: patients who underwent intra-pelvic surgery and had no endometriosis lesions (n = 53) | China | faeces | Content of yeast, Enterococci, and Enterobacteri: abortion group > control group > normal group; content of Lactobacillus and Bifidobacterium: normal group > control group > abortion group | / | All patients with RSA had high levels of gut microbiota; yeast, Enterococci, and Enterobacteria were positively correlated with RSA (p < 0.05); Lactobacillus and Bifidobacterium were negatively correlated with RSA (p < 0.05) | [18] |
| Yongjie Liu et al. | 2021 | Case group: RSA patients (n = 41); control group: normal early-pregnant women who chose abortion (n = 19) | China | faeces | The abortion group was rich in firmicutes and the control group was rich in Proteobacteria. In the control group, Prevotella, Prevotella _1, and Proteobacteria C were the most abundant microflora.Abortion group Spirochetes, Fibromyces, Softenicutes ↑*; Prevotella family NK3B31_ group, Bacteroideformes S24_7_Group, Eubacterium ruminant group, etc. ↑*; Prevotella (_1), Prevotella (UCG_003), Rothella (_1), and Selenomonas (_1) are among 48 species of bacteria ↓* | Bacterial abundance was significantly correlated with the changes in inflammatory factors and metabolites, such as IL-2, IFN-γ, IL-17A, 7-hydroxy-3-oxycholic acid, 1, 4-methylimidazolacetic acid, imidazolpropionic acid, etc | The abundance and uniformity of intestinal bacteria in abortion patients were low, and the gut microflora was obviously clustered. The proportion of some bacteria increased; Prevotella_1, Prevotella_UCG_003 and Selenomonas_1, which are the dominant bacterial groups in the gastrointestinal environment of healthy people, were significantly reduced | [21] |
| Zhi Li et al. | 2024 | Case group: RSA patients (n = 12); control group: normal early-pregnant women who chose abortion (n = 15) | China | faeces | The top 5 dominant genera in the case group were: Clostridium_sensu_stricto_1_unclassified, Escherichia—Shigella_unclassified, Klebsiella_pneumoniae, Streptococcus_salivarius, uncultured_Klebsiella_sp., Salivarius, uncultured _Klebsiella_sp. at the species level. The top 5 dominant genera in the control group were, in order, Megamonas_unclassified, Bacteroides _unclassified, Agathobacter_unclassified, Faecalibacterium_unclassified, and Bacteroides_uniformis | / | Gut microbiota of URSA patients has decreased diversity and changes in the dominant species. | [3] |
| Ying Cui et al. | 2021 | NR group: pregnant women who terminated their pregnancy and did not have a history of spontaneous abortion (n = 30) RSA group: RSA patients (n = 30) | China | faeces | The bacterial abundance index decreased in RSA patients, but the bacterial diversity index increased. They also found that Roseburia significantly decreased while Ruminococcus significantly increased in RSA patients. Also, in RSA patients with intrauterine adhesion, PCOS, and BMI > 23.9, Klebsiella significantly increased, and Prevotella.9 and Roseburia significantly decreased. | Functional prediction analysis indicated that gut microbiota may play their role through membrane transport, carbohydrate metabolism, amino acid metabolism, and other mechanisms. | RSA patients have abnormal gut microbiota compared with normal pregnant women. Butyrate-producing bacteria, like Roseburia, Prevotella.9, and Agathobacter, may play an important role in pregnant women, and are associated with RSA. | [22] |
| Metabolite | ||||||||
| Author | Year | Group | Nation | Specimen type | Constitution | Mechanism | Conclusion | Reference |
| Yongjie Liu et al. | 2021 | Case group: RSA patients (n = 41); control group: normal early-pregnant women who chose abortion (n = 19) | China | faeces | 239 differentiated metabolites were found in the miscarriage group compared to the control group. In miscarried patients, bile acids, methyl dihydrophosphonate, 3a, 7a, 12b-trihydroxy5b-cholic acid, 3a, 6a, 7b-trihydroxy5b-cholic acid, 3a, 6a, 7b-trihydroxy5b-cholic acid, 3α -hydroxy-5-β-chol-8,14-diene-24-cholic acid, 3,8-dihydroxy-6-methoxy-7(11)-dibenzyloxyphenol-12,8-lactone, d -urobilinogen, 1b,3a,7b-trihydroxy5b-cholic acid, THA and goose deoxycholic acid sulfate. ↑* | Metabolites such as 1,4-methylimidazole acetic acid in stool were positively correlated with IL-17A, IL-17F, TNF-α and IFN-γ. ROC analysis showed that imidazollic acid and 1,4-methylimidazolacetic acid were significantly associated with abortion | There were four broad clusters of differential metabolites: (1) glycerophospholipids and aryl alcohol lipids: control group > abortive group; (2) steroids and their derivatives; (3) amino acids and their derivatives; (4) alkaloids, drugs, and other metabolites: abortive group > control group. Differential metabolites were associated with the following metabolic processes: (1) bile secretion; (2) histidine metabolism; (3) glycerophospholipid metabolism; (4) arachidonic acid metabolic pathway; (5) steroid hormone biosynthesis. | [21] |
| Zhi Li et al. | 2024 | observation group: URSA patients (n = 12); control group: normal early-pregnant women who chose abortion (n = 15) | China | faeces | Acetic acid, propionic acid, butyric acid, deoxycholic acid (DCA) and glycolic acid (GLCA) ↓* in observation group. | DCA, GLCA, acetate, propionate, and butyrate were positively correlated with Tregs and Bregs frequencies. GLCA and butyrate were negatively correlated with Th1 and Th17 frequencies. Propionate and butyrate were negatively correlated with plasma B cell frequency | Levels of DCA, GLCA, acetate, propionate, and butyrate of intestinal microbial origin were decreased in URSA. | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Fang, R.; Xiong, T.; Li, W.; Yu, N. The Relationship Between Gut Microbiota and Recurrent Spontaneous Abortion. Microorganisms 2025, 13, 1073. https://doi.org/10.3390/microorganisms13051073
Huang Y, Fang R, Xiong T, Li W, Yu N. The Relationship Between Gut Microbiota and Recurrent Spontaneous Abortion. Microorganisms. 2025; 13(5):1073. https://doi.org/10.3390/microorganisms13051073
Chicago/Turabian StyleHuang, Yiyao, Ruijie Fang, Ting Xiong, Wei Li, and Nan Yu. 2025. "The Relationship Between Gut Microbiota and Recurrent Spontaneous Abortion" Microorganisms 13, no. 5: 1073. https://doi.org/10.3390/microorganisms13051073
APA StyleHuang, Y., Fang, R., Xiong, T., Li, W., & Yu, N. (2025). The Relationship Between Gut Microbiota and Recurrent Spontaneous Abortion. Microorganisms, 13(5), 1073. https://doi.org/10.3390/microorganisms13051073






