HABS-BLOCKS© Inhibited Microcystis and Planktothrix and Reduced Microcystin Concentrations in a Lake Water Mesocosm Study
Abstract
1. Introduction
2. Materials and Methods
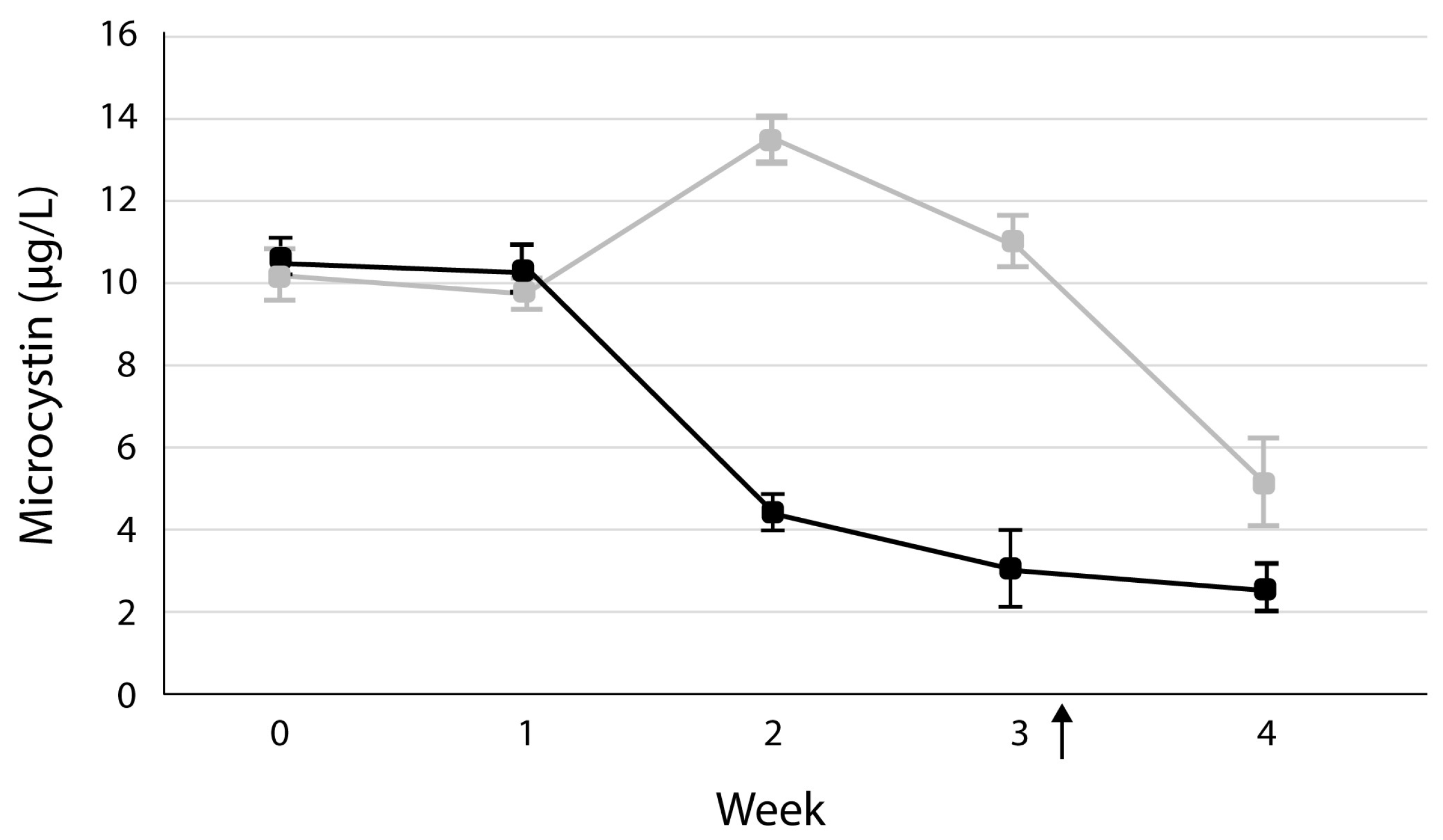
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCB | Harmful cyanobacteria bloom |
| HAB | Harmful algal bloom |
| P | Phosphorus |
| N | Nitrogen |
References
- Smucker, N.J.; Beaulieu, J.J.; Nietch, C.T.; Young, J.L. Increasingly severe cyanobacterial blooms and deep-water hypoxia coincide with warming water temperatures in reservoirs. Glob. Change Biol. 2021, 27, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Du, X.; Liu, H.; Meng, R.; Wu, C.; Zhang, Z.; Liang, X.; Yang, J.; Zhang, H. The cytotoxicity of microcystin-LR: Ultrastructural and functional damage of cells. Arch. Toxicol. 2024, 98, 663–687. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites: A short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Moura, A.D.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cyanobacterial toxins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; WHO/HEP/ECH/WSH/2020.1. [Google Scholar]
- United States Environmental Protection Agency. 2024. Available online: https://www.epa.gov/habs/epa-drinking-water-health-advisories-cyanotoxins (accessed on 15 July 2024).
- Mehdizadeh Allaf, M.; Erratt, K.J.; Peerhossaini, H. Comparative assessment of algaecide performance on freshwater phytoplankton: Understanding differential sensitivities to frame cyanobacteria management. Water Res. 2023, 234, 119811. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, K.; Shi, X.; Yang, G. Using hydrogen peroxide to control cyanobacterial blooms: A mesocosm study focused on the effects of algal density in Lake Chaohu, China. Environ. Pollut. 2021, 272, 115923. [Google Scholar] [CrossRef]
- Kim, W.; Park, Y.; Jung, J.; Jeon, C.O.; Toyofuku, M.; Lee, J.; Park, W. Biological and chemical approaches for controlling harmful microcystis blooms. J. Microbiol. 2024, 62, 249–260. [Google Scholar] [CrossRef]
- Piel, T.; Sandrini, G.; Weenink, E.F.J.; Qin, H.; Herk, M.J.V.; Morales-Grooters, M.L.; Schuurmans, J.M.; Slot, P.C.; Wijn, G.; Arntz, J.; et al. Shifts in phytoplankton and zooplankton communities in three cyanobacteria-dominated lakes after treatment with hydrogen peroxide. Harmful Algae 2024, 133, 102585. [Google Scholar] [CrossRef]
- Watson, S.E.; Taylor, C.H.; Bell, V.; Bellamy, T.R.; Hooper, A.S.; Taylor, H.; Jouault, M.; Kille, P.; Perkins, R.G. Impact of copper sulphate treatment on cyanobacterial blooms and subsequent water quality risks. J. Environ. Manag. 2024, 366, 121828. [Google Scholar] [CrossRef]
- Vesper, S.; Sienkiewicz, N.; Struewing, I.; Linz, D.; Lu, J. Prophylactic addition of glucose suppresses cyanobacterial abundance in lake water. Life 2022, 12, 385. [Google Scholar] [CrossRef]
- Linz, D.; Partridge, C.G.; Hassett, M.C.; Sienkiewicz, N.; Tyrrell, K.; Henderson, A.; Tardani, R.; Lu, J.; Steinman, A.D.; Vesper, S. Changes in cyanobacterial phytoplankton communities in lake-water mesocosms treated with either glucose or hydrogen peroxide. Microorganisms 2024, 12, 1925. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Struewing, I.; Sienkiewicz, N.; Steinman, A.D.; Partridge, C.G.; McIntosh, K.; Allen, J.; Lu, J.; Vesper, S. Periodic addition of glucose suppressed cyanobacterial abundance in additive lake water samples during the entire bloom season. J. Water Resour. Prot. 2024, 16, 140–155. [Google Scholar] [CrossRef]
- Vesper, S.; Linz, D.; Struewing, I.; Lu, J. HABS-BLOCKS©, a floating, slow-release glucose source, promoted the growth of heterotrophic bacteria relative to toxic cyanobacteria in lake water mesocosms. J. Water Resour. Prot. 2024, 16, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Allen, J.; Lu, J. Community structures of phytoplankton with emphasis on toxic cyanobacteria in an Ohio inland lake during bloom season. J. Water Resour. Prot. 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Bertone, E.; Chuang, A.; Burford, M.A.; Hamilton, D.P. In-situ fluorescence monitoring of cyanobacteria: Laboratory-based quantification of species-specific measurement accuracy. Harmful Algae 2019, 87, 101625. [Google Scholar] [CrossRef]
- Mirasbekov, Y.; Zhumakhanova, A.; Zhantuyakova, A.; Sarkytbayev, K.; Malashenkov, D.V.; Baishulakova, A.; Dashkova, V.; Davidson, T.A.; Vorobjev, I.A.; Jeppesen, E.; et al. Semi-automated classification of colonial Microcystis by FlowCAM imaging flow cytometry mesocosm experiment reveals high heterogeneity during seasonal bloom. Sci. Rep. 2021, 11, 9377. [Google Scholar] [CrossRef]
- Roache-Johnson, K.H.; Stephens, N.R. FlowCam 8400 and FlowCam Cyano phytoplankton classification and viability staining by imaging flow cytometry. Methods Mol. Biol. 2023, 2635, 219–244. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. 2016. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/method-546-determination-total-microcystins-nodularins-drinking-water-ambient-water-adda-enzyme-linked-immunosorbent-assay.pdf (accessed on 4 May 2025).
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Le Manach, S.; Duval, C.; Marie, A.; Djediat, C.; Catherine, A.; Edery, M.; Bernard, C.; Marie, B. Global metabolomic characterizations of Microcystis spp. highlights clonal diversity in natural bloom-forming populations and expands metabolite structural diversity. Front. Microbiol. 2019, 10, 791. [Google Scholar] [CrossRef]
- Pancrace, C.; Barny, M.A.; Ueoka, R.; Calteau, A.; Scalvenzi, T.; Pédron, J.; Barbe, V.; Piel, J.; Humbert, J.F.; Gugger, M. Insights into the Planktothrix genus: Genomic and metabolic comparison of benthic and planktic strains. Sci. Rep. 2017, 7, 41181. [Google Scholar] [CrossRef]
- Torres Cde, A.; Lürling, M.; Marinho, M.M. Assessment of the effects of light availability on growth and competition between strains of Planktothrix agardhii and Microcystis aeruginosa. Microb. Ecol. 2016, 71, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Trbojević, I.; Blagojević, A.; Kostić, D.; Marjanović, P.; Krizmanić, J.; Popović, S.; Simić, G.S. Periphyton development during summer stratification in the presence of a metalimnetic bloom of Planktothrix rubescens. Limnologica 2019, 78, 125709. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Anantapantula, S.S.; Wilson, A.E. Most treatments to control freshwater algal blooms are not effective: Meta-analysis of field experiments. Water Res. 2023, 243, 120342. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.; Li, W.; Wang, C.; Gin, K.Y.; Chai, X.; Wu, B. Dissolved organic carbon spurs bacterial-algal competition and phosphorus-paucity adaptation: Boosting Microcystis’ phosphorus uptake capacity. Water Res. 2024, 255, 121465. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Li, H.; Awasthi, M.K.; Kosolapov, D.B.; Ni, T.; Ma, B.; Liu, X.; Liu, X.; Zhi, W.; et al. Performance of aerobic denitrifying fungal community for promoting nitrogen reduction and its application in drinking water reservoirs. J. Environ. Manag. 2024, 351, 119842. [Google Scholar] [CrossRef]
- Brandenburg, K.; Siebers, L.; Keuskamp, J.; Jephcott, T.G.; Van de Waal, D.B. Effects of nutrient limitation on the synthesis of N-rich phytoplankton toxins: A meta-analysis. Toxins 2020, 12, 221. [Google Scholar] [CrossRef]
- Wagner, N.D.; Quach, E.; Buscho, S.; Ricciardelli, A.; Kannan, A.; Naung, S.W.; Phillip, G.; Sheppard, B.; Ferguson, L.; Allen, A.; et al. Nitrogen form, concentration, and micronutrient availability affect microcystin production in cyanobacterial blooms. Harmful Algae 2021, 103, 102002. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef]
- Yang, S.; Huang, T.; Zhang, H.; Tang, Y.; Guo, H.; Hu, R.; Cheng, Y. Promoting aerobic denitrification in reservoir water with iron-activated carbon: Enhanced nitrogen and organics removal efficiency, and biological mechanisms. Environ. Res. 2024, 240 Pt 2, 117452. [Google Scholar] [CrossRef]
- Li, Z.; Huang, T.; Wu, W.; Xu, X.; Wu, B.; Zhuang, J.; Yang, J.; Shi, H.; Zhang, Y.; Wang, B. Carbon slow-release and enhanced nitrogen removal performance of plant residue-based composite filler and ecological mechanisms in constructed wetland application. Bioresour. Technol. 2024, 402, 130795. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Ebrahimi, A.; Stubbusch, A.; Daniels, M.; Keegstra, J.; Stocker, R.; Cordero, O.; Ackermann, M. Cell aggregation is associated with enzyme secretion strategies in marine polysaccharide-degrading bacteria. Int. Soc. Microb. Ecol. J. 2023, 17, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, M.; Gerphagnon, M.; Bazin, P.; Colombet, J.; Tecchio, S.; Sime-Ngando, T.; Niquil, N. Microbial parasites make cyanobacteria blooms less of a trophic dead end than commonly assumed. Int. Soc. Microb. Ecol. J. 2018, 12, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Pomati, F.; Jokela, J.; Simona, M.; Veronesi, M.; Ibelings, B.W. An automated platform for phytoplankton ecology and aquatic ecosystem monitoring. Environ. Sci. Technol. 2011, 45, 9658–9665. [Google Scholar] [CrossRef]


| WEEK | 0 | 1 | 2 | 3 | 4 | 5 | 6 | T-Test |
|---|---|---|---|---|---|---|---|---|
| AVG Planktothrix × 102 Treated | 5 | 3 | 1 | 3 | 5 | 19 | 9 | p-value |
| AVG Planktothrix × 102 Control | 5 | 8 | 18 | 115 | 232 | 497 | 373 | |
| Planktothrix Treated vs. Control | 0.03 | |||||||
| AVG Microcystis × 102 Treated | 24 | 77 | 35 | 163 | 253 | 248 | 134 | |
| AVG Microcystis × 102 Control | 24 | 156 | 234 | 389 | 426 | 749 | 1260 | |
| Microcystis Treated vs. Control | 0.04 |
| Control | Treated | T-Test | |
|---|---|---|---|
| Test Measurements (Units) | Mean | Mean | p-Value |
| Total Dissolved Solids (mg/L) | 147 | 166 | 0.35 |
| Dissolved Oxygen (percent saturation) | 88 | 79 | 0.20 |
| Turbidity (formazin nephelometric units) | 135 | 101 | 0.52 |
| Conductivity (µS/CM) | 225 | 253 | 0.38 |
| Treated | Control | T-Test | ||||
|---|---|---|---|---|---|---|
| Analyte | Unit | Mean | SD | Mean | SD | p-Value |
| Total Nitrogen | mg-N/L | 4.930 | 0.801 | 8.37 | 0.338 | <0.001 |
| Total Phosphorus | mg P/L | 0.545 | 0.011 | 0.405 | 0.177 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastaldo, C.; Vesper, S. HABS-BLOCKS© Inhibited Microcystis and Planktothrix and Reduced Microcystin Concentrations in a Lake Water Mesocosm Study. Microorganisms 2025, 13, 1074. https://doi.org/10.3390/microorganisms13051074
Gastaldo C, Vesper S. HABS-BLOCKS© Inhibited Microcystis and Planktothrix and Reduced Microcystin Concentrations in a Lake Water Mesocosm Study. Microorganisms. 2025; 13(5):1074. https://doi.org/10.3390/microorganisms13051074
Chicago/Turabian StyleGastaldo, Cameron, and Stephen Vesper. 2025. "HABS-BLOCKS© Inhibited Microcystis and Planktothrix and Reduced Microcystin Concentrations in a Lake Water Mesocosm Study" Microorganisms 13, no. 5: 1074. https://doi.org/10.3390/microorganisms13051074
APA StyleGastaldo, C., & Vesper, S. (2025). HABS-BLOCKS© Inhibited Microcystis and Planktothrix and Reduced Microcystin Concentrations in a Lake Water Mesocosm Study. Microorganisms, 13(5), 1074. https://doi.org/10.3390/microorganisms13051074






