Abstract
Kytococcus belongs to the family Kytococcaceae, in the order Micrococcales, in the class Actinomycetes, and the phylum Actinomycetota. Kytococcus are aerobic, Gram-positive, non-spore forming bacteria that form coccoid, non-motile, non-encapsulated cells, and their colonies on agar have a yellow color. Infections by these species are increasingly identified nowadays. This narrative review aimed to present all available cases of Kytococcus spp. infections in humans, emphasizing data on the epidemiology, antimicrobial resistance, antimicrobial treatment, and mortality. A narrative review based on a literature search of the PubMed/MedLine and Scopus databases was performed. Results: In total, 26 articles providing data on 30 patients with Kytococcus spp. infections were included in this analysis. The median age was 59.5 years, while 56.7% were male. The presence of a prosthetic cardiac valve was the main predisposing factor in 36.7% of patients (100% among those with infective endocarditis), while immunosuppression due to underlying hematological malignancy under chemotherapy was the second most common. Bacteremia was the most common type of infection, with infective endocarditis being the most common subtype in this infection type, while respiratory tract infections and osteoarticular infections were also relatively common. K. schroeteri was the most commonly identified species. Microbial identification required the use of advanced molecular techniques such as 16s rRNA sequencing in most cases. Kytotoccus spp. was resistant to all beta-lactams with the exception of carbapenems and macrolides. The most commonly used antimicrobials were vancomycin and rifampicin. Mortality was significant (30%). Due to the potential of Kytotoccus spp. to cause infective endocarditis in patients with prosthetic cardiac valves and respiratory tract infections with concomitant bacteremia in patients with hematological malignancy under chemotherapy along with the difficulties in pathogen identification, clinicians and laboratory personnel should consider this pathogen in the differential diagnosis in patients with typical predisposing factors and clinical presentation, especially when traditional microbiological techniques are used for pathogen identification.
1. Introduction
The genus Micrococcus consists of Gram-positive, catalase-positive cocci, belongs to the group of actinomycete, and was first described more than a century ago [1,2]. Phylogenetic and chemotaxonomic analyses have led to several changes in the classification of bacteria from the genus Microoccus. The genus Kytococcus was first distinguished from the genus Micrococcus in 1995 [1]. Kytococcus belongs to the family Kytococcaceae, in the order Micrococcales, in the class Actinomycetes, and the phylum Actinomycetota [1,3]. Kytococcus are aerobic, Gram-positive, non-spore forming bacteria that form coccoid, non-motile, non-encapsulated cells, and their colonies on agar have a yellow color [3,4]. Its peptidoglycan is of the A4α type and contains L-lysine as the diamino acid. It does not contain teichoic acids or mycolic acids, and the genomic G+C content is 68–73% [3]. The genus includes three species, namely, Kyotococcus aerolatus, Kyotococcus schroeteri, and Kyotococcus sedentarius, while the NCBI taxonomy browser reports several unclassified and uncultured variants [5].
Infections by Kytococcus species are quite uncommon and are usually reported in the form of case reports with a literature review. Thus, data on the epidemiology, type of infection, diagnostic modalities, antimicrobial susceptibility, treatment, and outcomes remain scarce. To this end, the present study aimed to comprehensively review all the available information of human infections caused by Kytococcus spp. in the literature and to assess the clinical data, microbiology, treatment, and outcomes by performing individual patient data analysis from already published studies.
2. Materials and Methods
2.1. Search Strategy and Inclusion and Exclusion Criteria
This narrative review aimed to collect and present all of the published data on Kytococcus species infections in humans after performing individual patient data analysis from already published studies. The primary aim of the study was to present data on the patients’ epidemiology and mortality. The secondary aims were to collect data about the specific infection site, the clinical presentation, the microbiological features, and the treatment provided. For this review, the PubMed/Medline and Scopus databases were searched until 27 February 2025. Data were extracted using a predefined template. The following keywords were applied for the search strategy: “Kytococcus” AND “infection”. Studies providing original data, such as case series, case reports, and cohort studies providing information into the epidemiology and clinical outcomes of Kytococcus spp. infections in humans, were included. Studies in language other than English, reviews, and systematic reviews were excluded. Studies involving animals, articles without full-text access, and those lacking information on the patients’ mortality and epidemiology were also excluded from the analysis. Additionally, cases of colonization by Kytococcus species were excluded from the analysis, based on the clinical course depicted and the study authors’ consideration as to whether the species were pathogens or colonizers. The references of all included articles were examined to identify any studies potentially missed in the initial search.
2.2. Data Extraction and Definitions
The data extracted from each included study were publication year, article type, country of origin, patient demographics (age, gender), relevant medical history, details of infection, and key clinical characteristics such as specific infection site and complications as well as microbiological characteristics such as identified pathogen, antibiotic susceptibilities, and finally, treatment used and outcome (survival or mortality). The relationship between mortality and the initial infection was documented according to each study’s authors. This information from already published studies was transferred to a predefined datasheet and processed further by performing individual patient data analysis.
2.3. Statistical Analysis
Data are presented as numbers (%) for the categorical variables and the median (interquartile range, IQR) for continuous variables. Continuous variables were compared using the Mann–Whitney U-test for non-normally distributed variables or the t-test for normally distributed variables. All tests were two-tailed, and a p-value equal to or lower than 0.05 was considered significant. A univariate linear regression analysis including all patients, irrespective of age, was conducted to identify factors associated with all-cause mortality. Statistics were calculated with GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). A multivariate linear regression analysis was conducted in order to evaluate the effect of factors previously identified in the univariate analysis model to be associated with all-cause mortality with a p < 0.05. Multivariate analysis was performed using the SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Included Studies’ Characteristics
A total of 93 articles were screened from the PubMed and Scopus databases. Eventually, after duplicate removal, record screening, and applying the snowball procedure, only 26 articles met the inclusion criteria and were selected for analysis [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. These studies presented data on 30 patients. A flow diagram of the selection process is illustrated in Figure 1. Among the included cases, 16 occurred in Europe (61.6%), 5 in North and South America (19.2%), and 5 in Asia (19.2%). Among the 26 included articles, 23 (88.5%) were case reports. Table 1 shows the characteristics of the included studies in the present review.
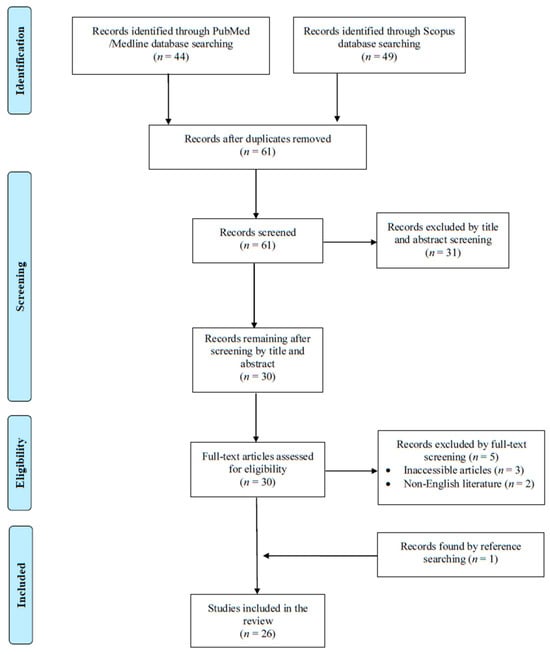
Figure 1.
Trial flow of this narrative review.

Table 1.
Characteristics of all of the included studies.
3.2. Epidemiology of Kytococcus spp. Infections
The median age of patients with Kytococcus spp. infections was 59.5 years, with a range of 0 to 83 years, while 56.7% (17 patients) were male. Regarding the patients’ medical history and predisposing risk factors, 11 out of 30 patients (36.7%) had a prosthetic cardiac valve, 8 (26.7%) had immunosuppression due to hematological malignancy undergoing treatment, 2 patients (6.7%) had a history of recent surgery within the previous three months, 1 (3.3%) had end stage renal disease on dialysis, 1 (3.3%) previously had infective endocarditis, and 1 (3.3%) had rheumatic fever. The demographic and clinical characteristics of patients with infections by Kytococcus spp. are shown in Table 2.

Table 2.
Characteristics of patients with Kytococcus species infection.
3.3. Microbiology and Antimicrobial Resistance of Kytococcus spp. Infections
Kytococcus spp. was isolated from blood cultures in 20 patients (66.7%), from bronchoalveolar fluid in 4 (13.3%), from tissue cultures in 4 (13.3%), from valve culture in 2 (6.7%), from bone culture in 2 (6.7%), and from joint fluid, peritoneal fluid, extracted port catheter, pus, and sputum in 1 (3.3%) each. K. schroeteri was the identified species in 25 patients (83.3%), K. sedentarius was identified in 4 (13.3%), and in 1 patient (3.3%) the species was not reported. In 17 cases (56.7%), identification was performed with 16s rRNA sequencing. In 10 cases (33.3%), identification was performed with matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALID-TOF MS). In one case (3.3%), identification was performed with VITEK2, and in one case (3.3%), the identification was performed with phenotypic and molecular methods. The means of pathogen identification was not mentioned in three cases (10%). The antimicrobial resistance of Kytococcus spp. is shown in Table 3. In 2 out of 22 patients (6.7%), the infection was polymicrobial.

Table 3.
Antimicrobial resistance rates of Kytococcus spp.
3.4. Clinical Presentation of Kytococcus spp. Infections
The most common type of Kytococcus spp. infections were those of the bloodstream in 20 patients (66.7%). Infective endocarditis was diagnosed in 12 patients (40%). Other infections were lower respiratory tract infections in six patients (20%), osteoarticular infections in five (16.7%), skin and soft tissue infections in two (6.7%), central nervous system infection in one (3.3%), vascular graft infection in one (3.3%), and peritoneal dialysis-associated infection in one (3.3%). The symptoms’ durations ranged from 0 (acute onset) to more than a year.
3.5. Treatment and Outcome of Kytococcus Infections
The treatment of patients with Kytococcus spp. infections in shown in detail in Table 1 and is also summarized in Table 2. Based on the available data, vancomycin was the most frequently administered antimicrobial used in 20 out of 28 patients with available data (71.4%), followed by rifampicin (used in combination with other antimicrobials) in 11 patients (39.3%), aminoglycosides in 7 (25%), quinolones in 6 (21.4%), carbapenems in 4 (14.3%), an aminopenicillin with an inhibitor in 2 (7.1%), cephalosporin in 2 (7.1%), daptomycin in 2 (7.1%), linezolid in 2 (7.1%), tetracycline in 1 (3.6%), trimethoprim with sulfamethoxazole in 1 (3.6%), anti-staphylococcal penicillin in 1 (3.6%), and teicoplanin in 1 (3.6%). Surgical interventions were applied in combination with antimicrobial treatment in 16 out of 30 patients (53.3%). The median treatment duration for survivors was 6 weeks. The overall mortality rate was estimated at 30% (9 out of 30 patients), with the mortality directly associated with the Kytococcys spp. infection being 26.7% (8 patients).
3.6. Bacteremia Due to Kytococcus
Bacteremia was diagnosed in 20 patients (66.7%). Among them, 12 (60%) were male and the median age was 51.5 years. Among these patients, six (30%) had immunosuppression due to hematological malignancy that was under treatment. Infective endocarditis was diagnosed in 11 patients (55%) with bacteremia while a lower respiratory tract infection was present in 4 (20%), and central nervous infection was present in 1 (5%). Only in one patient with an infection of the lower respiratory tract was the infection polymicrobial. Fever was present in 16 patients (80%), and sepsis was present in 11 (55%). Vancomycin, rifampicin, and aminogylcosides were the most commonly used antimicrobials. The overall mortality was 35% (7 patients).
3.7. Infective Endocarditis Due to Kytococcus
Infective endocarditis was diagnosed in 11 patients. The median age in this patient group was 64 years, and seven patients were male (63.6%). A prosthetic cardiac valve was present in all patients (100%), and was bioprosthetic in seven out of ten patients with available data (70%) and metallic in three (30%). Previous infective endocarditis was noted in the history of one patient (9.1%), rheumatic fever in one (9.1%), and one patient (9.1%) had a history of recent surgery in the three months before presentation. All patients had bacteremia, and the diagnosis was made with blood cultures in all patients, valve culture in three patients (27.3%), polymerase chain reaction (PCR) in the valve in two patients (18.2%), valve histology in one patient (9.1%), transesophageal echocardiography in nine patients (81.8%), and transthoracic echocardiography in two (18.2%). The infected intracardiac site was the aortic valve in nine patients (81.8%), and the mitral valve in two (18.2%). Fever was present in eight patients (72.7%), and sepsis was present in five (45.5%). Embolic phenomena developed in three patients (27.3%), and heart failure developed in one patient (9.1%). A paravalvular abscess was noted in six patients (54.5%). The median treatment duration was 6 weeks. The most commonly used antimicrobial agents were vancomycin in ten patients (90.9%), rifampicin in eight (72.7%), and aminoglycosides in seven (63.6%). The overall mortality was 9.1%, with only one patient dying due to the episode of Kytococcus infective endocarditis.
3.8. Lower Respiratory Tract Infection Due to Kytococcus
A lower respiratory tract infection was diagnosed in seven patients (23.3%). Among them, four (57.1%) were male, and the median age was 52 years. Among patients with this infection, six (85.7%) had immunosuppression due to hematological malignancy that was under treatment, and five (71.4%) had bacteremia along with the respiratory tract infection. One patient with hematological malignancy had a skin and soft tissue infection that was confirmed to be due to Kytococcus; at the same time, he had a respiratory tract infection. Only in one patient was the infection polymicrobial. Fever was present in six patients (85.7%), and sepsis in two (28.6%). Vancomycin and cephalosporins were the most commonly used antimicrobials. The overall mortality was 85.7% (six patients).
3.9. Osteoarticular Infection Due to Kytococcus
An osteoarticular infection was diagnosed in five patients (16.7%). Among them, one (20%) was male, and the median age was 71 years. Among patients with this infection, one (20%) had surgery in the three months preceding the diagnosis. Fever was present in one patient (20%). Quinolone was the most commonly used antimicrobial for treatment, and surgical management was performed in four patients (80%). No patient died.
3.10. Characteristics of Patients with Kytococcus spp. with Regard to Survival
Table 4 shows a comparison of the characteristics of patients with Kytococcus spp. who lived with those who died. Patients who died were more likely to have had immunosuppression due to hematological malignancy on chemotherapy, more likely to have had an infection of the lower respiratory tract, and were less likely to have had treatment with rifampicin or have had surgical treatment along with antimicrobials. These results more likely reflect the high mortality rate of respiratory tract infections in patients with immunosuppression due to hematological malignancy, the use of rifampicin in patients with infective endocarditis and vascular graft infections, and the high rate of surgical intervention in patients with infective endocarditis and osteoarticular infections that had a small mortality rate.

Table 4.
Characteristics of patients with Kytococcus species infection with regard to outcome.
To identify factors independently associated with overall mortality, we first performed a linear regression of gender, age, presence of a prosthetic valve, type of infection, presence of fever or sepsis, and antimicrobial treatment provided. Infection of the lower respiratory tract and presence of immunosuppression were identified as being positively associated with mortality (p < 0.0001 and p = 0.0006, respectively). However, a multivariate logistic regression analysis did not identify any factor to be independently associated with mortality. The results of the multivariate regression can be seen in Table 5.

Table 5.
Logistic regression analysis of the overall mortality of patients with Kytococcus species infection.
4. Discussion
This review summarized the clinical and microbiological characteristics of human infections by Kytococcus spp. by evaluating all published studies providing relevant data in the literature and by presenting data from individual patient data analysis from these already published studies. The most common types of infections were bacteremia, infective endocarditis, respiratory tract infections, and osteoarticular infections. Antimicrobial resistance to first-line beta-lactams was almost universal while vancomycin, daptomycin, linezolid, and cabapenems were active in almost all cases, and vancomycin was the most commonly used antimicrobial for treating infections by these pathogens. Mortality from infections by Kytococcus spp. was high, especially for immunosuppressed patients and those with respiratory tract infections.
Kytococcus species were dissected from the genus Micrococcus after the work of Stackebrandt et al. in 1995 [1]. Ever since, several reports of infections have been reported with identification requiring advanced molecular techniques such as 16s rRNA sequencing or MALDI-TOF MS. Indeed, there seem to be several difficulties with the correct identification of this microorganism. For example, a recent study in Japan revealed that cases of human infection by Kytococcus spp. may have been missed due to misidentification as Micrococcus spp. [32]. Moreover, due to the fact that Kytoococcus spp. can represent human skin microbiota, further difficulties in its identification may also occur since specimens could be discarded as being considered to have been contaminated [27]. Thus, cases of Kytococcus spp. human infection could be underreported. In the present review, 16s rRNA sequencing was the most common means of pathogen identification, and was used in more than half the cases, followed by MALDI-TOF MS. These techniques have led to a revolution in microbiology, facilitating the identification of uncommon microorganisms that would be very difficult to identify based on the use of traditional biochemical and morphological pathogen characteristics [33,34,35]. To this end, only one study included in the present review performed identification solely based on the biochemical and morphological characteristics.
The most common conditions in the past medical history of patients suffering an infection by Kytococcus spp. were the presence of a prosthetic cardiac valve and immunosuppression, which was due to hematological malignancy that was under treatment in all immunosuppressed patients. The presence of a prosthetic cardiac valve was clearly a predisposing factor for the patients with infective endocarditis, with all patients with this infection having a prosthetic cardiac valve. This is reasonable since the presence of a prosthetic cardiac valve is a well-known factor for the development of infective endocarditis [36,37,38]. However, the fact that all patients with infective endocarditis had a prosthetic cardiac valve is intriguing. This could be associated with the fact that Kytococcus spp. is part of the skin microbiome, thus increasing the likelihood that a short episode of bacteremia due to a brief duration of lysis of the epithelial barrier due to trauma or other reasons could lead to inoculation of the prosthetic valve and the development of infective endocarditis. On the other hand, given that the pathogenicity of this microorganism is not clear, it could be reasonable to think that the predilection that Kytococcus spp. shows for prosthetic cardiac valves could have a pathophysiological basis. Thus, it could be that this pathogen produces specific substances that allow it to more easily adhere to the prosthetic material of prosthetic cardiac valves, leading to infective endocarditis. However, this has to be shown in studies directly evaluating the pathogenicity of this species.
Immunosuppression due to treatment for hematological malignancy was the second most common condition identified in patients with infection due to Kytococcus spp. These patients were more likely to have a respiratory tract infection and bacteremia, while the outcome was commonly fatal. On the other hand, patients with immunosuppression are well-known to be more susceptible to infection and are also more likely to suffer more severe complications, such as sepsis, if infected [39,40]. Since the field of infectious diseases has changed a lot in the last years, and not all immunosuppressed patients are considered identical, it is of utmost importance to understand the exact mechanism leading to immunosuppression and to understand the ‘net state of immunosuppression’ for each particular patient [39]. Patients with hematological malignancies are at a particularly high risk for infections due to the neutropenia that is common in patients on myeloablative chemotherapy or even due to the disease, the hypogammaglobulinemia that is often seen in these patients, the mucosal damage due to therapy, T-cell dysfunction, and the common use of intravascular devices [41,42]. Despite the fact that these patients with such immunosuppression usually had bacteremia and frequently had sepsis, it is unclear as to why they are more likely to have a respiratory tract infection, and since there are scarce data available regarding its pathophysiology, little can be said regarding the route of infection. Future studies should focus on the exact pathogenetic mechanisms leading to these infections in immunosuppressed individuals.
Among the different clinical presentations, bacteremia was the most common presentation and was associated with infective endocarditis in the majority of cases, and second, respiratory tract infections. However, given the rarity of the pathogen, the possible underreporting in the literature, the problems related to the identification of the pathogen, and the possibility of rejecting the diagnosis of bacteremia by discarding a blood culture as a contaminant, it could be that cases of bacteremia without a respiratory tract infection or infective endocarditis, especially in patients with hematological malignancy that have indwelling devices and are under chemotherapy, could have been missed. Other infections noted in the present narrative review include osteoarticular infections, peritoneal dialysis-associated peritonitis, skin and soft tissue infections, and vascular graft infections. These infections are commonly seen with bacteria that are commensals of the skin such as staphylococci [43,44,45,46,47,48]. Kytococcus spp. have been also identified in other conditions that do not constitute invasive human infections such as pitted keratolysis [49]. For example, in a case report from a Greek center, clinical specimens from an adolescent with pitted keratolysis revealed the growth of several microorganisms, with two species of Kytococcus being among them [49]. Such studies were excluded from the present review, since they do not constitute deep seated infections, while underreporting would have probably been extensive.
Studies regarding the antimicrobial susceptibility of Kytococcus spp. are scarce, and studies regarding the genes and the molecular mechanisms underlying this resistance are even scarcer. In a recent study analyzing a Kytococcus sedentarius strain isolated from a dehumidifier in a university, several genes associated with the development of antimicrobial resistance were identified [50]. For example, that strain was resistant to ciprofloxacin, gentamicin, and erythromycin. Notably, in that study, the presence of genes encoding multi-drug-resistance pumps was noted, however, the resistance to erythromycin could not be explained based on those genes, implying the presence of other genes coding for proteins and mechanisms associated with resistance to that antimicrobial [50]. In the present study, antimicrobial resistance to macrolides, penicillin, oxacillin, and cephalosporins was higher than 90%. Antimicrobial resistance to vancomycin, linezolid, quinolones, daptomycin, tetracyclines, rifampicin, and carbapenems was low, thus these antimicrobials could be used to treat these infections. Indeed, in most of the studies included in the present review, vancomycin and rifampicin were the two most commonly used antimicrobials, with rifampicin being used as an adjunct antimicrobial mostly in patients with infective endocarditis. Even though this review cannot provide strong recommendations given the rarity of the infection and the very few reports included in the present analysis, appropriate empirical treatment is of paramount importance, especially in severe infections, as in immunosuppressed patients. However, given the inability for international scientific societies to provide guidelines for treating infections by these microorganisms, it is reasonable to consider vancomycin, daptomycin, tetracyclines, quinolones, and linezolid for treatment.
Notably, the treatment duration of the patients included in the present analysis was quite long, and this could be associated with the diagnosis of infective endocarditis in many of them. Moreover, the duration of treatment herein was calculated only for patients who survived, and since patients with infective endocarditis had very low mortality, the duration of their treatment contributed significantly to the duration of treatment of all survivors. More studies are warranted to identify the optimal treatment, and even more so, the optimal duration of treatment for these patients.
The mortality of Kytococcus infections was high, with about 30% of patients dying from the infection. Importantly, 67% of patients who died had a hematological malignancy under treatment, 78% of patients who died had bacteremia, and 67% had a respiratory tract infection. These factors were among those that were different in those who died versus those who survived in a statistically significant manner. Moreover, patients who survived were given rifampicin more frequently and were also treated with surgery more frequently; both of these factors can be associated with the treatment of infective endocarditis that was more frequent in those who survived. The discrepancy in the mortality of infective endocarditis, which is traditionally considered a more deadly infection, and that of patients with respiratory tract infection with bacteremia, mostly seen in patients with hematological malignancy, could be partly associated with the particularities noted in patients with underlying immunosuppression and their higher likelihood for worse clinical outcomes in the case of an infection [51]. Further studies are needed to better understand the differences in the clinical syndromes of Kytococcus spp. infections.
This study had some limitations. Firstly, the published studies may not have adequately represented the infections by Kytococcus spp. due to problems associated with the identification, publication bias, or other reasons. Moreover, some studies may have been missed during the screening process. Additionally, the analysis herein was based exclusively on case reports, which depend on highly accurate record-keeping. Thus, this review only presented findings from studies that provided complete data for the parameters that were evaluated, especially for mortality and epidemiology. Moreover, this review identified only a small number of studies providing data for only a small number of patients, thus limiting the credibility of the conclusions of the study.
5. Conclusions
The present review provides important information regarding the epidemiology, clinical presentation, antimicrobial resistance, and treatment and outcomes of Kytococcus spp. infections in humans. K. schroeteri was the most commonly identified species, while the most common infections were those of the bloodstream, infective endocarditis, and respiratory tract infections. Kytococcus was resistant to all beta-lactams with the exception of carbapenems and macrolides, while antimicrobial resistance to vancomycin, daptomycin, linezolid, and tetracyclines was minimal. Vancomycin was the most commonly used for treating infections by these pathogens. Mortality was significant, but mostly for patients with hematological malignancy under treatment that had a respiratory tract infection along with bacteremia. Kytococcus spp. infections should be considered in patients with a prosthetic cardiac valve suffering infective endocarditis, or in patients with hematological malignancy and a respiratory tract infection with bacteremia when Gram-positive cocci are isolated, especially when their features resemble those of Micrococcus, even though advanced molecular techniques such as MALDI-TOF MS or 16s rRNA sequencing will probably be needed for the correctly identification of this pathogen.
Author Contributions
Conceptualization, P.I.; Methodology, P.I., S.B. and D.K.; Software, P.I.; Validation, P.I.; Formal analysis, P.I. and A.G.T.; Investigation, P.I., E.K., S.K., A.G.T. and S.B.; Resources, P.I.; Data curation, P.I.; Writing—original draft preparation, P.I. and S.B.; Writing—review and editing, E.K., S.K., A.G.T. and D.K.; Visualization, P.I.; Supervision, P.I.; Project administration, P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stackebrandt, E.; Koch, C.; Gvozdiak, O.; Schumann, P. Taxonomic Dissection of the Genus Micrococcus: Kocuria Gen. Nov., Nesterenkonia Gen. Nov., Kytococcus Gen. Nov., Dermacoccus Gen. Nov., and Micrococcus Cohn 1872 Gen. Emend. Int. J. Syst. Bacteriol. 1995, 45, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Lewis, B.J.; Woese, C.R. The Phylogenetic Structure of the Coryneform Group of Bacteria. Zentralblatt Für Bakteriol. Abt Orig. C Allg. Angew. Ökol. Mikrobiol. 1980, 1, 137–149. [Google Scholar] [CrossRef]
- Nouioui, I.; Carro, L.; García-López, M.; Meier-Kolthoff, J.P.; Woyke, T.; Kyrpides, N.C.; Pukall, R.; Klenk, H.-P.; Goodfellow, M.; Göker, M. Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 2018, 9, 2007. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Robert, M.; Teteneva, N.A.; Danilova, N.D.; Zhurina, M.V.; Mart’yanov, S.V.; Plakunov, V.K.; Feuilloley, M.G.J.; Gannesen, A.V. Draft Genome Sequence of Kytococcus schroeteri Strain H01, Isolated from Human Skin. Microbiol. Resour. Announc. 2019, 8, e01081-19. [Google Scholar] [CrossRef] [PubMed]
- NCBI Taxonomy Browser for Kytococcus. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=57499 (accessed on 3 May 2025).
- Becker, K.; Wüllenweber, J.; Odenthal, H.-J.; Moeller, M.; Schumann, P.; Peters, G.; Von Eiff, C. Prosthetic Valve Endocarditis Due to Kytococcus schroeteri. Emerg. Infect. Dis. 2003, 9, 1493–1495. [Google Scholar] [CrossRef]
- Levenga, H.; Donnelly, P.; Blijlevens, N.; Verweij, P.; Shirango, H.; De Pauw, B. Fatal Hemorrhagic Pneumonia Caused by Infection Due to Kytococcus sedentarius—A Pathogen or Passenger? Ann. Hematol. 2004, 83, 447–449. [Google Scholar] [CrossRef]
- Le Brun, C.; Bouet, J.; Gautier, P.; Avril, J.-L.; Gaillot, O. Kytococcus schroeteri Endocarditis. Emerg. Infect. Dis. 2005, 11, 179–180. [Google Scholar] [CrossRef]
- Mohammedi, I.; Berchiche, C.; Becker, K.; Belkhouja, K.; Robert, D.; Voneiff, C.; Etienne, J. Fatal Bacteremic Pneumonia. J. Infect. 2005, 51, E11–E13. [Google Scholar] [CrossRef] [PubMed]
- Mnif, B.; Boujelbène, I.; Mahjoubi, F.; Gdoura, R.; Trabelsi, I.; Moalla, S.; Frikha, I.; Kammoun, S.; Hammami, A. Endocarditis Due to Kytococcus schroeteri: Case Report and Review of the Literature. J. Clin. Microbiol. 2006, 44, 1187–1189. [Google Scholar] [CrossRef]
- Renvoise, A.; Roux, V.; Thuny, F.; Riberi, A.; Casalta, J.-P. Kytococcus schroeteri, a Rare Agent of Endocarditis. Int. J. Infect. Dis. 2008, 12, 223–227. [Google Scholar] [CrossRef]
- Aepinus, C.; Adolph, E.; Von Eiff, C.; Podbielski, A.; Petzsch, M. Kytococcus schroeteri: A Probably Underdiagnosed Pathogen Involved in Prosthetic Valve Endocarditis. Wien. Klin. Wochenschr. 2008, 120, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, S.; Miendje Deyi, V.Y.; Musampa, K.; Wauters, G.; Denis, O.; Lepage, P.; Vergison, A. Kytococcus schroeteri Infection of a Ventriculoperitoneal Shunt in a Child. Int. J. Infect. Dis. 2009, 13, e153–e155. [Google Scholar] [CrossRef]
- Yousri, T.; Hawari, M.; Saad, R.; Langley, S. Kytococcus schroeteri Prosthetic Valve Endocarditis. BMJ Case Rep. 2010, 2010, bcr0620103064. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, H.; Allard, A.; Richette, P.; Ea, H.K.; Sanson-Le Pors, M.J.; Berçot, B. Postoperative Spondylodiscitis Due to Kytococcus schroeteri in a Diabetic Woman. J. Med. Microbiol. 2010, 59, 127–129. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Huisman, C.; Spanjaard, L.; Van Ketel, R.J. Kytococcus schroeteri Pneumonia in Two Patients with a Hematological Malignancy. Infection 2010, 38, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Finkle, S.N. Peritoneal Dialysis-Associated Peritonitis Due to Kytococcus sedentarius. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2010, 30, 252–253. [Google Scholar] [CrossRef]
- Nagler, A.R. Fatal Kytococcus schroeteri Infection with Crusted Papules and Distinctive Histologic Plump Tetrads. Arch. Dermatol. 2011, 147, 1121–1122. [Google Scholar] [CrossRef]
- Dainese, L.; Saccu, C.; Zoli, S.; Trabattoni, P.; Guarino, A.; Ca Vallero, A.; Spirito, R. Vascular Homograft Use in a Femoropopliteal Rare Bacterial Infection Bypass. Int. J. Artif. Organs 2012, 35, 1077–1079. [Google Scholar] [CrossRef]
- Liu, J.C.; Jenkins, D.R.; Malnick, H.; Kovac, J.; Szostek, J. Kytococcus schroeteri Endocarditis Successfully Managed with Daptomycin: A Case Report and Review of the Literature. J. Med. Microbiol. 2012, 61, 750–753. [Google Scholar] [CrossRef]
- Blennow, O.; Westling, K.; Fröding, I.; Özenci, V. Pneumonia and Bacteremia Due to Kytococcus schroeteri. J. Clin. Microbiol. 2012, 50, 522–524. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Wong, S.S.Y.; Leung, S.S.M.; Fan, R.Y.Y.; Ngan, A.H.Y.; To, K.K.W.; Lau, S.K.P.; Yuen, K.-Y.; Woo, P.C.Y. First Report of Chronic Implant-Related Septic Arthritis and Osteomyelitis Due to Kytococcus schroeteri and a Review of Human K. Schroeteri Infections. Infection 2012, 40, 567–573. [Google Scholar] [CrossRef]
- Amaraneni, A.; Malik, D.; Jasra, S.; Chandana, S.R.; Garg, D. Kytococcus schroeteri Bacteremia in a Patient with Hairy Cell Leukemia: A Case Report and Review of the Literature. Case Rep. Infect. Dis. 2015, 2015, 217307. [Google Scholar] [CrossRef] [PubMed]
- DeMartini, T.; Ericson, J.; Ceneviva, G. 1771: Kytococcus bacteremia in a pediatric patient with myelodysplastic syndrome. Crit. Care Med. 2016, 44, 518. [Google Scholar] [CrossRef]
- Bayraktar, B.; Dalgic, N.; Duman, N.; Petmezci, E. First Case of Bacteremia Caused by Kytococcus schroeteri in a Child With Congenital Adrenal Hyperplasia. Pediatr. Infect. Dis. J. 2018, 37, e304–e305. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Vijayvargiya, P.; Jung, S.; Wilson, J.W. Postoperative Hardware-Related Infection from Kytococcus schroeteri: Its Association with Prosthetic Material and Hematological Malignancies—A Report of a Case and Review of Existing Literature. Case Rep. Infect. Dis. 2019, 2019, 6936472. [Google Scholar] [CrossRef]
- Bagelman, S.; Zvigule-Neidere, G. Insight into Kytococcus schroeteri Infection Management: A Case Report and Review. Infect. Dis. Rep. 2021, 13, 230–238. [Google Scholar] [CrossRef]
- Lim, K.R.; Son, J.S.; Moon, S. Case Report of Infectious Spondylitis Caused by Kytococcus sedentarius. Medicina 2021, 57, 797. [Google Scholar] [CrossRef]
- Zellner, A.A.; Hischebeth, G.T.; Molitor, E.; Wirtz, D.C.; Randau, T.M. Periprosthetic Joint Infection Caused by Kytococcus schroeteri: The First Reported Case and a Review of the Literature. Diagn. Microbiol. Infect. Dis. 2023, 106, 115922. [Google Scholar] [CrossRef]
- Shah, S.; Thakkar, P.; Poojary, S.; Singhal, T. A Case of Kytococcus schroeteri Prosthetic Valve Endocarditis in a Patient with COVID-19 Infection. Indian J. Med. Microbiol. 2023, 42, 89–91. [Google Scholar] [CrossRef]
- Pandey, I.; Pai, S.; Purmessur, R.; Kappeler, R.; Ali, J.M. Kytococcus schroeteri: An Emerging Cause of Prosthetic Valve Endocarditis. Infect. Dis. Clin. Pract. 2024, 22, e1344. [Google Scholar] [CrossRef]
- Noguchi, K.; Nishimura, R.; Ikawa, Y.; Mase, S.; Matsuda, Y.; Fujiki, T.; Kuroda, R.; Araki, R.; Maeba, H.; Yachie, A. Half of “Micrococcus Spp.” Cases Identified by Conventional Methods Are Revealed as Other Life-Threatening Bacteria with Different Drug Susceptibility Patterns by 16S Ribosomal RNA Gene Sequencing. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2020, 26, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Schröttner, P.; Gunzer, F.; Schüppel, J.; Rudolph, W.W. Identification of Rare Bacterial Pathogens by 16S rRNA Gene Sequencing and MALDI-TOF MS. J. Vis. Exp. JoVE 2016, 113, e53176. [Google Scholar] [CrossRef]
- Seng, P.; Abat, C.; Rolain, J.M.; Colson, P.; Lagier, J.-C.; Gouriet, F.; Fournier, P.E.; Drancourt, M.; La Scola, B.; Raoult, D. Identification of Rare Pathogenic Bacteria in a Clinical Microbiology Laboratory: Impact of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2013, 51, 2182–2194. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.-M. Use of MALDI-TOF Mass Spectrometry for Identification of Bacteria That Are Difficult to Culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Tzoumas, A.; Sagris, M.; Xenos, D.; Ntoumaziou, A.; Kyriakoulis, I.; Kakargias, F.; Liaqat, W.; Nagraj, S.; Patel, R.; Korosoglou, G.; et al. Epidemiological Profile and Mortality of Infective Endocarditis Over the Past Decade: A Systematic Review and Meta-Analysis of 133 Studies. Am. J. Cardiol. 2025, 244, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Samonis, G.; Andrianaki, A.M.; Christofaki, M.; Dimopoulou, D.; Papadakis, J.; Gikas, A.; Kofteridis, D.P. Epidemiology, Microbiological and Clinical Features, Treatment, and Outcomes of Infective Endocarditis in Crete, Greece. Infect. Chemother. 2018, 50, 21–28. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- McCreery, R.J.; Florescu, D.F.; Kalil, A.C. Sepsis in Immunocompromised Patients Without Human Immunodeficiency Virus. J. Infect. Dis. 2020, 222, S156–S165. [Google Scholar] [CrossRef]
- O’Brien, S.N.; Blijlevens, N.M.A.; Mahfouz, T.H.; Anaissie, E.J. Infections in Patients with Hematological Cancer: Recent Developments. Hematology 2003, 2003, 438–472. [Google Scholar] [CrossRef]
- Chen, S.; Lin, K.; Li, Q.; Luo, X.; Xiao, M.; Chen, M.; Zhu, H.; Chen, Y.; Wu, X.; Zeng, Y.; et al. A Practical Update on the Epidemiology and Risk Factors for the Emergence and Mortality of Bloodstream Infections from Real-World Data of 3014 Hematological Malignancy Patients Receiving Chemotherapy. J. Cancer 2021, 12, 5494–5505. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, S.H.; Atkins, B.A.; Bejon, P.; Byren, I.; Wyllie, D.; Athanasou, N.A.; Berendt, A.R.; McNally, M.A. The Microbiology of Chronic Osteomyelitis: Prevalence of Resistance to Common Empirical Anti-Microbial Regimens. J. Infect. 2010, 60, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Tsagkaraki, E.; Athanasaki, A.; Tsioutis, C.; Gikas, A. Gram-Negative Bacteria as Emerging Pathogens Affecting Mortality in Skin and Soft Tissue Infections. Hippokratia 2018, 22, 23–28. [Google Scholar]
- Nessim, S.J.; Nisenbaum, R.; Bargman, J.M.; Jassal, S.V. Microbiology of Peritonitis in Peritoneal Dialysis Patients with Multiple Episodes. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2012, 32, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Huang, C.-H.; Kuo, M.-C.; Lin, S.-Y.; Hsu, C.-H.; Lee, C.-Y.; Chiu, Y.-W.; Chen, Y.-H.; Lu, P.-L. Microbiology of Peritoneal Dialysis-Related Infection and Factors of Refractory Peritoneal Dialysis Related Peritonitis: A Ten-Year Single-Center Study in Taiwan. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2019, 52, 752–759. [Google Scholar] [CrossRef]
- Gouveia E Melo, R.; Martins, B.; Pedro, D.M.; Santos, C.M.; Duarte, A.; Fernandes E Fernandes, R.; Garrido, P.; Mendes Pedro, L. Microbial Evolution of Vascular Graft Infections in a Tertiary Hospital Based on Positive Graft Cultures. J. Vasc. Surg. 2021, 74, 276–284.e4. [Google Scholar] [CrossRef]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Papaparaskevas, J.; Stathi, A.; Alexandrou-Athanassoulis, H.; Charisiadou, A.; Petropoulou, N.; Tsakris, A.; Valari, M. Pitted Keratolysis in an Adolescent, Diagnosed Using Conventional and Molecular Microbiology and Successfully Treated with Fusidic Acid. Eur. J. Dermatol. EJD 2014, 24, 499–500. [Google Scholar] [CrossRef]
- Alhadlaq, M.A.; Green, J.; Kudhair, B.K. Analysis of Kytococcus sedentarius Strain Isolated from a Dehumidifier Operating in a University Lecture Theatre: Systems for Aerobic Respiration, Resisting Osmotic Stress, and Sensing Nitric Oxide. Microb. Physiol. 2021, 31, 135–145. [Google Scholar] [CrossRef]
- Yin, X.; Hu, X.; Tong, H.; You, L. Trends in Mortality from Infection among Patients with Hematologic Malignancies: Differences According to Hematologic Malignancy Subtype. Ther. Adv. Chronic Dis. 2023, 14, 20406223231173891. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).