Vaginal Capsules: A Viable Alternative for the Delivery of Lactobacillus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Maintenance of Strains
2.2. Preparation of Formulas
2.3. Pharmaceutical Analysis
2.3.1. Average Weight Analysis
2.3.2. Disintegration Test
2.3.3. Organoleptic Analysis
2.4. Viability Testing of Strains of Lactobacillus spp.
2.5. Culturability Assay of Recovered Strains of Lactobacillus spp. from Vaginal Preparations
2.6. Data Analysis
3. Results
3.1. Pharmacotechnical Evaluation of Vaginal Formulations
3.1.1. Average Weight
3.1.2. Disintegration Tests
3.1.3. Organoleptic Evaluation of the Vaginal Ointment
3.2. Microbiological Evaluation of Vaginal Formulations
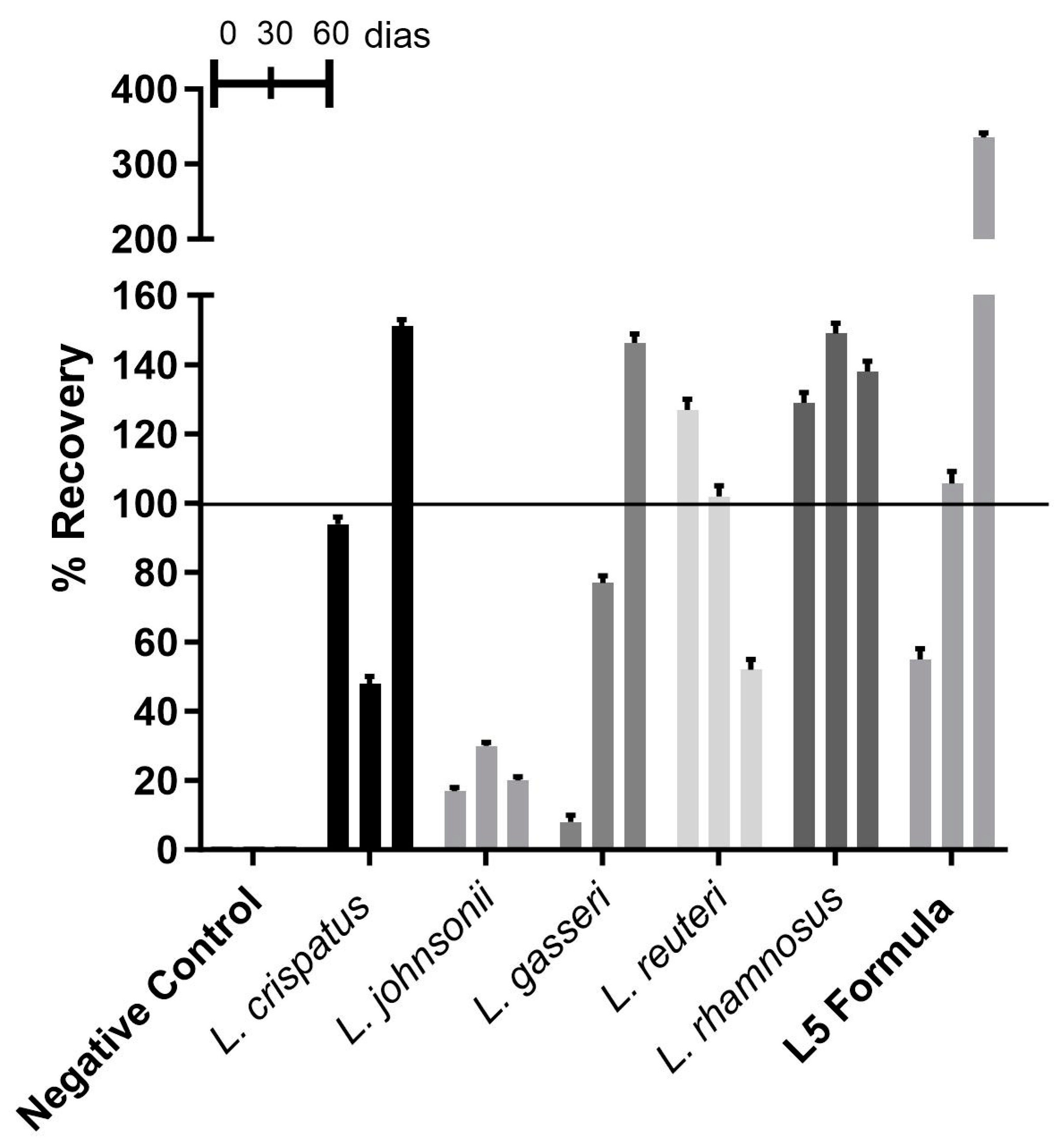
3.2.1. Recovery of Lactobacillus spp. from Vaginal Capsules
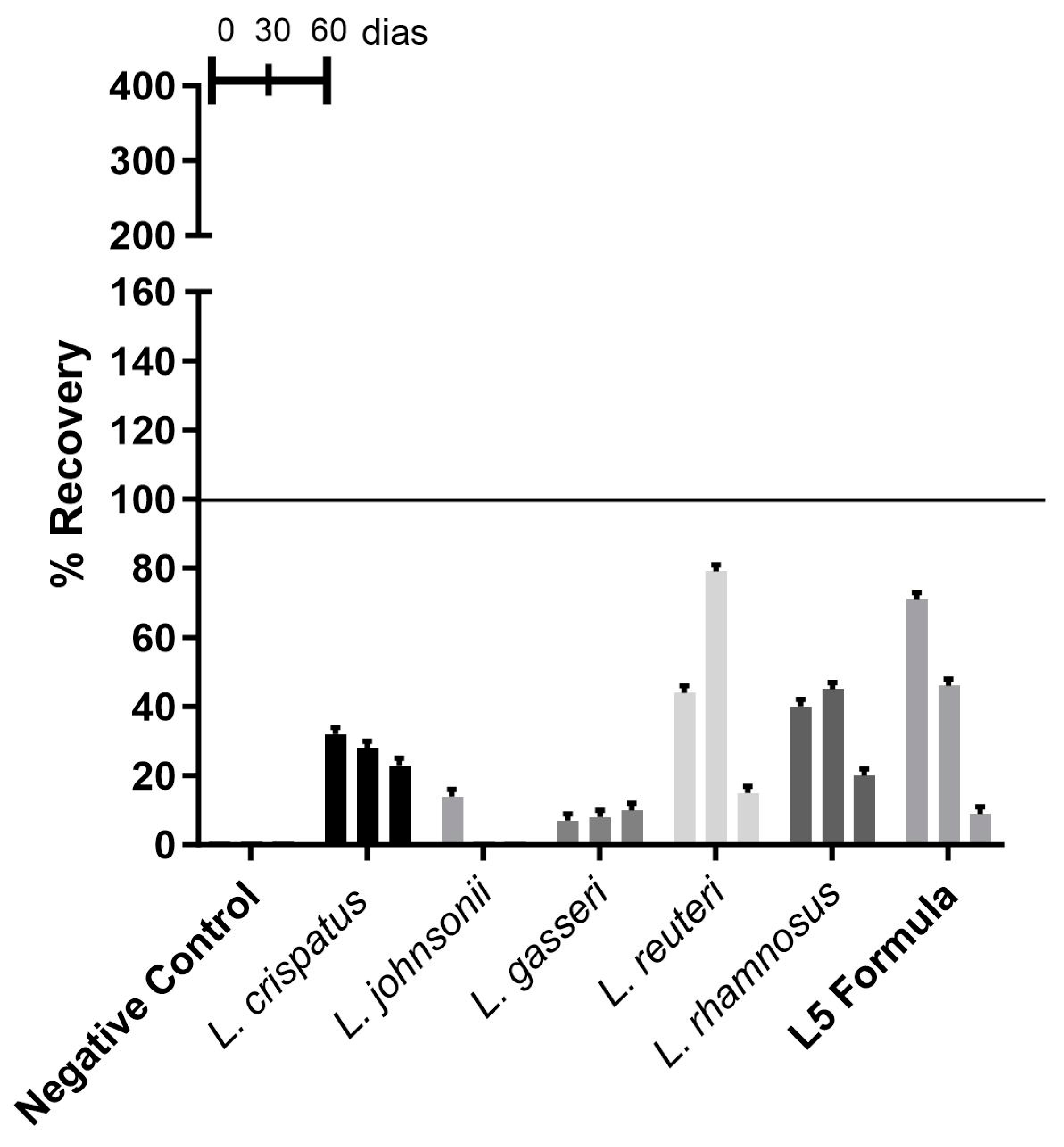
3.2.2. Recovery of Lactobacillus spp. from Vaginal Ointment
3.2.3. Recovery of Lactobacillus spp. from Waxy Ovules
3.2.4. Recovery of Lactobacillus spp. from Gelatinous Ovules
3.3. Potential Validity of the Dosage Forms Studied
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal Microbiome: Normalcy vs Dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus Iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Aguilera, M. Vaginal Probiotics for Reproductive Health and Related Dysbiosis: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1461. [Google Scholar] [CrossRef]
- Tomusiak, A.; Strus, M.; Heczko, P.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and Safety of a Vaginal Medicinal Product Containing Three Strains of Probiotic Bacteria: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Drug Des. Dev. Ther. 2015, 9, 5345. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial Vaginosis: A Review on Clinical Trials with Probiotics. New Microbiol. 2013, 36, 229–238. [Google Scholar] [CrossRef]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Chen, H.; Mei, L.; Wang, X.; Fang, F. Probiotics for Vulvovaginal Candidiasis in Non-Pregnant Women. Cochrane Database Syst. Rev. 2013, 2017, CD010496. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of Bacterial Viability: A Comprehensive Review on Recent Advances and Challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Camilletti, A.L.; Ruíz, F.O.; Pascual, L.M.; Barberis, I.L. First Steps towards the Pharmaceutical Development of Ovules Containing Lactobacillus Strains: Viability and Antimicrobial Activity as Basic First Parameters in Vaginal Formulations. AAPS PharmSciTech 2018, 19, 886–895. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Tomás, M.; Terraf, M.; Nader-Macías, M. In Vitro and In Vivo Effects of Beneficial Vaginal Lactobacilli on Pathogens Responsible for Urogenital Tract Infections. J. Med. Microbiol. 2014, 63, 685–696. [Google Scholar] [CrossRef]
- Falagas, M.E.; Betsi, G.I.; Athanasiou, S. Probiotics for Prevention of Recurrent Vulvovaginal Candidiasis: A Review. J. Antimicrob. Chemother. 2006, 58, 266–272. [Google Scholar] [CrossRef]
- Charoo, N.A. Critical Excipient Attributes Relevant to Solid Dosage Formulation Manufacturing. J. Pharm. Innov. 2020, 15, 163–181. [Google Scholar] [CrossRef]
- Pingitore, E.; Tomás, M.; Wiese, B.; Nader-Macías, M. Design of Novel Urogenital Pharmabiotic Formulations Containing Lactobacilli, Salivaricin CRL 1328 and Non-Microbial Compounds with Different Functionalities. Drug Dev. Ind. Pharm. 2015, 41, 942–952. [Google Scholar] [CrossRef]
- dos Santos Santiago, G.L.; Verstraelen, H.; Poelvoorde, N.; De Corte, S.; Claeys, G.; Trog, M.; De Backer, E.; Saerens, B.; Vervaet, C.; De Boeck, F.; et al. A Pilot Study Evaluating the Safety of Vaginal Administration of a Multi-Particulate Pellet Formulation. Eur. J. Pharm. Biopharm. 2009, 73, 399–403. [Google Scholar] [CrossRef]
- Afrassiabian, Z.; Guessasma, M.; Saleh, K. A Study on the Caking Behaviour of Binary Mixtures of Lactose Due to Solid-State Crystallisation of the Amorphous Phase. Chem. Eng. Res. Des. 2019, 147, 354–366. [Google Scholar] [CrossRef]
- Vaessen, E.M.J.; den Besten, H.M.W.; Esveld, E.D.C.; Schutyser, M.A.I. Accumulation of Intracellular Trehalose and Lactose in Lactobacillus Plantarum WCFS1 during Pulsed Electric Field Treatment and Subsequent Freeze and Spray Drying. LWT 2019, 115, 108478. [Google Scholar] [CrossRef]
- Draelos, Z.D. The Science behind Skin Care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Lamb, B.; Jafelice, S.; Reid, G. Topical Probiotics for Women’s Urogenital Health: Selection of an Oil-Based Carrier. OBM Integr. Complement. Med. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (Brasil); Coordenação da Farmacopeia. Formulário Nacional Da Farmacopeia Brasileira 2a Edição. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/formulario-nacional/arquivos/8065json-file-1 (accessed on 18 June 2022).
- Agência Nacional de Vigilância Sanitária—Anvisa. Farmacopeia Brasileira, 6a Edição. Volume I. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira (accessed on 18 June 2022).
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of Probiotics on the Recurrence of Bacterial Vaginosis. J. Low. Genit. Tract. Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Polova, Z.; Aleinyk, S.; Kazak, A. Formulation and Technology Development of Vaginal Pessaries with Probiotic Activity. Ceska Slov. Farm. 2020, 69, 90–99. [Google Scholar]
- Yang, S.; Reid, G.; Challis, J.R.G.; Gloor, G.B.; Asztalos, E.; Money, D.; Seney, S.; Bocking, A.D. Effect of Oral Probiotic Lactobacillus Rhamnosus GR-1 and Lactobacillus Reuteri RC-14 on the Vaginal Microbiota, Cytokines and Chemokines in Pregnant Women. Nutrients 2020, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Paniágua, A.L.; Correia, A.F.; Pereira, L.C.; de Alencar, B.M.; Silva, F.B.A.; Almeida, R.M.; de Medeiros Nóbrega, Y.K. Inhibitory Effects of Lactobacillus Casei Shirota against Both Candida Auris and Candida Spp. Isolates That Cause Vulvovaginal Candidiasis and Are Resistant to Antifungals. BMC Complement. Med. Ther. 2021, 21, 237. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/handle/10665/42682 (accessed on 18 June 2022).
- Georgieva, R.; Iliev, I.; Haertlé, T.; Chobert, J.-M.; Ivanova, I.; Danova, S. Technological Properties of Candidate Probiotic Lactobacillus Plantarum Strains. Int. Dairy J. 2009, 19, 696–702. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, Source, Extraction and Industrial Applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Silva, J.A.; Marchesi, A.; Wiese, B.; Nader-Macias, M.E.F. Screening of Autochthonous Vaginal Beneficial Lactobacilli Strains by Their Growth at High Temperatures for Technological Applications. Antonie van Leeuwenhoek 2020, 113, 1393–1409. [Google Scholar] [CrossRef]
- Kurtmann, L.; Carlsen, C.U.; Risbo, J.; Skibsted, L.H. Storage Stability of Freeze-Dried Lactobacillus Acidophilus (La-5) in Relation to Water Activity and Presence of Oxygen and Ascorbate. Cryobiology 2009, 58, 175–180. [Google Scholar] [CrossRef]
- De Gregorio, P.; Maldonado, N.C.; Pingitore, E.V.; Terraf, M.C.L.; Tomás, M.S.J.; de Ruiz, C.S.; Santos, V.; Wiese, B.; Bru, E.; Paiz, M.C.; et al. Intravaginal Administration of Gelatine Capsules Containing Freeze-Dried Autochthonous Lactobacilli: A Double-Blind, Randomised Clinical Trial of Safety. Benef. Microbes. 2020, 11, 5–17. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Li, C.; Shu, G. Response Surface Optimization of Lyoprotectant for Lactobacillus Bulgaricus During Vacuum Freeze-Drying. Prep. Biochem. Biotechnol. 2015, 45, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; de Paula, J.A.; et al. World Gastroenterology Organisation Global Guidelines. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef] [PubMed]

| Species | Strain | Lot | Distributor |
|---|---|---|---|
| Lactobacillus crispatus (synonymous with Lactobacillus acidophilus group A2 of Johnson) | LCr86 | Wk20200908004 | Active Pharmaceutica |
| Lactobacillus johnsonii (synonymous with L. acidophilus group B2 of Johnson) | LJ-G55-81 | IK2004 | Organic Compounding |
| Lactobacillus gasseri | LG08 | WK20201021001 | Active Pharmaceutica |
| Limosilactobacillus reuteri | LR-G100 | IL2301 | Lemma Solutions |
| Lacticaseibacillusrhamnosus | LRa05 | WK20200321012 | Active Pharmaceutica |
| Strains of Lactobacillus spp. | Quantity, in CFU, of Each Pharmaceutical Form | Composition and Excipients | |||
|---|---|---|---|---|---|
| Vaginal Capsule | Gelatinous Ovule | Waxy Ovule | Vaginal Ointment | ||
| L. crispatus | 109 CFU | 1 gelatinous capsule lactose s.q. 500 mg | 1 ovule s.q. 3 g (gelatin powder 14.3%, potassium sorbate 0.02%, purified water 43%, vegetable glycerin s.q. 100%) | 1 ovule s.q. 3g beeswax (Appis melífera linnaeus) 5.0% and cacao butter (Theobroma cacao 95.0%) | Petrolatum s.q. 1 g |
| L. gasseri | 109 CFU | ||||
| L. johnsonii | 109 CFU | ||||
| Limosilactobacillusreuteri | 109 CFU | ||||
| Lacticaseibacillusrhamnosus | 109 CFU | ||||
| L5 formula | 5 × 109 CFU | ||||
| Vaginal Capsules | Weight | Standard Deviation | Total Weight | Yield Percentage | Average Weight | Results | |
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | ||||||
| L. crispatus | 0.543 g | 0.508 g | 0.012 | 0.511 g | 103.3% | 0.528 g | As per |
| L. johnsonii | 0.528 g | 0.506 g | 0.007 | 0.528 g | 97.7% | 0.516 g | As per |
| L. gasseri | 0.529 g | 0.509 g | 0.006 | 0.519 g | 99.9% | 0.518 g | As per |
| Limosilactobacillusreuteri | 0.535 g | 0.508 g | 0.009 | 0.517 g | 100.0% | 0.517 g | As per |
| Lacticaseibacillusrhamnosus | 0.529 g | 0.515 g | 0.005 | 0.516 g | 101.4% | 0.523 g | As per |
| L5 formula | 0.549 g | 0.495 g | 0.016 | 0.549 g | 96.8% | 0.531 g | As per |
| Pharmaceutical Form | Average Disintegration Time at 37 °C | ||
|---|---|---|---|
| Gelatinous Ovules | Waxy Ovules | Vaginal Capsules | |
| Strains | Average Time (±SD) | ||
| L. crispatus | 8 ± 2.0 | 10.6 ± 0.6 | 4.3 ± 0.6 |
| L. johnsonii | 13.6 ± 2.9 | 11.6 ± 0.6 | 3.6 ± 0.6 |
| L. gasseri | 13 ± 1.7 | 9.6 ± 0.6 | 4.0 ± 1.0 |
| Limosilactobacillusreuteri | 23.3 ± 0.6 | 10.6 ± 0.6 | 4.6 ± 0.6 |
| Lacticaseibacillusrhamnosus | 23.3 ± 0.6 | 11.3 ± 1.0 | 3.3 ± 0.6 |
| L5 formula | 23.3 ± 1.2 | 11 ± 1.0 | 4.3 ± 0.6 |
| Mean | 17.5 ± 1.5 | 10.8 ± 0.7 | 4.1 ± 0.6 |
| Pharmaceutical Formula | Probiotic | CFU | Real-Time Validity |
|---|---|---|---|
| Vaginal capsules | L. crispatus | 1 × 108 CFU | 60 days |
| L. johnsonii | 1 × 108 CFU | 60 days | |
| L. gasseri | 1 × 107 CFU | 60 days | |
| Limosilactobacillusreuteri | 5 × 108 CFU | 60 days | |
| Lacticaseibacillusrhamnosus | 1 × 109 CFU | 60 days | |
| L5 formula | 5 × 108 CFU | 60 days | |
| Vaginal ointment | L. crispatus | 2 × 108 CFU | 60 days |
| L. johnsonii | 0 from 30 days | Infeasible | |
| L. gasseri | 1 × 108 CFU | 60 days | |
| Limosilactobacillusreuteri | 1 × 108 CFU | 60 days | |
| Lacticaseibacillusrhamnosus | 2 × 108 CFU | 60 days | |
| L5 formula | 1 × 108 CFU | 60 days | |
| Waxy ovule | L. crispatus | 1 × 107 CFU | 60 days |
| L. johnsonii | 2 × 108 CFU | 60 days | |
| L. gasseri | 2 × 108 CFU | 60 days | |
| Limosilactobacillusreuteri | 4 × 108 CFU | 60 days | |
| Lacticaseibacillusrhamnosus | 3 × 108 CFU | 60 days | |
| L5 formula | 3 × 108 CFU | 60 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.S.d.; Pereira, L.C.; Almeida, R.M.; Nóbrega, Y.K.d.M. Vaginal Capsules: A Viable Alternative for the Delivery of Lactobacillus spp. Microorganisms 2025, 13, 1056. https://doi.org/10.3390/microorganisms13051056
Lima LSd, Pereira LC, Almeida RM, Nóbrega YKdM. Vaginal Capsules: A Viable Alternative for the Delivery of Lactobacillus spp. Microorganisms. 2025; 13(5):1056. https://doi.org/10.3390/microorganisms13051056
Chicago/Turabian StyleLima, Leandra Sá de, Lívia Custódio Pereira, Rosane Mansan Almeida, and Yanna Karla de Medeiros Nóbrega. 2025. "Vaginal Capsules: A Viable Alternative for the Delivery of Lactobacillus spp." Microorganisms 13, no. 5: 1056. https://doi.org/10.3390/microorganisms13051056
APA StyleLima, L. S. d., Pereira, L. C., Almeida, R. M., & Nóbrega, Y. K. d. M. (2025). Vaginal Capsules: A Viable Alternative for the Delivery of Lactobacillus spp. Microorganisms, 13(5), 1056. https://doi.org/10.3390/microorganisms13051056






