Molecular Typing of Tick-Borne Pathogens in Ixodids of Bosnia and Herzegovina
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection Sites
2.2. Nucleic Acid Extraction
2.3. Morphological and Molecular Tick Identification
2.4. DNA-Based Pathogen Detection
2.5. RNA-Based Pathogen Detection
2.6. Borrelia Burgdorferi Sensu Lato Species Discrimination by Reverse Line Blotting (RLB)
2.7. Statistical Analysis
3. Results
3.1. Tick Collection
3.2. Tick Barcoding
3.3. DNA-Based Pathogen Screening
3.4. Detected Pathogens by Location
3.5. Pathogen Typing
4. Discussion
4.1. Pathogens in Questing Ticks
4.2. Co-Infections
4.3. Pathogen Absence
4.4. Sympatric Occurrence of Dermacentor spp.
4.5. Differences in Pathogen Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, 3–14. [Google Scholar] [CrossRef]
- Gray, J.S. The ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 1998, 22, 249–258. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; De La Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. Forecasting habitat suitability for ticks and prevention of tick-borne diseases. Vet. Parasitol. 2001, 98, 111–132. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Farkas, R.; Jaenson, T.G.T.; Koenen, F.; Madder, M.; Pascucci, I.; Salman, M.; Tarrés-Call, J.; Jongejan, F. Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the western Palearctic. A digital data set. Exp. Appl. Acarol. 2012, 59, 351. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.-K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Ticks as vectors: Taxonomy, biology and ecology. Rev. Sci. Tech. 2015, 34, 53–65. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M. (Eds.) Biology of Ticks; Oxford University Press: Oxford, UK, 2014; Volume 2, ISBN 9780199744053. [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L.; Rieß, R.; Kahl, O.; Wölfel, S.; Dobler, G.; Nava, S.; Estrada-Peña, A. Ixodes inopinatus—Occurring also outside the Mediterranean region. Ticks Tick-Borne Dis. 2018, 9, 196–200. [Google Scholar] [CrossRef]
- Zając, Z.; Bartosik, K.; Woźniak, A. Monitoring Dermacentor reticulatus Host-Seeking Activity in Natural Conditions. Insects 2020, 11, 264. [Google Scholar] [CrossRef]
- Bilbija, B.; Spitzweg, C.; Papoušek, I.; Fritz, U.; Földvári, G.; Mullett, M.; Ihlow, F.; Sprong, H.; Civáňová Křížová, K.; Anisimov, N.; et al. Dermacentor reticulatus—A tick on its way from glacial refugia to a panmictic Eurasian population. Int. J. Parasitol. 2023, 53, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick-Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex-clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Hofmann, H.; Fingerle, V.; Huppertz, H.I.; Hunfeld, K.P.; Krause, A.; Ruf, B.; Dersch, R. Guidelines for diagnosis and treatment in neurology—Lyme neuroborreliosis. Ger. Med. Sci. 2020, 18, Doc03. [Google Scholar] [CrossRef]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef]
- Fingerle, V.; Schulte-Spechtel, U.C.; Ruzic-Sabljic, E.; Leonhard, S.; Hofmann, H.; Weber, K.; Pfister, K.; Strle, F.; Wilske, B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 2008, 298, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Slovák, M.; Drexler, J.F.; Klempa, B. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick-Borne Dis. 2020, 11, 101414. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L. TBEV-transmission and natural cycles. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Chitimia-Dobler, L., Schmitt, H.J., Eds.; Global Health Press: Singapore, 2024; Chapter 5. [Google Scholar]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in Tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Jado, I.; Oteo, J.A.; Aldámiz, M.; Gil, H.; Escudero, R.; Ibarra, V.; Portu, J.; Portillo, A.; Lezaun, M.J.; García-Amil, C.; et al. Rickettsia monacensis and Human Disease, Spain. Emerg. Infect. Dis. 2007, 13, 1405–1407. [Google Scholar] [CrossRef]
- de Sousa, R.; Pereira, B.I.; Nazareth, C.; Cabral, S.; Ventura, C.; Crespo, P.; Marques, N.; da Cunha, S. Rickettsia slovaca Infection in Humans, Portugal. Emerg. Infect. Dis. 2013, 19, 1627. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Parola, P.; Fournier, P.E.; Raoult, D. Spotted fever rickettsioses in southern and eastern Europe. FEMS Immunol. Med. Microbiol. 2007, 49, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef]
- Kapo, N.; Zuber Bogdanović, I.; Gagović, E.; Žekić, M.; Veinović, G.; Sukara, R.; Mihaljica, D.; Adžić, B.; Kadriaj, P.; Cvetkovikj, A.; et al. Ixodid ticks and zoonotic tick-borne pathogens of the Western Balkans. Parasit. Vectors 2024, 17, 45. [Google Scholar] [CrossRef]
- Omeragić, J.; Šerić-Haračić, S.; Klarić Soldo, D.; Kapo, N.; Fejzić, N.; Škapur, V.; Medlock, J. Distribution of ticks in Bosnia and Herzegovina. Ticks Tick-Borne Dis. 2022, 13, 101870. [Google Scholar] [CrossRef]
- Omeragić, J.; Kapo, N.; Goletić, Š.; Softić, A.; Terzić, I.; Šabić, E.; Škapur, V.; Klarić Soldo, D.; Goletić, T. Investigation of Tick-Borne Pathogens in Ixodes Ticks from Bosnia and Herzegovina. Animals 2024, 14, 2190. [Google Scholar] [CrossRef]
- Goletić, T.; Klarić Soldo, D.; Kapo, N.; Goletić, Š.; Koro-Spahić, A.; Alispahić, A.; Softić, A.; Škapur, V.; Omeragić, J. Tick-Borne Pathogens in Dermacentor reticulatus Ticks from Bosnia and Herzegovina. Pathogens 2024, 13, 421. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Berlin, Germany, 2018. [Google Scholar]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Nicholas, K.B. Genedoc: A Tool for Editing and Annoting Multiple Sequence Alignments. 1997. Available online: https://nrbsc.org/gfx/genedoc/ (accessed on 5 January 2025).
- Schouls, L.M.; Van De Pol, I.; Rijpkema, S.G.T.; Schot, C.S. Detection and Identification of Ehrlichia, Borrelia burgdorferi Sensu Lato, and Bartonella Species in Dutch Ixodes ricinus Ticks. J. Clin. Microbiol. 1999, 37, 2215–2222. [Google Scholar] [CrossRef]
- Bekker, C.P.; de Vos, S.; Taoufik, A.; Sparagano, O.A.; Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002, 89, 223–238. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Han, R.; Liu, G.; Shi, Y.; Luo, J.; Yin, H. Molecular survey and characterization of a novel Anaplasma species closely related to Anaplasma capra in ticks, northwestern China. Parasit. Vectors 2016, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, S.; Wang, K.; Song, J.; Yan, Y.; Zhou, Y.; Shi, K.; Jian, F.; Wang, R.; Zhang, L.; et al. A Multiplex PCR Detection Assay for the Identification of Clinically Relevant Anaplasma Species in Field Blood Samples. Front. Microbiol. 2020, 11, 511546. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Agnone, A.; Blanda, V.; Alongi, A.; D’Agostino, R.; Caracappa, S.; Marino, A.M.F.; Di Marco, V.; de la Fuente, J. Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks Tick-Borne Dis. 2012, 3, 283–287. [Google Scholar] [CrossRef]
- Liebisch, G.; Sohns, B.; Bautsch, W. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J. Clin. Microbiol. 1998, 36, 3355–3358. [Google Scholar] [CrossRef]
- Margos, G.; Gatewood, A.G.; Aanensen, D.M.; Hanincová, K.; Terekhova, D.; Vollmer, S.A.; Cornet, M.; Piesman, J.; Donaghy, M.; Bormane, A.; et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2008, 105, 8730–8735. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, A.; Eriksson, U.; Berglund, L.; Tärnvik, A. Detection of Francisella tularensis in ulcers of patients with tularemia by PCR. J. Clin. Microbiol. 1997, 35, 1045–1048. [Google Scholar] [CrossRef]
- Bonnet, S.; Jouglin, M.; L’Hostis, M.; Chauvin, A. Babesia sp. EU1 from Roe Deer and Transmission within Ixodes ricinus. Emerg. Infect. Dis. 2007, 13, 1208. [Google Scholar] [CrossRef]
- Zintl, A.; Finnerty, E.J.; Murphy, T.M.; de Waal, T.; Gray, J.S. Babesias of red deer (Cervus elaphus) in Ireland. Vet. Res. 2011, 42, 7. [Google Scholar] [CrossRef]
- Vitorino, L.; Zé-Zé, L.; Sousa, A.; Bacellar, F.; Tenreiro, R. rRNA Intergenic Spacer Regions for Phylogenetic Analysis of Rickettsia Species. Ann. N. Y. Acad. Sci. 2003, 990, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Moureau, G.; Temmam, S.; Gonzalez, J.P.; Charrel, R.N.; Grard, G.; De Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector-Borne Zoonotic Dis. 2007, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Schötta, A.-M.; Wijnveld, M.; Stockinger, H.; Stanek, G. Approaches for Reverse Line Blot-Based Detection of Microbial Pathogens in Ixodes ricinus Ticks Collected in Austria and Impact of the Chosen Method. Appl. Environ. Microbiol. 2017, 83, e00489-17. [Google Scholar] [CrossRef] [PubMed]
- Wijnveld, M.; Schötta, A.-M.; Pintér, A.; Stockinger, H.; Stanek, G. Novel Rickettsia raoultii strain isolated and propagated from Austrian Dermacentor reticulatus ticks. Parasit. Vectors 2016, 9, 567. [Google Scholar] [CrossRef]
- Wijnveld, M.; Schötta, A.M.; Stelzer, T.; Duscher, G.; Leschnik, M.; Stockinger, H.; Lindgren, P.E.; Stanek, G. Novel Protozoans in Austria Revealed through the Use of Dogs as Sentinels for Ticks and Tick-Borne Pathogens. Microorganisms 2021, 9, 1392. [Google Scholar] [CrossRef]
- Gubbels, J.M.; De Vos, A.P.; Van Der Weide, M.; Viseras, J.; Schouls, L.M.; De Vries, E.; Jongejan, F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999, 37, 1782–1789. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 5 January 2025).
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef]
- Hubálek, Z. Epidemiology of Lyme Borreliosis. In Lyme Borreliosis; Lipsker, D., Jaulhac, B., Eds.; Karger: Basel, Switzerland, 2009; Volume 37, pp. 31–50. [Google Scholar]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef]
- Lasić, L.; Ušanović, L.; Ćakić, S.; Hanjalić, J.; Stroil, B.K. First molecular detection of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from humans in the Sarajevo Canton (Bosnia and Herzegovina). Syst. Appl. Acarol. 2020, 25, 169–172. [Google Scholar] [CrossRef]
- World Health Organization. The Institute of Public Health of the Federation of Bosnia and Herzegovina. 2025. Available online: https://www.phi.rs.ba/ (accessed on 19 February 2025).
- Hodžić, A.; Fuehrer, H.P.; Duscher, G.G. First Molecular Evidence of Zoonotic Bacteria in Ticks in Bosnia and Herzegovina. Transbound. Emerg. Dis. 2017, 64, 1313–1316. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Foucault-Simonin, A.; Mateos-Hernandez, L.; Wu-Chuang, A.; Galon, C.; Simin, V.; Mijatović, D.; Bogdan, I.; Corona-González, B.; et al. Emerging tick-borne spotted fever group rickettsioses in the Balkans. Infect. Genet. Evol. 2023, 107, 105400. [Google Scholar] [CrossRef] [PubMed]
- Boretti, F.S.; Perreten, A.; Meli, M.L.; Cattori, V.; Willi, B.; Wengi, N.; Hornok, S.; Honegger, H.; Hegglin, D.; Woelfel, R.; et al. Molecular Investigations of Rickettsia helvetica Infection in Dogs, Foxes, Humans, and Ixodes Ticks. Appl. Environ. Microbiol. 2009, 75, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Davoust, B.; Raoult, D. Tick- and flea-borne rickettsial emerging zoonoses. Vet. Res. 2005, 36, 469–492. [Google Scholar] [CrossRef]
- Walker, D.H. Rickettsiae and Rickettsial Infections: The Current State of Knowledge. Clin. Infect. Dis. 2007, 45, S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Biernat, B.; Stańczak, J.; Michalik, J.; Sikora, B.; Cieniuch, S. Rickettsia helvetica and R. monacensis infections in immature Ixodes ricinus ticks derived from sylvatic passerine birds in west-central Poland. Parasitol. Res. 2016, 115, 3469–3477. [Google Scholar] [CrossRef]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Maksimović, Z.; Dervišević, M.; Zahirović, A.; Rifatbegović, M. Seroprevalence of Anaplasma spp. and Ehrlichia spp. and molecular detection of Anaplasma phagocytophilum and Anaplasma platys in stray dogs in Bosnia and Herzegovina. Ticks Tick. Borne. Dis. 2022, 13, 101875. [Google Scholar] [CrossRef]
- Jahfari, S.; Fonville, M.; Hengeveld, P.; Reusken, C.; Scholte, E.-J.; Takken, W.; Heyman, P.; Medlock, J.M.; Heylen, D.; Kleve, J.; et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasit. Vectors 2012, 5, 74. [Google Scholar] [CrossRef]
- Plantard, O.; Bouju-Albert, A.; Malard, M.A.; Hermouet, A.; Capron, G.; Verheyden, H. Detection of Wolbachia in the Tick Ixodes ricinus is Due to the Presence of the Hymenoptera Endoparasitoid Ixodiphagus hookeri. PLoS One 2012, 7, e30692. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 271096. [Google Scholar] [CrossRef]
- Grunwaldt, E.; Barbour, A.; Benach, J. High Potassium in Low-Sodium Soups. N. Engl. J. Med. 1983, 308, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Raileanu, C.; Moutailler, S.; Pavel, I.; Porea, D.; Mihalca, A.D.; Savuta, G.; Vayssier-Taussat, M. Borrelia Diversity and Co-infection with Other Tick Borne Pathogens in Ticks. Front. Cell. Infect. Microbiol. 2017, 7, 244580. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, I.; Xhekaj, B.; Halimi, G.; Wijnveld, M.; Ruivo, M.; Çaushi, D.; Matoshi, A.; Obwaller, A.G.; Jäger, B.; Weiler, M.; et al. Zoonotic Tick-Borne Pathogens in Ixodes ricinus Complex (Acari: Ixodidae) From Urban and Peri-Urban Areas of Kosovo. Zoonoses Public Health 2025, 72, 174–183. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Hodžić, A.; Georges, I.; Postl, M.; Duscher, G.G.; Jeschke, D.; Szentiks, C.A.; Ansorge, H.; Heddergott, M. Molecular survey of tick-borne pathogens reveals a high prevalence and low genetic variability of Hepatozoon canis in free-ranging grey wolves (Canis lupus) in Germany. Ticks Tick. Borne. Dis. 2020, 11, 101389. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antiviral Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick. Borne. Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The Spatial Distribution of Dermacentor Ticks (Ixodidae) in Germany—Evidence of a Continuing Spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 578220. [Google Scholar] [CrossRef]
- Halos, L.; Bord, S.; Cotté, V.; Gasqui, P.; Abrial, D.; Barnouin, J.; Boulouis, H.-J.; Vayssier-Taussat, M.; Vourc’h, G. Ecological Factors Characterizing the Prevalence of Bacterial Tick-Borne Pathogens in Ixodes ricinus Ticks in Pastures and Woodlands. Appl. Environ. Microbiol. 2010, 76, 4413–4420. [Google Scholar] [CrossRef]
- Shaw, G.; Lilly, M.; Mai, V.; Clark, J.; Summers, S.; Slater, K.; Karpathy, S.; Nakano, A.; Crews, A.; Lawrence, A.; et al. The roles of habitat isolation, landscape connectivity and host community in tick-borne pathogen ecology. R. Soc. Open Sci. 2024, 11, 240837. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Gray, J.S.; Kahl, O.; Lane, R.S.; Nijhof, A.M. Research on the ecology of ticks and tick-borne pathogens—Methodological principles and caveats. Front. Cell. Infect. Microbiol. 2013, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; Lasters, R.; Adriaensen, F.; Fonville, M.; Sprong, H.; Matthysen, E. Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci. Total Environ. 2019, 670, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Evidence that climate change has caused ‘emergence’ of tick-borne diseases in Europe? Int. J. Med. Microbiol. Suppl. 2004, 293, 5–15. [Google Scholar] [CrossRef]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
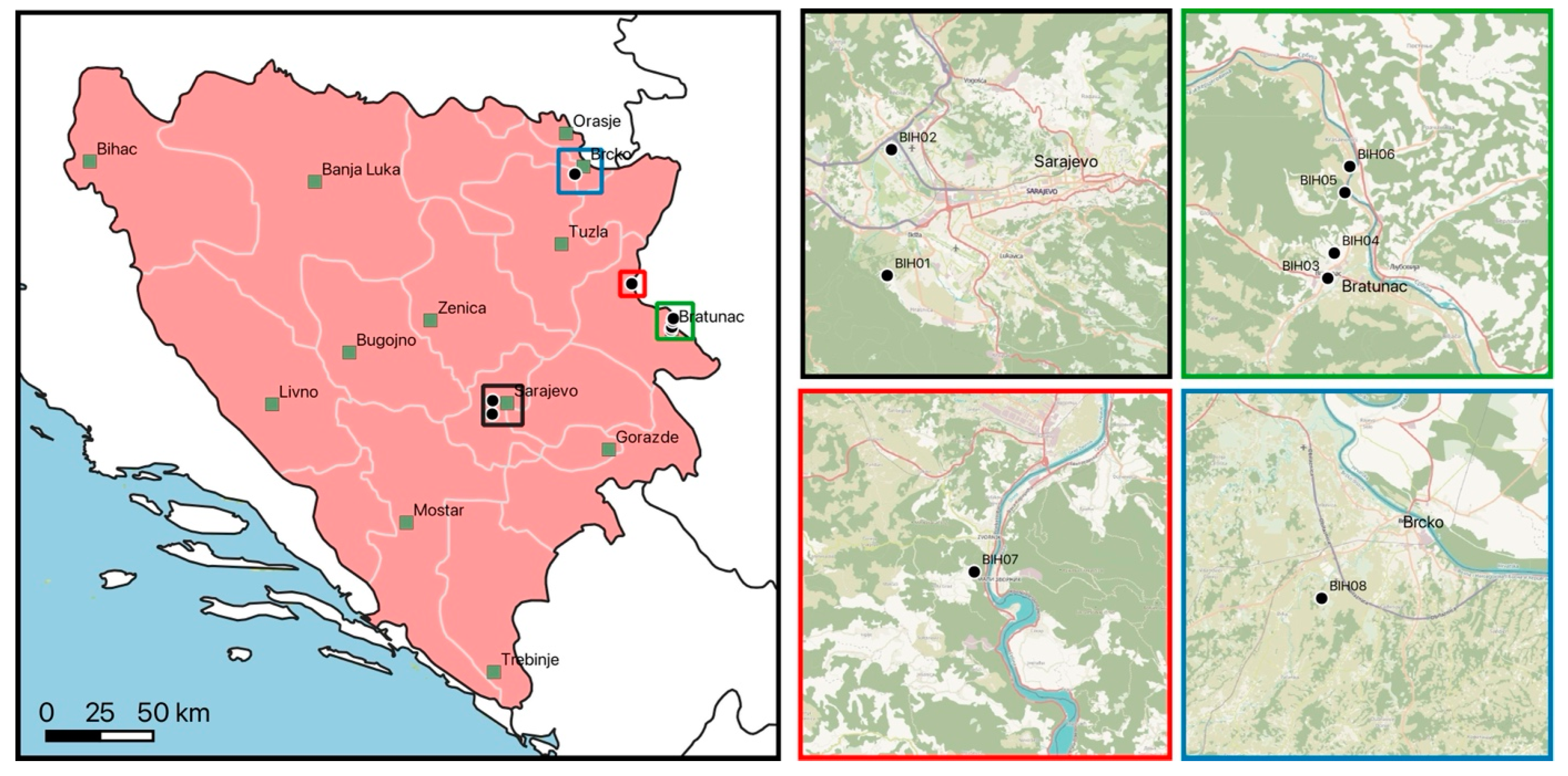

| Location | Site | Type | Attributes | Collection Method |
|---|---|---|---|---|
| BIH1 | public | peri-urban | large public recreational park with large meadows and forest close by, sheep close by | flagging, from host |
| BIH2 | agricultural | rural | surroundings of agricultural area with high grass and bushes | flagging |
| BIH3 | private | peri-urban | private property with uncut grass in small garden | flagging |
| BIH4 | private | peri-urban | private property with large meadows and forest close by | flagging |
| BIH5 | private | peri-urban | private property with large meadows and forest close by | flagging |
| BIH6 | public | rural | riverbank along Drina, regular floodings | flagging |
| BIH7 | public | rural | uphill forest around Zvornik castle | flagging |
| BIH8 | private | peri-urban | private property with large meadows and forest close by | flagging |
| Pathogen, Target Gene (Length) | Primer Sequence (5′-3′) | PCR Protocol | References |
|---|---|---|---|
| Anaplasmataceae 16S rRNA gene (345 bp) | EHR16SD-for: GGTACCYACAGAAGAAGTCC EHR16SR-rev: TAGCACTCATCGTTTACAGC | 95 °C/2 min; 35×: 94 °C/1 min, 54 °C/30 s, 72 °C/30 s; 72 °C 5 min | [35,36] |
| Anaplasma spp. typing A. capra groEL gene (874 bp) | groEL for: TGAAGAGCATCAAACCCGAAG groEL rev: CTGCTCGATGCTATCGG | 94 °C/5 min; 35×: 94 °C/30 s, 63 °C/30 s, 72 °C/1 min; 72 °C 10 min | [37] |
| A. bovis groEL gene (529 bp) | groEL for: GTGGGATGTACTGCTGACC groEL rev: ATGGGGAGATATCCGCGA | 94 °C/5 min; 35×: 94 °C/30 s, 63 °C/30 s, 72 °C/1 min; 72 °C 10 min | [38] |
| A. ovis msp4 gene (347 bp) | msp4 for: TGAAGGGAGCGGGTCATGGG msp4 rev: GAGTAATTGCAGCCAGGCACTCT | 94 °C/5 min; 35×: 94 °C/30 s, 63 °C/30 s, 72 °C/1 min; 72 °C 10 min | [39] |
| A. phagocytophilum 16S rRNA gene (172 bp) | 16S-for: AGTGCTGAATGTGGGGATAATTTATCTCCGTG 16S-rev: CTAATCTCCATGTCAAGAGTGGTAAGGTTT | 94 °C/5 min; 35×: 94 °C/30 s, 63 °C/30 s, 72 °C/1 min; 72 °C 10 min | [38] |
| Borrelia spp. 16S rRNA gene (674 bp) | BORR_ALLG_for: ACGCTGGCAGTGCGTCTT BORR_ALLG_rev: CTGATATCAACAGATTCC | 94 °C/5 min; 40×: 94 °C/1.5 min, 63 °C/2 min, 72 °C/2 min; 72 °C 10 min | [40] |
| B. garinii/ B. bavariensis typing * clpA gene (849 bp) clpA gene (706 bp) | clpAF1237: AAAGATAGATTTCTTCCAGAC clpAR2218: GAATTTCATCTATTAAAAGCTTTC clpAF1255: GACAAAGCTTTTGATATTTTAG clpAR2104: CAAAAAAAACATCAAATTTTCTATCTC | 98 °C/1 min; 10×: 98 °C/5 s, (60–1 °C/cycle)/5 s, 72 °C/15 s; 40×: 98 °C/5 s, 50 °C/5 s, 72 °C/15 s; 72 °C 1 min 98 °C/1 min; 10×: 98 °C/5 s, (60 –1 °C/cycle)/5 s, 72 °C/15 s; 45×: 98 °C/5 s, 50 °C/5 s, 72 °C/15 s; 72 °C 1 min | [41] |
| Francisella 16S rRNA gene (400 bp) | TUL4-435: GCTGTATCATCATTTAATAAACTGCTG TUL4-863: TTGGGAAGCTTGTATCATGGCACT | 94 °C/5 min; 40×: 94 °C/1 min, 54 °C/1 min, 72 °C/1 min; 72 °C/10 min | [42] |
| Piroplasmida * 18S rRNA gene (700 bp) 18S rRNA gene (561 bp) | BTH-1F: CCTGAGAAACGGCTACCACATCT BTH-1R: TTGCGACCATACTCCCCCCA GF2: GYYTTGTAATTGGAATGATGG GR2: CCAAAGACTTTGATTTCTCTC | 94 °C/2 min; 40×: 95 °C/30 s, 68 °C/1 min, 2 °C/1 min; 72 °C 10 min 94 °C/2 min; 40×: 95 °C/30 s, 60 °C/1 min, 72 °C/1 min; 72 °C 10 min | [43,44] |
| Rickettsia 23S/5S rRNA gene (350–550 bp) | ITS_F: GATAGGTCGGGTGTGGAAG IST_R: TCGGGATGGGATCGTGTG | 96 °C/4 min; 35×: 94 °C/1 min, 52 °C/1 min, 72 °C/2 min; 72 °C 3 min | [45] |
| Nymph | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| Questing | Host | Questing | Host a | Engorged b | Questing | Host | |
| I. ricinus | 211 | - | 152 | - | 16 | 132 | - |
| D. marginatus | - | - | 7 | - | 17 | 2 | 16 |
| D. reticulatus | - | - | 3 | - | - | - | - |
| Total | 211 | 0 | 162 | 0 | 33 | 134 | 16 |
| BIH1 | BIH2 | BIH3 | BIH4 | BIH5 | BIH6 | BIH7 | BIH8 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| I. ricinus | 72 | - | 5 | 196 | 148 | 3 | 46 | 41 | 511 |
| D. marginatus | 34 | 6 | - | - | - | - | - | 2 | 42 |
| D. reticulatus | - | 3 | - | - | - | - | - | - | 3 |
| Total | 106 | 9 | 5 | 196 | 148 | 3 | 46 | 43 | 556 |
| Species | Barcodes | Haplotypes | Accession Numbers | BLAST Identity |
|---|---|---|---|---|
| I. ricinus | 23 | 12 | PV203446 to PV203468 | 99.75% (MK671578) to 100% (KY039161) |
| D. marginatus | 34 | 8 | PV203469 to PV203502 | 99.52% (OM368304) to 100% (MT229170) |
| D. reticulatus | 3 | 1 | PV203503 to PV203505 | 100% (OR936112) |
| Status | Negative | Positive | Single | Double | Triple |
|---|---|---|---|---|---|
| questing (507) | 395 (77.9%) | 112 (22.1%) | 105 (20.6%) | 11 (2.2%) | 5 (1.0%) |
| unfed from host (16) | 7 (43.8%) | 9 (56.3%) | 7 (43.8%) | 2 (12.5%) | - |
| engorged (33) | 33 (87.9%) | 4 (12.1%) | 4 (12.1%) | - | - |
| Pathogen | Nymphs (n = 211) a | Female (n = 152) a | Male (n = 132) a | Total (n = 495) b |
|---|---|---|---|---|
| Single infection | ||||
| A. phagocytophilum | 3 (1.4%) | 8 (5.3%) | 4 (3.0%) | 15 (3.0%) |
| B. burgdorferi s.l. c | 1 (0.5%) | - | - | 1 (0.2%) |
| B. afzelii | 2 (1.0%) | 5 (3.3%) | 3 (2.3%) | 10 (2.0%) |
| B. burgdorferi s.s. | 8 (3.8%) | 4 (2.6%) | 2 (1.5%) | 14 (2.8%) |
| B. garinii | 1 (0.5%) | - | 1 (0.8%) | 2 (0.4%) |
| B. lusitaniae | 2 (1.0%) | 9 (5.9%) | 7 (5.3%) | 18 (3.6%) |
| B. spielmanii | - | - | 2 (1.5%) | 2 (0.4%) |
| B. valaisiana | 2 (1.0%) | 1 (0.7%) | 2 (1.5%) | 5 (1.0%) |
| B. miyamotoi | 2 (1.0%) | - | - | 2 (0.4%) |
| N. mikurensis | 1 (0.5%) | - | - | 1 (0.2%) |
| R. helvetica | 11 (5.2%) | 17 (11.2%) | 14 (10.6%) | 42 (8.5%) |
| R. monacensis | 7 (3.3%) | 3 (2.0%) | 6 (4.6%) | 16 (3.2%) |
| Double infection | ||||
| B. burgdorferi s.s. + B. valaisiana | 1 (0.5%) | - | - | 1 (0.2%) |
| B. burgdorferi s.s. + B. afzelii | - | 1 (0.7%) | - | 1 (0.2%) |
| B. burgdorferi s.s. + B. lusitaniae | - | 1 (0.7%) | 1 (0.8%) | 2 (0.4%) |
| B. afzelii + B. lusitaniae | - | 2 (1.3%) | - | 2 (0.4%) |
| B. lusitaniae + B. valaisiana | - | - | 1 (0.8%) | 1 (0.2%) |
| B. burgdorferi s.s. + N. mikurensis | 1 (0.5%) | - | - | 1 (0.2%) |
| B. lusitaniae + R. helvetica | - | 1 (0.7%) | 2 (1.5%) | 3 (0.6%) |
| Triple infection | ||||
| B. burgdorferi s.s. + B. afzeli + B. lusitaniae | 2 (1.0%) | - | - | 2 (0.4%) |
| B. afzeli + B. valaisiana + B. spielmanii | - | - | 1 (0.8%) | 1 (0.2%) |
| R. monacensis + B. burgdorferi s.s. + B. lusitaniae | - | - | 1 (0.8%) | 1 (0.2%) |
| A. phagocytophilum + B. burgdorferi s.s. + B. lusitaniae | - | 1 (0.7%) | - | 1 (0.2%) |
| Pathogen | Sequences | Haplotypes | Accession Numbers | BLAST Identity |
|---|---|---|---|---|
| A. phagocythophilum | 14 | 1 | PV203568 to PV203581 | 100% (MW922753, HG916766, OL690564) |
| A. ovis a | 5 | 1 | PV203582 to PV203586 | 100% (PQ616034, MH908943) |
| B. myamotoi | 1 | 1 | PV203587 | 100% (KJ847049, KF422749). |
| N. mikurensis | 1 | 1 | PV203588 | 100% (OP269946, OP269947) |
| R. monacensis | 6 | 2 | PV231331 to PV231336 | 99.71% to 100% (JQ796867, LN794217) |
| R. helvetica | 4 | 2 | PV231337 to PV231340 | 99.8% to 100% (JQ796866, EU057990) |
| R. raoultii | 5 | 1 | PV231341 to PV231345 | 100% (CP010969) |
| R. slovaca | 3 | 1 | PV231346 to PV231348 | 100% (MN581971, AY125009) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoxha, I.; Dervović, J.; Ruivo, M.; Wijnveld, M.; Obwaller, A.G.; Jäger, B.; Weiler, M.; Walochnik, J.; Kniha, E.; Alić, A. Molecular Typing of Tick-Borne Pathogens in Ixodids of Bosnia and Herzegovina. Microorganisms 2025, 13, 1054. https://doi.org/10.3390/microorganisms13051054
Hoxha I, Dervović J, Ruivo M, Wijnveld M, Obwaller AG, Jäger B, Weiler M, Walochnik J, Kniha E, Alić A. Molecular Typing of Tick-Borne Pathogens in Ixodids of Bosnia and Herzegovina. Microorganisms. 2025; 13(5):1054. https://doi.org/10.3390/microorganisms13051054
Chicago/Turabian StyleHoxha, Ina, Jovana Dervović, Margarida Ruivo, Michiel Wijnveld, Adelheid G. Obwaller, Bernhard Jäger, Martin Weiler, Julia Walochnik, Edwin Kniha, and Amer Alić. 2025. "Molecular Typing of Tick-Borne Pathogens in Ixodids of Bosnia and Herzegovina" Microorganisms 13, no. 5: 1054. https://doi.org/10.3390/microorganisms13051054
APA StyleHoxha, I., Dervović, J., Ruivo, M., Wijnveld, M., Obwaller, A. G., Jäger, B., Weiler, M., Walochnik, J., Kniha, E., & Alić, A. (2025). Molecular Typing of Tick-Borne Pathogens in Ixodids of Bosnia and Herzegovina. Microorganisms, 13(5), 1054. https://doi.org/10.3390/microorganisms13051054







