Mechanistic Study on the Alleviation of Endometritis in Mice Through Inhibition of NF-κB and MAPK Signaling Pathways by Berberine and Carvacrol
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. In Vitro Bacteriostatic Assay of Berberine and Carvacrol
2.3. Drug Safety Tests of Berberine and Carvacrol
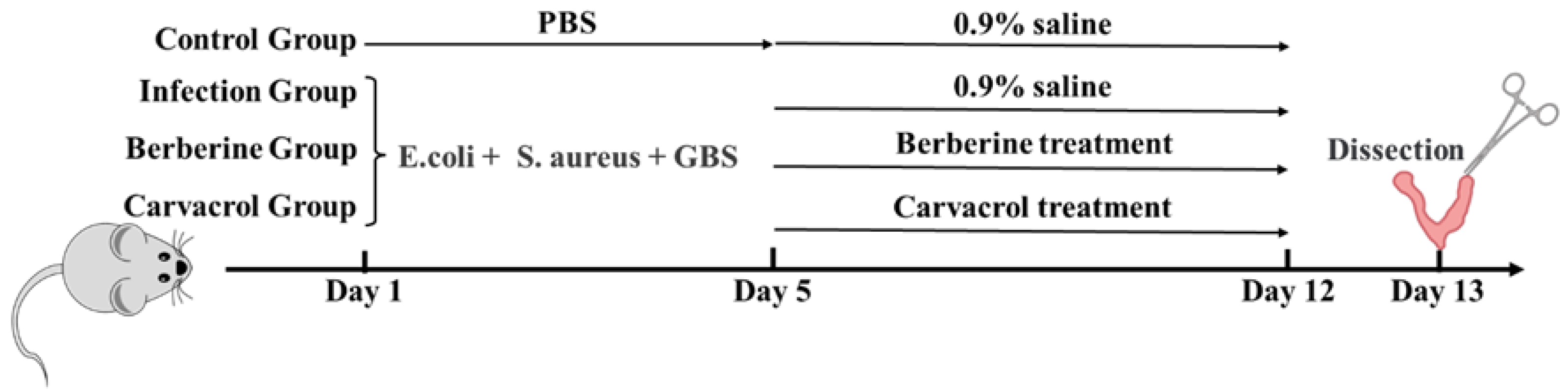
2.4. Modeling of Bacterial Infectious Endometritis
2.5. Treatment of Bacterial Endometritis with Berberine and Carvacrol
2.5.1. Treatment of Bacterial Endometritis in Mice with Berberine and Carvacrol
2.5.2. Measurement of Uterine Index and Histomorphological Observation
2.5.3. Enzyme-Linked Immunosorbent Assay
2.5.4. DNA Extraction and Real-Time Fluorescence Quantitative Polymerase Chain Reaction
2.5.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. In Vitro Antibacterial Activity of Berberine and Carvacrol
3.2. Safety Assessment Results for Berberine and Carvacrol
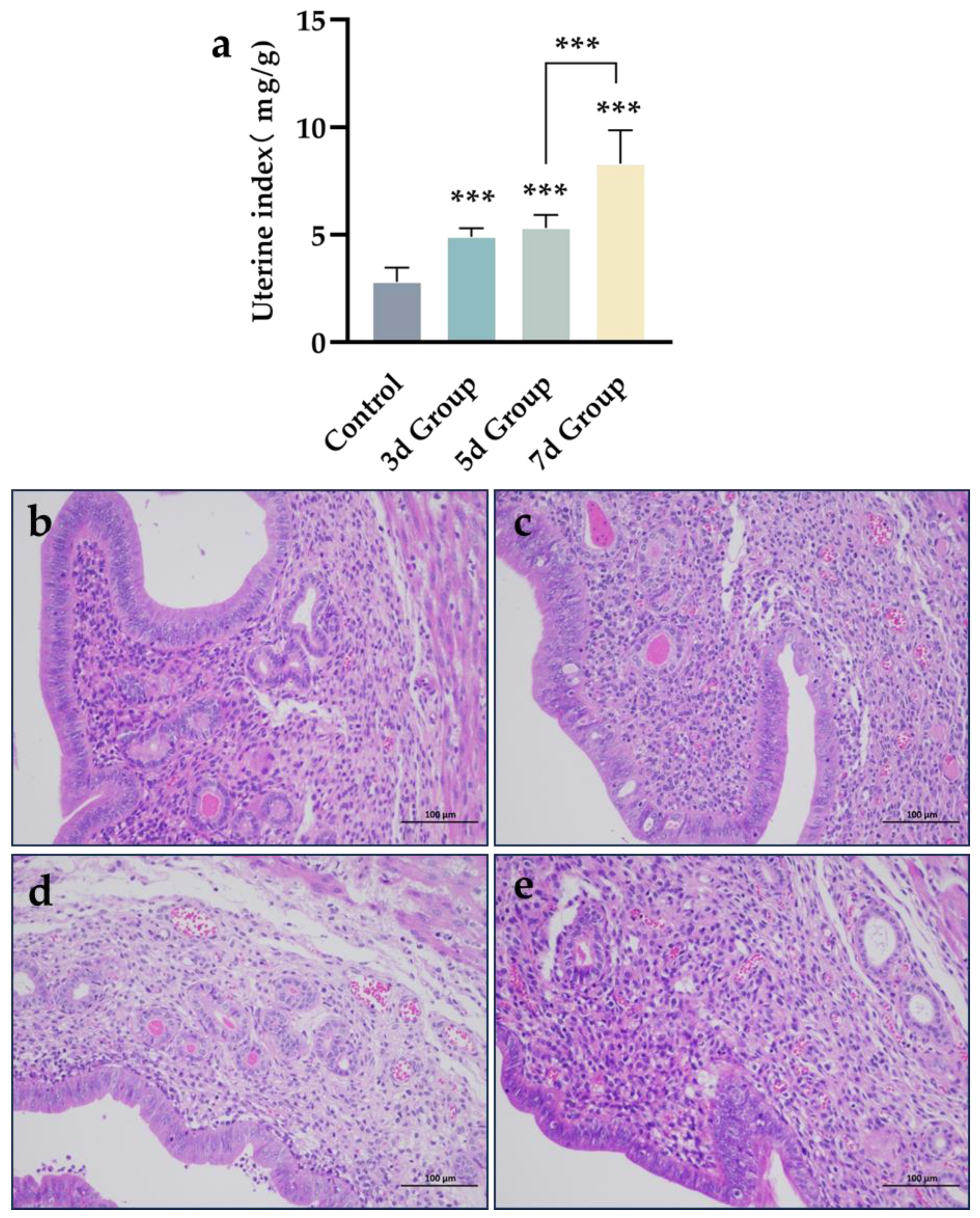
3.3. Modeling Outcomes of Bacterial Infectious Endometritis
3.4. Therapeutic Efficacy of Berberine and Carvacrol in Treating Bacterial Endometritis
3.4.1. Impact of Berberine and Carvacrol on Uterine Index and Histopathological Changes in Mice
3.4.2. Effects of Berberine and Carvacrol on TLR2 and TLR4 mRNA Expression Levels in the Uterus
3.4.3. Effects of Berberine and Carvacrol on the Phosphorylation Levels of Proteins Associated with NF-κB and MAPK Signaling Pathways in the Uterus
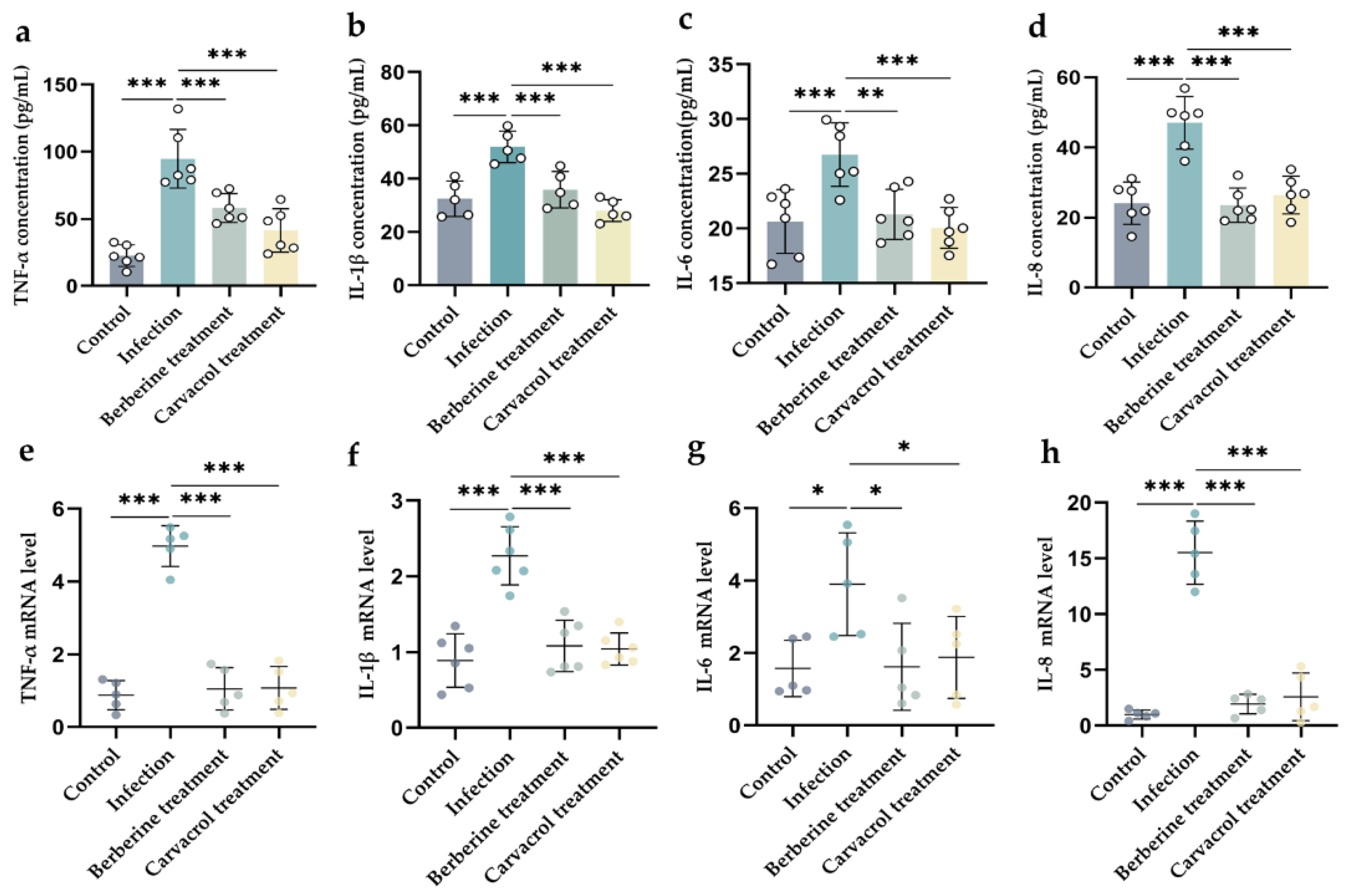
3.4.4. Effects of Berberine and Carvacrol on the Expression of Pro- and Anti-Inflammatory Markers Induced by Bacterial Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Chapwanya, A. A Review of the Diversity of the Genital Tract Microbiome and Implications for Fertility of Cattle. Animals 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Nyabinwa, P.; Kashongwe, O.B.; Habimana, J.P.; Hirwa, C.D.; Bebe, B.O. Estimating prevalence of endometritis in smallholder zero-grazed dairy cows in Rwanda. Trop. Anim. Health Prod. 2020, 52, 3135–3145. [Google Scholar] [CrossRef]
- Bijmholt, S.; Müller, K.; Leiding, C.; Hoedemaker, M.; Bollwein, H.; Kaske, M. [Lactational incidences of production diseases in German Fleckvieh cows of six Bavarian dairy farms]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2012, 40, 347–358. [Google Scholar] [CrossRef]
- Barański, W.; Baryczka, A.; Zduńczyk, S.; Tobolski, D.; Janowski, T. Prevalence of subclinical endometritis in dairy cows that recovered after treatment of clinical endometritis with cephapirin and PGF(2α). Theriogenology 2022, 192, 166–171. [Google Scholar] [CrossRef]
- Raheel, I.; Hassan, W.H.; Salem, S.S.R.; Salam, H.S.H. Biofilm forming potentiality of Escherichia coli isolated from bovine endometritis and their antibiotic resistance profiles. J. Adv. Vet. Anim. Res. 2020, 7, 442–451. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.; Sousa-Neto, B.P.; Machado, F.D.; Quintans-Júnior, L.J.; Arcanjo, D.D.; Oliveira, F.A.; Oliveira, R.C. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- Gong, T.; Si, K.; Liu, H.; Zhang, X. Research advances in the role of MAPK cascade in regulation of cell growth, immunity, inflammation, and cancer. J. Cent. South Univ. Med. Sci. 2022, 47, 1721–1728. [Google Scholar] [CrossRef]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zeng, Q.; Wang, Y.; Guo, X.; Fan, T.; Li, Y.; Deng, H.; Zhao, L.; Zhang, X.; Liu, Y.; et al. Discovery and identification of EIF2AK2 as a direct key target of berberine for anti-inflammatory effects. Acta Pharm. Sin. B 2023, 13, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Shara, M.; Yasmin, T.; Kincaid, A.E.; Limpach, A.L.; Bartz, J.; Brenneman, K.A.; Chatterjee, A.; Bagchi, M.; Stohs, S.J.; Bagchi, D. Safety and toxicological evaluation of a novel niacin-bound chromium (III) complex. J. Inorg. Biochem. 2005, 99, 2161–2183. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Runciman, D.J.; Anderson, G.A.; Malmo, J.; Davis, G.M. Effect of intrauterine treatment with cephapirin on the reproductive performance of seasonally calving dairy cows at risk of endometritis following periparturient disease. Aust. Vet. J. 2008, 86, 250–258. [Google Scholar] [CrossRef]
- Lefebvre, R.C.; Stock, A.E. Therapeutic efficiency of antibiotics and prostaglandin F2α in postpartum dairy cows with clinical endometritis: An evidence-based evaluation. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 79–96. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Wang, J.; Zhang, X.; Yao, D.; Ding, X.; Zhao, X.; Zhang, Y. Melatonin Alleviates Lipopolysaccharide-Induced Endometritis by Inhibiting the Activation of NLRP3 Inflammasome through Autophagy. Animals 2023, 13, 2449. [Google Scholar] [CrossRef]
- Kocamüftüoğlu, M.; Bozkurt, G.; Ağaoğlu, A.R.; Özmen, Ö.; Öztürk, D. The curative effect of ozonated bidistilled water on Escherichia coli-induced endometritis in rats. Vet. Med. Sci. 2023, 9, 2352–2358. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Yin, J.; Liu, X.; Liu, S.; Sun, W.; Wang, X.; Yao, H.; Xiao, L. Dihydrotestosterone mediates the inflammation effect under lipopolysaccharides in bovine endometrial epithelial cells via AR blockading TLR4/MyD88 signaling pathway. Anim. Reprod. Sci. 2023, 255, 107292. [Google Scholar] [CrossRef]
- Shaukat, A.; Shaukat, I.; Rajput, S.A.; Shukat, R.; Hanif, S.; Huang, S.; Aleem, M.T.; Li, K.; Li, Q.; Chen, C.; et al. Icariin Alleviates Escherichia coli Lipopolysaccharide-Mediated Endometritis in Mice by Inhibiting Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 10219. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ishfaq, M.; Liu, Y.; Wu, Z.; Wang, J.; Li, R.; Qian, F.; Ding, L.; Li, J. Baicalin attenuates endometritis in a rabbit model induced by infection with Escherichia coli and Staphylococcus aureus via NF-κB and JNK signaling pathways. Domest. Anim. Endocrinol. 2021, 74, 106508. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, Y.; Li, Z.; Li, Q.; Liu, H.; Zhao, J.; Lu, W.; Wang, J. Baogong decoction treats endometritis in mice by regulating uterine microbiota structure and metabolites. Microb. Biotechnol. 2022, 15, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yan, C.; Deng, L.; Peng, Z.; Yang, D.; Hu, W.; Ding, X.; Tong, C.; Wang, X. Role of MicroRNAs in Protective Effects of Forsythoside A Against Lipopolysaccharide-Induced Inflammation in Bovine Endometrial Stromal Cells. Front. Vet. Sci. 2021, 8, 642913. [Google Scholar] [CrossRef]
- Wei, X.Y.; Tao, J.H.; Cui, X.; Jiang, S.; Qian, D.W.; Duan, J.A. Comparative pharmacokinetics of six major bioactive components in normal and type 2 diabetic rats after oral administration of Sanhuang Xiexin Decoction extracts by UPLC-TQ MS/MS. J. Chromatogr. B 2017, 1061–1062, 248–255. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- Zhao, W.; Deng, C.; Han, Q.; Xu, H.; Chen, Y. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020, 46, 977–988. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol-A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Chu, M.; Ding, R.; Chu, Z.Y.; Zhang, M.B.; Liu, X.Y.; Xie, S.H.; Zhai, Y.J.; Wang, Y.D. Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement. Altern. Med. 2014, 14, 89. [Google Scholar] [CrossRef]
- Wang, K.; Wang, K.; Wang, J.; Yu, F.; Ye, C. Protective Effect of Clostridium butyricum on Escherichia coli-Induced Endometritis in Mice via Ameliorating Endometrial Barrier and Inhibiting Inflammatory Response. Microbiol. Spectr. 2022, 10, e0328622. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Pospiech, M.; Turner, M.L. Symposium review: Mechanisms linking metabolic stress with innate immunity in the endometrium. J. Dairy Sci. 2018, 101, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wu, L.; Yuan, S.; Liu, G.; Wang, Y.; Fang, L.; Xu, D. Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur. J. Pharmacol. 2021, 898, 173982. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Ding, N.; He, Y.; Li, C.; Li, M.; Ding, X.; Ding, H.; Li, J.; Wu, J.; et al. Anti-Inflammatory Effects of Berberine Hydrochloride in an LPS-Induced Murine Model of Mastitis. Evid. Based Complement. Alternat. Med. 2018, 2018, 5164314. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, B.; Liu, J.; Ma, X. Carvacrol ameliorates inflammatory response in interleukin 1β-stimulated human chondrocytes. Mol. Med. Rep. 2018, 17, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Canli, K.; Bozyel, M.E.; Turu, D.; Benek, A.; Simsek, O.; Altuner, E.M. Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites. Microorganisms 2023, 11, 1987. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciardiello, F.; Romano, M.; Federico, A. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B(3)/B(8) vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: An ex vivo study. Front. Immunol. 2023, 14, 1215329. [Google Scholar] [CrossRef]



| Target Gene | Orientations | Primer Sequence (5′-3′) | Lengths | Product Key |
|---|---|---|---|---|
| TNF-α | F | TCCAGAAGTTGCTTGTGCCT | 144 | NM_173966.3 |
| R | CAGAGGGCTGTTGATGGAGG | |||
| IL-1β | F | CCTCGGTTCCATGGGAGATG | 119 | NM_174093.1 |
| R | AGGCACTGTTCCTCAGCTTC | |||
| IL-6 | F | GCTGAATCTTCCAAAAATGGAGG | 215 | NM_173923.2 |
| R | GCTTCAGGATCTGGATCAGTG | |||
| IL-8 | F | ACACATTCCACACCTTTCCAC | 149 | AF232704 |
| R | ACCTTCTGCACCGACTTTTC | |||
| TLR2 | F | TTTGCTCCTGCGAACTCC | 109 | |
| R | GCCACGCCCACATCATTC | |||
| TLR4 | F | TATGAACCACTCCACTCGCTC | 207 | DQ839566 |
| R | CATCATTTGCTCAGCTCCCAC | |||
| GAPDH | F | GTCTTCACTACCATGGAGAAGG | 201 | NM_001034034 |
| R | TCATGGATGACCTTGGCCAG |
| Reagent Name | Dosages |
|---|---|
| 2 × SuperReal PreMix Plus | 10 μL |
| Forward primer (10 μM) | 0.6 μL |
| Reverse primer (10 μM) | 0.6 μL |
| cDNA templates | 2.0 μL |
| RNase-free ddH2O | Replenish to 20 μL |
| Drug Name | Drug Concentration (mg/Tablet) | Inhibition Circle Diameter (mm) | ||
|---|---|---|---|---|
| E. coli | S. aureus | GBS | ||
| Control group | 0 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| Berberine group | 3.2 | 16.36 ± 0.12 | 13.50 ± 0.60 | 15.40 ± 0.29 |
| Carvacrol group | 3.2 | 51.36 ± 0.86 | 46.90 ± 0.76 | 23.97 ± 0.32 |
| Bacterial Species | Drug Treatment (mg/mL) | |||
|---|---|---|---|---|
| Berberine | Carvacrol | |||
| MIC | MBC | MIC | MBC | |
| E. coli | 8 | 2 | 0.5 | 0.5 |
| S. aureus | 8 | 2 | 0.5 | 0.0625 |
| GBS | 16 | 4 | 0.25 | 0.0625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, Y.; Li, T.; Li, P.; Jiang, G. Mechanistic Study on the Alleviation of Endometritis in Mice Through Inhibition of NF-κB and MAPK Signaling Pathways by Berberine and Carvacrol. Microorganisms 2025, 13, 1051. https://doi.org/10.3390/microorganisms13051051
Liang X, Wang Y, Li T, Li P, Jiang G. Mechanistic Study on the Alleviation of Endometritis in Mice Through Inhibition of NF-κB and MAPK Signaling Pathways by Berberine and Carvacrol. Microorganisms. 2025; 13(5):1051. https://doi.org/10.3390/microorganisms13051051
Chicago/Turabian StyleLiang, Xiaoshan, Yabo Wang, Tianyi Li, Peilong Li, and Guojun Jiang. 2025. "Mechanistic Study on the Alleviation of Endometritis in Mice Through Inhibition of NF-κB and MAPK Signaling Pathways by Berberine and Carvacrol" Microorganisms 13, no. 5: 1051. https://doi.org/10.3390/microorganisms13051051
APA StyleLiang, X., Wang, Y., Li, T., Li, P., & Jiang, G. (2025). Mechanistic Study on the Alleviation of Endometritis in Mice Through Inhibition of NF-κB and MAPK Signaling Pathways by Berberine and Carvacrol. Microorganisms, 13(5), 1051. https://doi.org/10.3390/microorganisms13051051







