Microbial Carbon Limitation Mediates Soil Organic Carbon Sequestration in Sugarcane–Watermelon Intercropping System
Abstract
1. Introduction
2. Materials and Methods
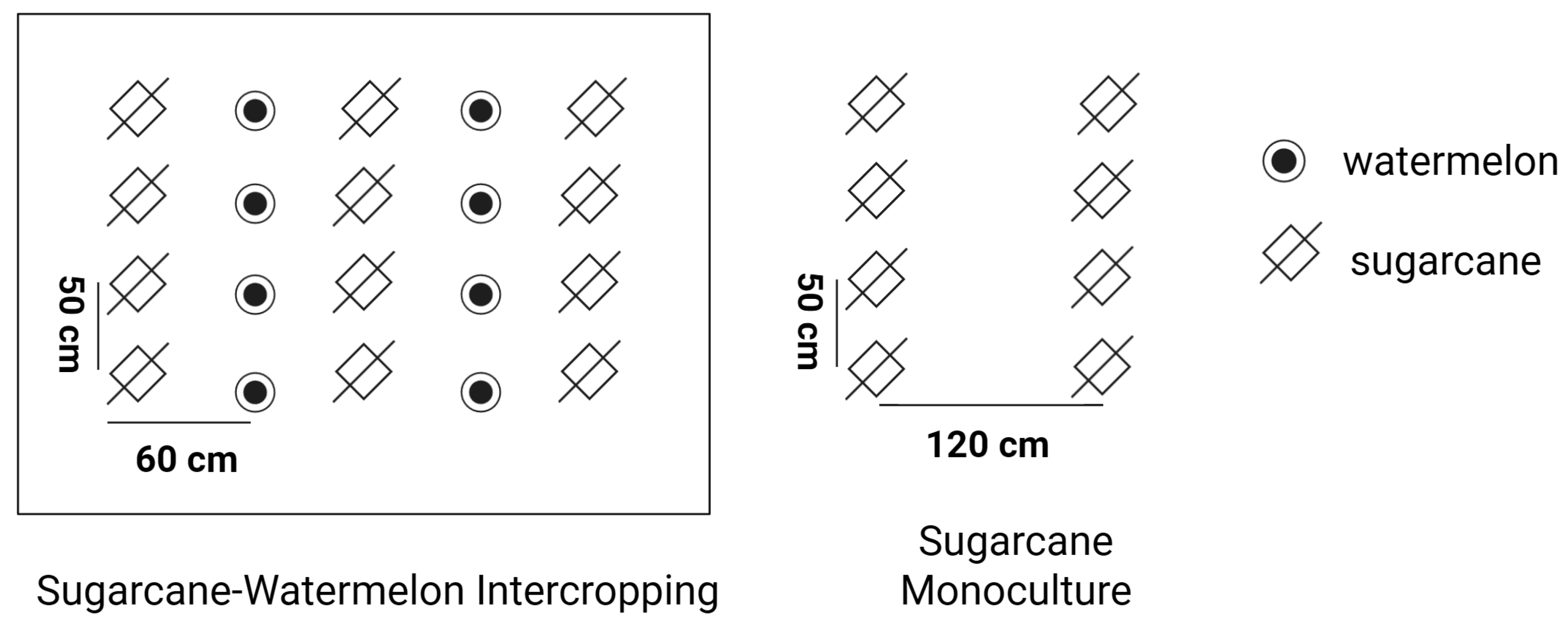
2.1. Study Area and Site Selection
2.2. Soil Sampling
2.3. Soil Physicochemical Properties
2.4. Soil Enzyme Activity
2.5. SOC Fractions
2.6. Soil Respiration
2.7. Statistical Analysis
3. Results
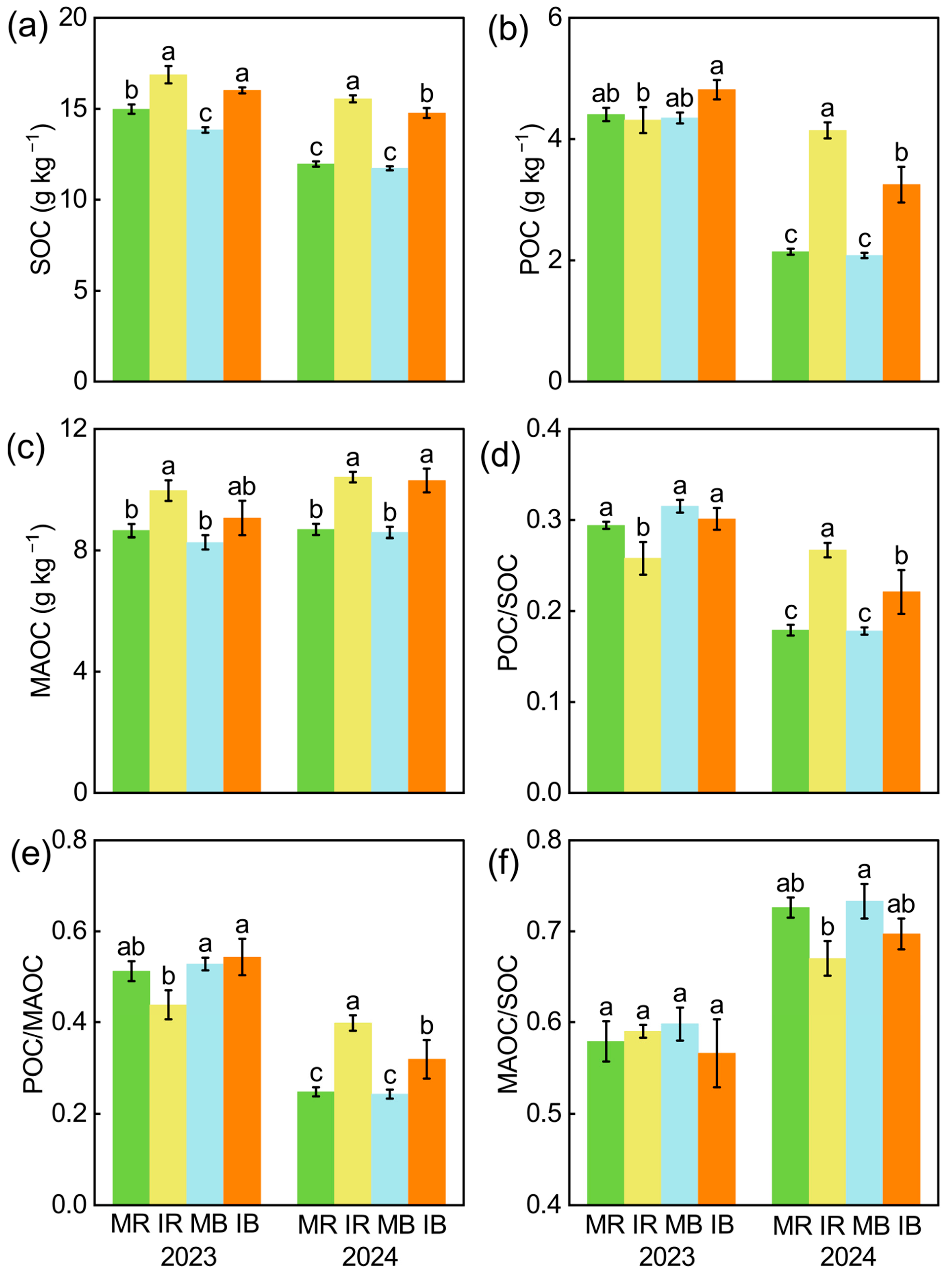
3.1. Intercropping Effects on Soil Respiration and SOC Fractions
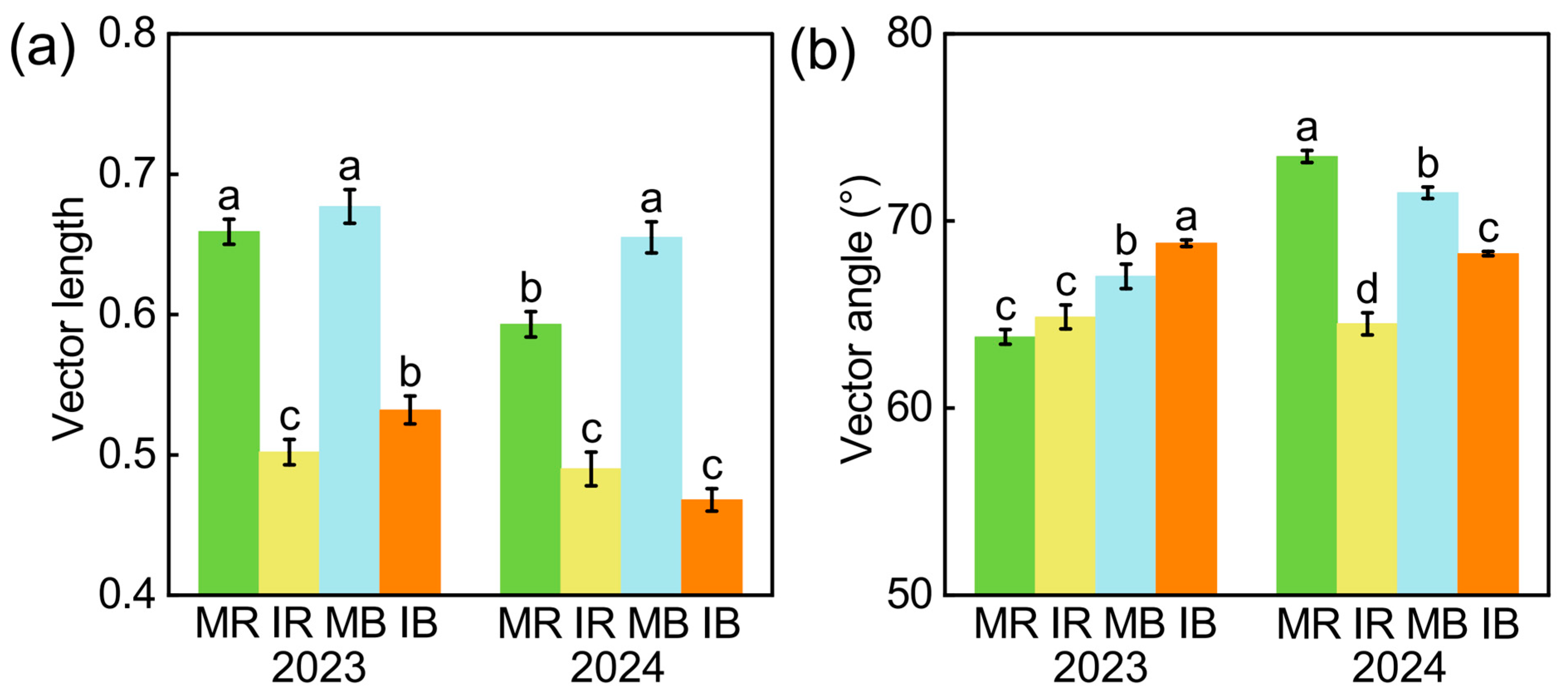
3.2. Intercropping Effect on Soil Enzyme Activities
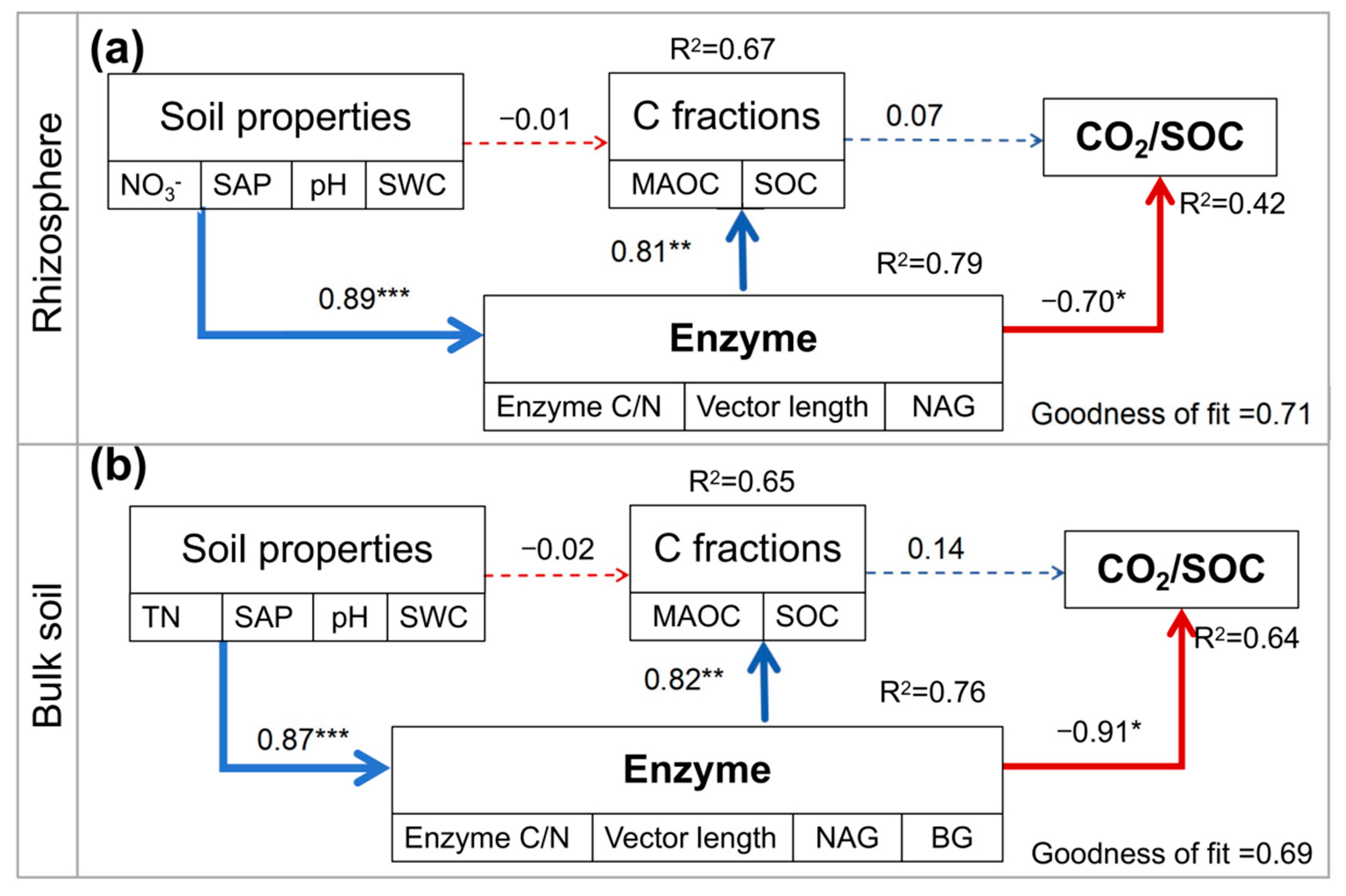
3.3. Correlations Among Soil Respiration, Enzymes, and SOC Fractions
4. Discussion
4.1. Intercropping Reduced Soil Respiration
4.2. Contributors to Soil Respiration with Intercropping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOC | Soil organic carbon |
| PLS-PM | Partial least squares path model |
| MAOC | Mineral-associated organic carbon |
| POC | Particulate organic carbon |
| N | Nitrogen |
| P | Phosphorus |
| SOM | Soil organic matter |
| SWC | Soil water content |
| TN | Total nitrogen |
| NO3− | Nitrate |
| SAP | Soil available phosphorus |
| TP | Total phosphorus |
| BG | Β-1,4-glucosidase |
| AP | Acid phosphatase |
| NAG | Β-1,4-nacetylglucosaminidase |
| LAP | Leucine aminopeptidase |
| WHC | Water holding capacity |
| ANOVA | One-way analysis of variance |
| GOF | Goodness of fit |
| MR | Monoculture rhizosphere |
| IR | Intercropping rhizosphere |
| MB | Monoculture bulk soil |
| IB | Intercropping bulk soil |
| CUE | Microbial carbon use efficiency |
References
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Haaf, D.; Six, J.; Doetterl, S. Global patterns of geo-ecological controls on the response of soil respiration to warming. Nat. Clim. Change 2021, 11, 623–627. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Dai, T.; Fan, Z.; Luo, Y.; Li, S.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.; et al. Depth-dependent soil organic carbon dynamics of croplands across the Chengdu Plain of China from the 1980s to the 2010s. Glob. Change Biol. 2020, 26, 4134–4146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Zhang, J.; Zhou, G.; Chang, D.; Chai, Q.; Cao, W. Maize and legume intercropping enhanced crop growth and soil carbon and nutrient cycling through regulating soil enzyme activities. Eur. J. Agron. 2024, 159, 127237. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, L.; Hao, X.; Li, N.; Shi, X.; Shi, F.; Tian, Y.; Wang, W.; Luo, H. Optimizing plant density to improve the soil microenvironment and enhance crop productivity in cotton/cumin intercropping systems. Front. Plant Sci. 2025, 16, 1533211. [Google Scholar] [CrossRef]
- Gonçalves e Silva, J.A.; Habermann, E.; de Pinho Costa, K.A.; da Silva, L.M.; da Costa Severiano, E.; Costa, A.C.; Silva, F.G.; de Oliveira, T.C.; Dário, B.M.M.; Vilela, L.; et al. Integration crop-livestock system increases the sustainability of soybean cultivation through improved soil health and plant physiology. Agric. Ecosyst. Environ. 2024, 359, 108770. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.Y.; Wen, Q.H.; Mo, F.; Ren, A.T.; Duan, H.X.; Tao, H.Y.; Li, J.M.; Cao, J.; Sheteiwy, M.S.; et al. Plant-Plant Interactions Drive the Decomposition of Soil Organic Carbon via Nutrition Competition in Dryland. Plant Cell Environ. 2025, 1–14. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Yao, S.; Ye, Y.; Zhang, B. Responses of soil mineral-associated and particulate organic carbon to carbon input: A meta-analysis. Sci. Total Environ. 2022, 829, 154626. [Google Scholar] [CrossRef]
- Guo, X.; Viscarra Rossel, R.A.; Wang, G.; Xiao, L.; Wang, M.; Zhang, S.; Luo, Z. Particulate and mineral-associated organic carbon turnover revealed by modelling their long-term dynamics. Soil Biol. Biochem. 2022, 173, 108780. [Google Scholar] [CrossRef]
- Sun, H.; Sun, F.; Deng, X.; Storn, N.; Wan, S. Unfolding the Roles of Particulate- and Mineral-Associated Organic Carbon in Soil Microbial Communities. Forests 2024, 16, 27. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Rehman, S.; Oetting, J.N. Unraveling the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef] [PubMed]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Zheng, H.; Vesterdal, L.; Schmidt, I.K.; Rousk, J. Ecoenzymatic stoichiometry can reflect microbial resource limitation, substrate quality, or both in forest soils. Soil Biol. Biochem. 2022, 167, 108613. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, J.; Shi, L.; Feng, W.; Dippold, M.A.; Zang, H.; Kurganova, I.; de Gerenyu, V.L.; Kalinina, O.; Giani, L.; et al. Microbial growth rates, carbon use efficiency and enzyme activities during post-agricultural soil restoration. Catena 2022, 214, 106226. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, T.; Shi, L.; Kurganova, I.; Lopes de Gerenyu, V.; Kalinina, O.; Giani, L.; Kuzyakov, Y. Organic carbon accumulation and microbial activities in arable soils after abandonment: A chronosequence study. Geoderma 2023, 435, 116496. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Xia, Z.; Zhang, X.; Zhu, M.; Gao, Y.; An, S.; Yu, W.; Ma, Q. The mechanism of the dose effect of straw on soil respiration: Evidence from enzymatic stoichiometry and functional genes. Soil Biol. Biochem. 2022, 168, 108636. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, A.; Zhang, Z.; Wang, F.; Chen, J.; Hao, Y.; Cui, X. Extracellular enzyme activities response to nitrogen addition in the rhizosphere and bulk soil: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 356, 108630. [Google Scholar] [CrossRef]
- Pang, Z.; Fallah, N.; Weng, P.; Zhou, Y.; Tang, X.; Tayyab, M.; Liu, Y.; Liu, Q.; Xiao, Y.; Hu, C.; et al. Sugarcane–Peanut Intercropping System Enhances Bacteria Abundance, Diversity, and Sugarcane Parameters in Rhizospheric and Bulk Soils. Front. Microbiol. 2022, 12, 815129. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, B.; Wei, X.; Yu, M.; Cheng, X. Root functional traits mediate rhizosphere soil carbon stability in a subtropical forest. Soil Biol. Biochem. 2021, 162, 108431. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, J.; Fu, Y.; Wu, L.; Zhang, T. Soil nitrogen availability mediates the positive effects of intercropping on soil organic carbon at global scales. Soil Tillage Res. 2024, 239, 106063. [Google Scholar] [CrossRef]
- Bastos, T.R.d.S.; Barreto-Garcia, P.A.B.; Mendes, I.d.C.; Monroe, P.H.M.; de Carvalho, F.F. Response of soil microbial biomass and enzyme activity in coffee-based agroforestry systems in a high-altitude tropical climate region of Brazil. Catena 2023, 230, 107270. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Intercropping increases soil extracellular enzyme activity: A meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107489. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, Y.; Guo, P.; Ren, J.; Zhang, H.; Dong, Q.; Jiang, C.; Zhong, C.; Zhang, Z.; Wan, S.; et al. Peanut/sorghum intercropping drives specific variation in peanut rhizosphere soil properties and microbiomes under salt stress. Land Degrad. Dev. 2022, 34, 736–750. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, X.; Sun, T.; Lin, L.; Wu, L.; Zhang, T.; Gong, Y.; Li, Y.; Wu, H.; Xiong, J.; et al. Rare taxa mediate microbial carbon and nutrient limitation in the rhizosphere and bulk soil under sugarcane–peanut intercropping systems. Front. Microbiol. 2024, 15, 1403338. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Cappelli, S.L.; Shrestha, R.; Gerin, S.; Lohila, A.K.; Heinonsalo, J.; Nelson, D.B.; Kahmen, A.; Duan, P.; Sebag, D.; et al. Plant diversity drives positive microbial associations in the rhizosphere enhancing carbon use efficiency in agricultural soils. Nat. Commun. 2024, 15, 8065. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Dai, L.; Guo, H.; Huang, Z.; Chen, T.; Huang, Y.; Li, J.; Yang, C.; Abegunrina, T.P. Control of sugarcane planting patterns on slope erosion-induced nitrogen and phosphorus loss and their export coefficients from the watershed. Agric. Ecosyst. Environ. 2022, 336, 108030. [Google Scholar] [CrossRef]
- Li, Y.; Mo, Y.; Are, K.S.; Huang, Z.; Guo, H.; Tang, C.; Abegunrin, T.P.; Qin, Z.; Kang, Z.; Wang, X. Sugarcane planting patterns control ephemeral gully erosion and associated nutrient losses: Evidence from hillslope observation. Agric. Ecosyst. Environ. 2021, 309, 107289. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, M.; Zhang, J.; Chen, Y.; Chen, X.; Chen, L.; Li, H.; Zhang, X.-X.; Sun, B. Nematode grazing promotes bacterial community dynamics in soil at the aggregate level. ISME J. 2017, 11, 2705–2717. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorous. In Methods of Soil Analysis, Part 2, Chemical and Microbial Properties; Agronomy Monograph 9; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Page, A.L. (Ed.) Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Qi, J.Y.; Yao, X.B.; Duan, M.Y.; Huang, X.W.; Fan, M.Y.; Yang, Y.; Luo, H.W.; Tang, X.R. Effects of contrasting tillage managements on the vertical distribution of plant- and microbial-derived carbon in rice paddy. Sci. Total Environ. 2023, 892, 164348. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Guo, Y.; Jing, X.; Feng, W. Contrasting elevational patterns of microbial carbon and nutrient limitation in soil from alpine meadow to desert. Catena 2023, 223, 106901. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Wu, J.; Cui, Y.; Dong, L.; Liu, Y.; Hai, X.; Li, Y.; Shangguan, Z.; Wang, K.; et al. Soil microbial respiration is regulated by stoichiometric imbalances: Evidence from a humidity gradient case. Pedosphere 2023, 33, 905–915. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, W.; Lu, X.; Gu, Y.; Wu, S.; Shen, Z.; Han, X.; Yang, G.; Ren, C. Adaptive pathways of soil microorganisms to stoichiometric imbalances regulate microbial respiration following afforestation in the Loess Plateau, China. Soil Biol. Biochem. 2020, 151, 108048. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhang, Y.; Fei, J.; Rong, X.; Peng, J.; Yin, L.; Zhou, X.; Luo, G. Enhanced productivity of maize through intercropping is associated with community composition, core species, and network complexity of abundant microbiota in rhizosphere soil. Geoderma 2024, 442, 116786. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Hui, D.; Jing, X.; Feng, W. Soil properties rather than climate and ecosystem type control the vertical variations of soil organic carbon, microbial carbon, and microbial quotient. Soil Biol. Biochem. 2020, 148, 107905. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Tools for Partial Least Squares Path Modeling (PLS-PM). R Package Version 0.4.7 edn. 2016. Available online: https://CRAN.R-project.org/package=plspm (accessed on 1 June 2024).
- Sun, T.; Mao, X.; Han, K.; Wang, X.; Cheng, Q.; Liu, X.; Zhou, J.; Ma, Q.; Ni, Z.; Wu, L. Nitrogen addition increased soil particulate organic carbon via plant carbon input whereas reduced mineral−associated organic carbon through attenuating mineral protection in agroecosystem. Sci. Total Environ. 2023, 899, 165705. [Google Scholar] [CrossRef]
- Li, H.; Fan, Z.; Wang, Q.; Wang, G.; Yin, W.; Zhao, C.; Yu, A.; Cao, W.; Chai, Q.; Hu, F. Green manure and maize intercropping with reduced chemical N enhances productivity and carbon mitigation of farmland in arid areas. Eur. J. Agron. 2023, 145, 126788. [Google Scholar] [CrossRef]
- Xu, K.; Hu, F.; Fan, Z.; Yin, W.; Niu, Y.; Wang, Q.; Chai, Q. Delayed application of N fertilizer mitigates the carbon emissions of pea/maize intercropping via altering soil microbial diversity. Front. Microbiol. 2022, 13, 1002009. [Google Scholar] [CrossRef]
- Zhou, Q.; Gunina, A.; Chen, J.; Xing, Y.; Xiong, Y.; Guo, Z.; Wang, L. Reduction in soil CO2 efflux through alteration of hydrothermal factor in milk vetch (Astragalus sinicus L.)-rapeseed (Brassica napus L.) intercropping system. Front. Plant Sci. 2023, 13, 1093507. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Fu, Q.; Qiu, Y.; Zhao, J.; Li, J.; Wu, X.; Yang, Y.; Liu, H.; Yang, X.; et al. Critical transition of soil microbial diversity and composition triggered by plant rhizosphere effects. Front. Plant Sci. 2023, 14, 1252821. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Sietiö, O.-M.; Meyer, N.; Poeplau, C.; Adamczyk, S.; Biasi, C.; Kalu, S.; Räsänen, M.; Ambus, P.; Fritze, H.; et al. Microbial carbon use efficiency along an altitudinal gradient. Soil Biol. Biochem. 2022, 173, 108799. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.Y.; Zhu, S.G.; Khan, A.; Tao, X.P.; Huang, G.F.; Liu, H.Y.; Zhang, W.; Tao, H.Y.; Gong, D.S.; et al. Plant facilitation improves carbon production efficiency while reducing nitrogen input in semiarid agroecosystem. Catena 2023, 230, 107247. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; McIntosh, M. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 2006, 38, 1451–1461. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, J.; Ge, J.; Nie, J.; Zhao, J.; Xue, Z.; Hu, Y.; Yang, Y.; Peixoto, L.; Zang, H.; et al. Intercropping improves soil ecosystem multifunctionality through enhanced available nutrients but depends on regional factors. Plant Soil 2022, 480, 71–84. [Google Scholar] [CrossRef]
- Kuczynski, J.; Liu, Z.; Lozupone, C.; McDonald, D.; Fierer, N.; Knight, R. Microbial community resemblance methods differ in their ability to detect biologically relevant patterns. Nat. Methods 2010, 7, 813–819. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2013, 14, 538–548. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Hafner, B.D.; Schweizer, S.A.; Höschen, C.; Possinger, A.; Lehmann, J.; Bauerle, T. Root exudates simultaneously form and disrupt soil organo-mineral associations. Commun. Earth Environ. 2024, 5, 699. [Google Scholar] [CrossRef]
- Xiao, S.; Luo, M.; Liu, Y.; Bai, J.; Yang, Y.; Zhai, Z.; Huang, J. Rhizosphere effect and its associated soil-microbe interactions drive iron fraction dynamics in tidal wetland soils. Sci. Total Environ. 2021, 756, 144056. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, W.; Li, L.; Li, Y. Interactions between organic matter and Fe oxides at soil micro-interfaces: Quantification, associations, and influencing factors. Sci. Total Environ. 2023, 855, 158710. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Liang, G.; Reed, S.C.; Stark, J.M.; Waring, B.G. Unraveling mechanisms underlying effects of wetting–drying cycles on soil respiration in a dryland. Biogeochemistry 2023, 166, 23–37. [Google Scholar] [CrossRef]
- Zhang, C.J.; Delgado-Baquerizo, M.; Drake, J.E.; Reich, P.B.; Tjoelker, M.G.; Tissue, D.T.; Wang, J.T.; He, J.Z.; Singh, B.K. Intraspecies variation in a widely distributed tree species regulates the responses of soil microbiome to different temperature regimes. Environ. Microbiol. Rep. 2018, 10, 167–178. [Google Scholar] [CrossRef]
- Ji, H.; Yuan, G.; Liu, Y.; Yu, J.; Li, S.; Wu, Q.; Qin, H.; Chen, J. Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation. Forests 2023, 14, 853. [Google Scholar] [CrossRef]
- Ning, Q.; Hättenschwiler, S.; Lü, X.; Kardol, P.; Zhang, Y.; Wei, C.; Xu, C.; Huang, J.; Li, A.; Yang, J.; et al. Carbon limitation overrides acidification in mediating soil microbial activity to nitrogen enrichment in a temperate grassland. Glob. Change Biol. 2021, 27, 5976–5988. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Luo, G. Regulation of soil C–N–P stoichiometry by intercropping mitigates microbial resource limitations and contributes to maize productivity. Plant Soil 2023, 498, 21–38. [Google Scholar] [CrossRef]
- Ndabankulu, K.; Egbewale, S.O.; Tsvuura, Z.; Magadlela, A. Soil microbes and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soils. Sci. Rep. 2022, 12, 12601. [Google Scholar] [CrossRef]
- Huang, C.; He, Y.; Zhou, L.; Liu, R.; Chen, H.; Du, Z.; Fu, Y.; Zhu, Y.; Zhou, Y.; Wu, C.; et al. Opposite effects of soil pH on bacteria and fungi β diversity in forests at a continental scale. J. Environ. Manag. 2024, 370, 122428. [Google Scholar] [CrossRef] [PubMed]
- de Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Y.; Lou, M.; Cheng, Z.; Ma, R.; Guo, H.; Zhou, J.; Jia, H.; Fan, L.; Wang, T. Microbial transformation mechanisms of particulate organic carbon to mineral-associated organic carbon at the chemical molecular level: Highlighting the effects of ambient temperature and soil moisture. Soil Biol. Biochem. 2024, 195, 109454. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, X.; Huang, Q.; Liu, C.; Jiao, L.; Yang, Y.; Dou, Y.; Wang, B.; Qu, T.; Zhou, Z.; et al. Advance in the formation and stabilization mechanisms of soil mineral-associated organic carbon. J. Soil Water Conserv. 2023, 37, 12–23. [Google Scholar] [CrossRef]
- Shi, J.; Lv, J.; Peng, Y.; Yao, Y.; Wei, X.; Wang, X. Mechanisms controlling the stability and sequestration of mineral associated organic carbon upon erosion and deposition. Catena 2024, 242, 108119. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Q.; Zhang, Y.; Jiang, P.; Wang, Y.; Fei, J.; Rong, X.; Peng, J.; Wei, X.; Luo, G. Soil carbon storage and accessibility drive microbial carbon use efficiency by regulating microbial diversity and key taxa in intercropping ecosystems. Biol. Fertil. Soils 2024, 60, 437–453. [Google Scholar] [CrossRef]
- Possinger, A.R.; Zachman, M.J.; Enders, A.; Levin, B.D.A.; Muller, D.A.; Kourkoutis, L.F.; Lehmann, J. Organo–organic and organo–mineral interfaces in soil at the nanometer scale. Nat. Commun. 2020, 11, 6103. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, L.; Chen, S.; Chen, Y.; Ma, J.; Zhang, Y.; Pang, D.; Li, X. Soil microbial carbon and nitrogen limitation constraints soil organic carbon stability in arid and semi-arid grasslands. J. Environ. Manag. 2025, 373, 123675. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Ma, J.; Zhang, Y.; Li, X.; Pang, D. Aggregate-associated carbon contributes to soil organic carbon accumulation along the elevation gradient of Helan Mountains. Soil Biol. Biochem. 2023, 178, 108926. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2019, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Feng, J.; Jiang, D.; Zhu, B. Forest management causes soil carbon loss by reducing particulate organic carbon in Guangxi, Southern China. For. Ecosyst. 2023, 10, 100092. [Google Scholar] [CrossRef]
- Wu, G.; Huang, G.; Lin, S.; Huang, Z.; Cheng, H.; Su, Y. Changes in soil organic carbon stocks and its physical fractions along an elevation in a subtropical mountain forest. J. Environ. Manag. 2024, 351, 119823. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, Z.; Zhang, Y.; Wang, Y.; Fei, J.; Rong, X.; Peng, J.; Wei, X.; Luo, G. Intercropping regulates plant- and microbe-derived carbon accumulation by influencing soil physicochemical and microbial physiological properties. Agric. Ecosyst. Environ. 2024, 364, 108880. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Cui, S.; Liang, G.; Zhang, Q. Microbial-derived carbon components are critical for enhancing soil organic carbon in no-tillage croplands: A global perspective. Soil Tillage Res. 2021, 205, 104758. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Fu, Y.; Zhang, T.; Sun, T. Microbial Carbon Limitation Mediates Soil Organic Carbon Sequestration in Sugarcane–Watermelon Intercropping System. Microorganisms 2025, 13, 1049. https://doi.org/10.3390/microorganisms13051049
Wu L, Fu Y, Zhang T, Sun T. Microbial Carbon Limitation Mediates Soil Organic Carbon Sequestration in Sugarcane–Watermelon Intercropping System. Microorganisms. 2025; 13(5):1049. https://doi.org/10.3390/microorganisms13051049
Chicago/Turabian StyleWu, Lixue, Yue Fu, Tian Zhang, and Tingting Sun. 2025. "Microbial Carbon Limitation Mediates Soil Organic Carbon Sequestration in Sugarcane–Watermelon Intercropping System" Microorganisms 13, no. 5: 1049. https://doi.org/10.3390/microorganisms13051049
APA StyleWu, L., Fu, Y., Zhang, T., & Sun, T. (2025). Microbial Carbon Limitation Mediates Soil Organic Carbon Sequestration in Sugarcane–Watermelon Intercropping System. Microorganisms, 13(5), 1049. https://doi.org/10.3390/microorganisms13051049





