New Insights into the Synergistic Interaction Between Pseudomonas qingdaonensis NZ 1 and Silicon to Mitigate Drought Stress in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Isolation, and Identification of PGPR Isolates
2.2. Preliminary Screening of the Microbial Isolates
2.3. Quantification of Secondary Metabolites
2.4. Drought Stress Tolerance
2.5. Molecular Identification
2.6. Screening for the Optimum Si Concentration and Isolate Growth Conditions
2.7. Pot Experiment
2.8. Assessment of Plant Morphological Parameters and Non-Invasive Physiological Responses
2.9. Determination of Hydrogen Peroxide, Superoxide Anion, and Malondialdehyde Production
2.10. Quantification of the Plants’ Antioxidant Enzyme Activities
2.11. Quantification of the Plants’ Soluble Sugars and Amino Acids Content
2.12. Quantification of the Plants’ Nutrient Levels
2.13. Quantification of the Plants’ Endogenous Phytohormones ABA, JA, and SA
2.14. Relative Gene Expression
2.15. Statistical Analysis
3. Results
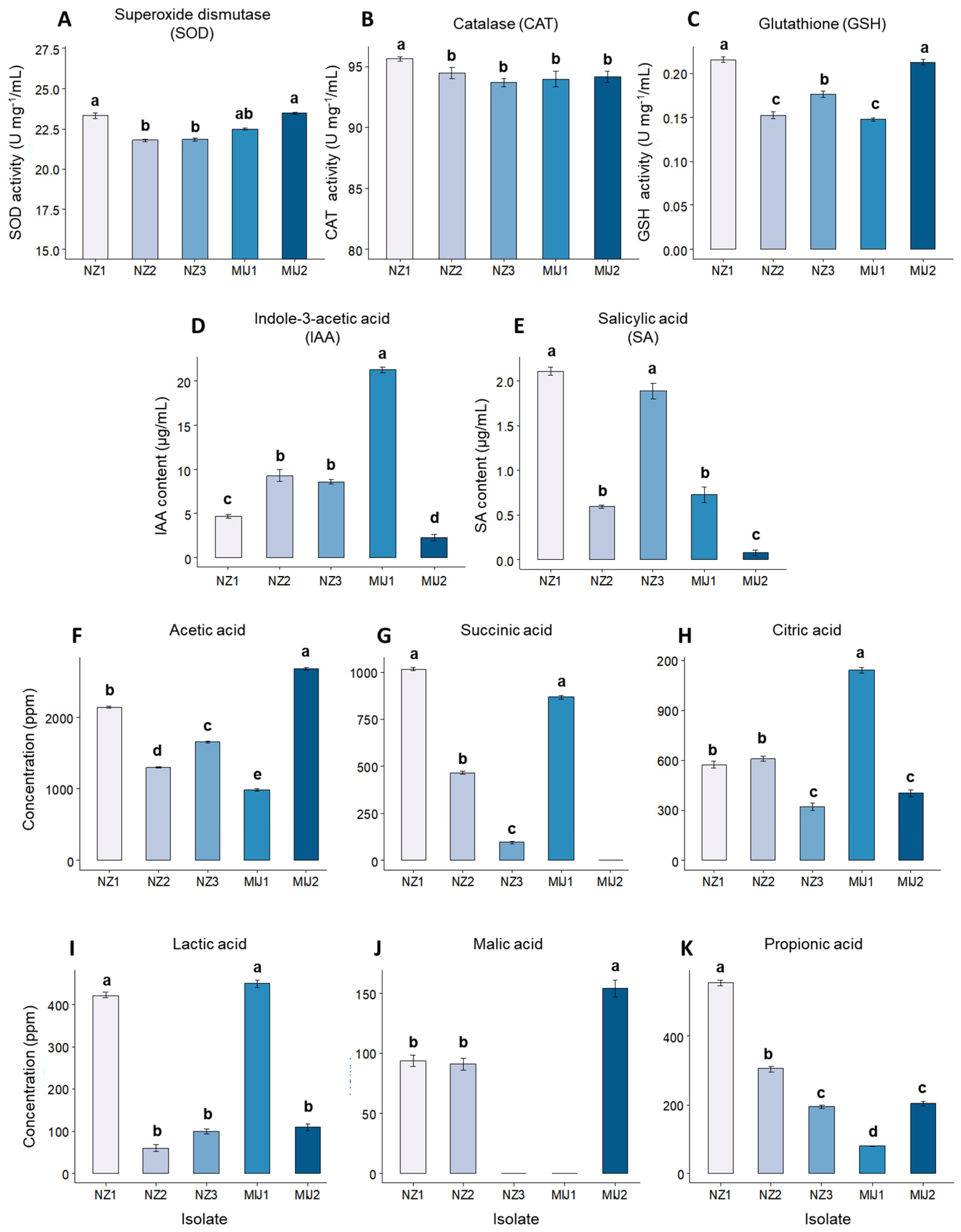
3.1. In Vitro Microbial Antioxidant, Phytohormone, Organic Acid, and Amino Acids Production and Drought Stress Tolerance
3.2. Phylogenetic Identification of Isolate NZ 1
3.3. Plant Morphological Analysis
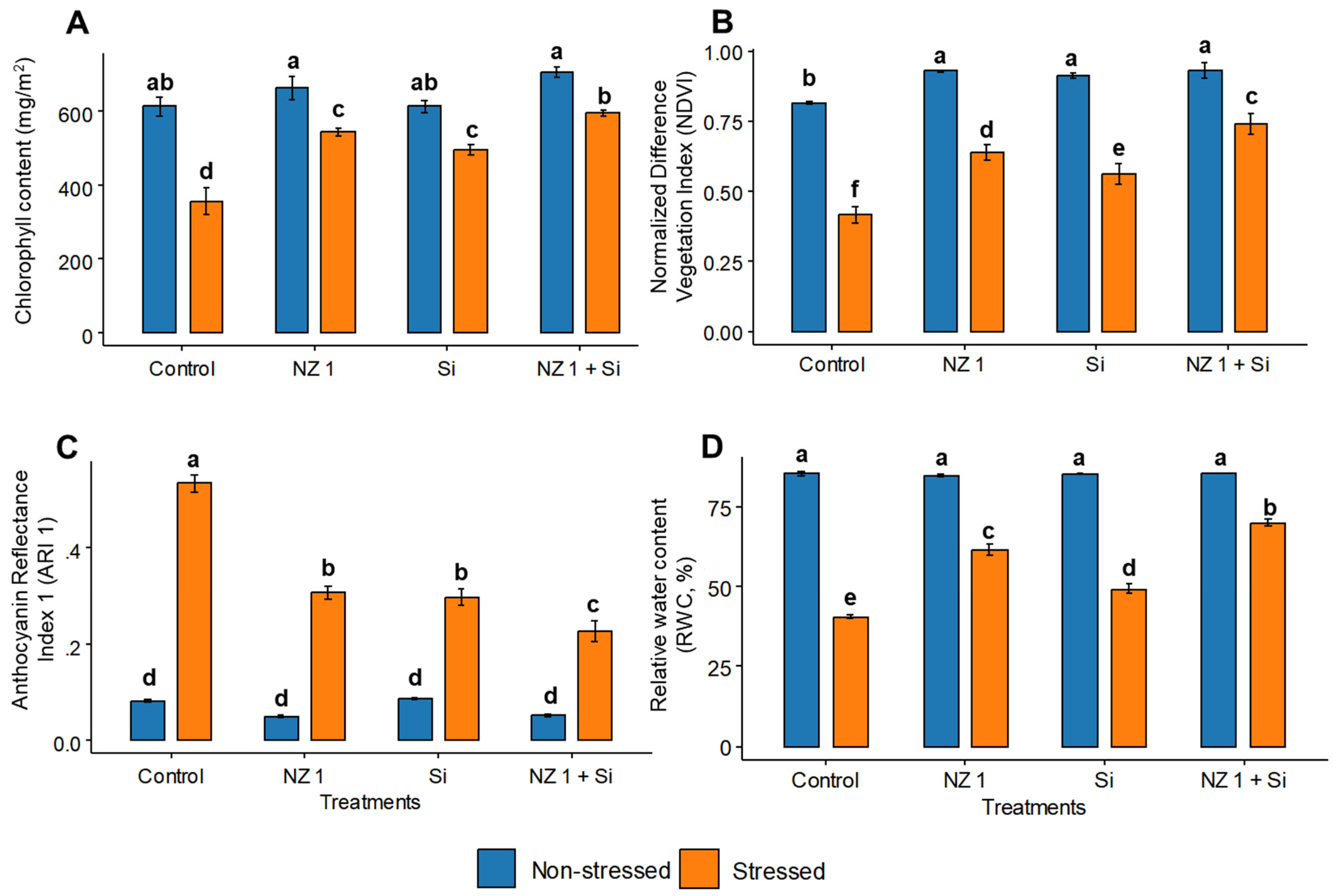
3.4. Chlorophyll Content, NDVI, ARI1, and RWC
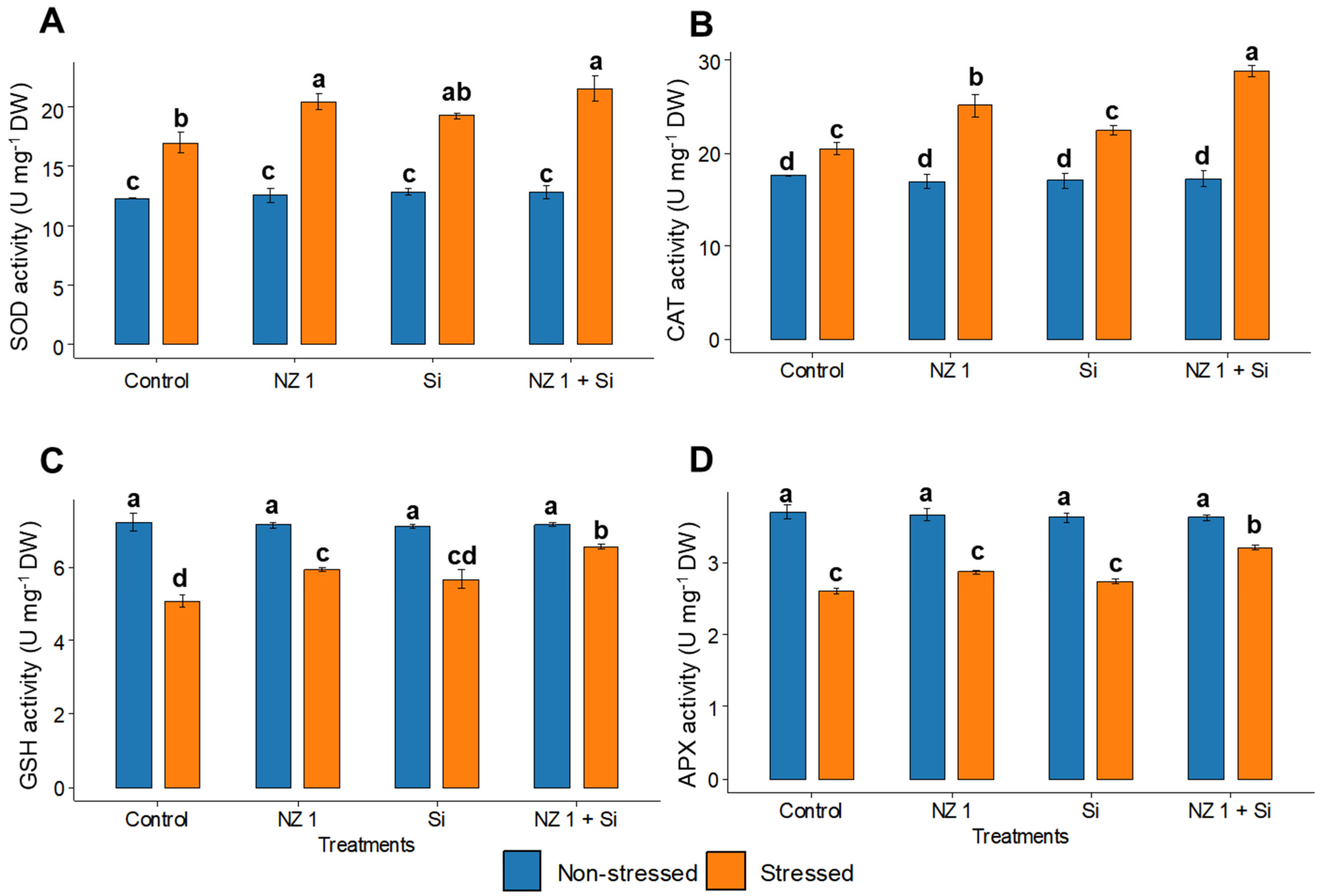
3.5. ROS and Antioxidants Contents
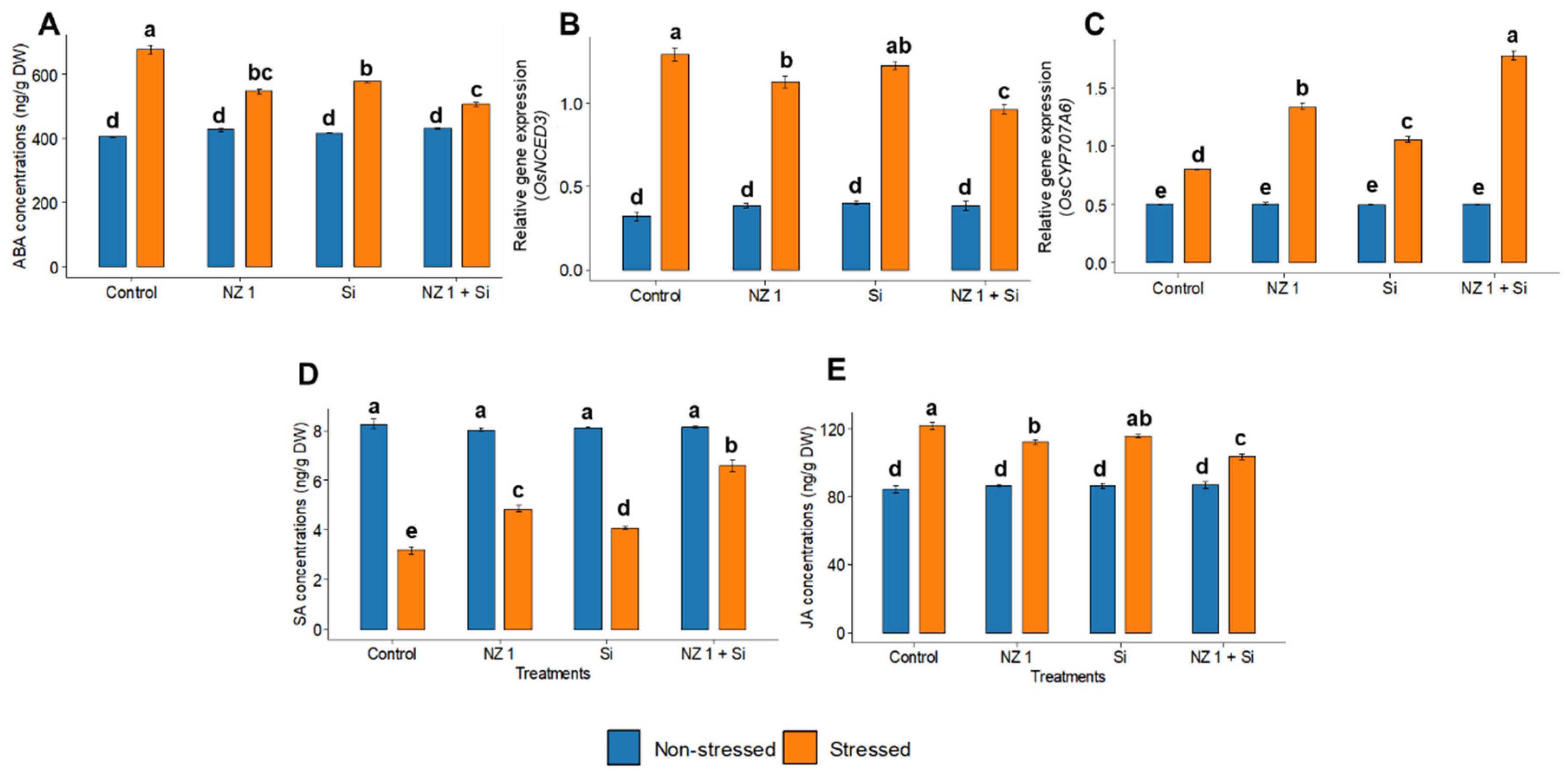
3.6. Phytohormones and ABA Metabolic Gene Expression
3.7. Osmolytes Content
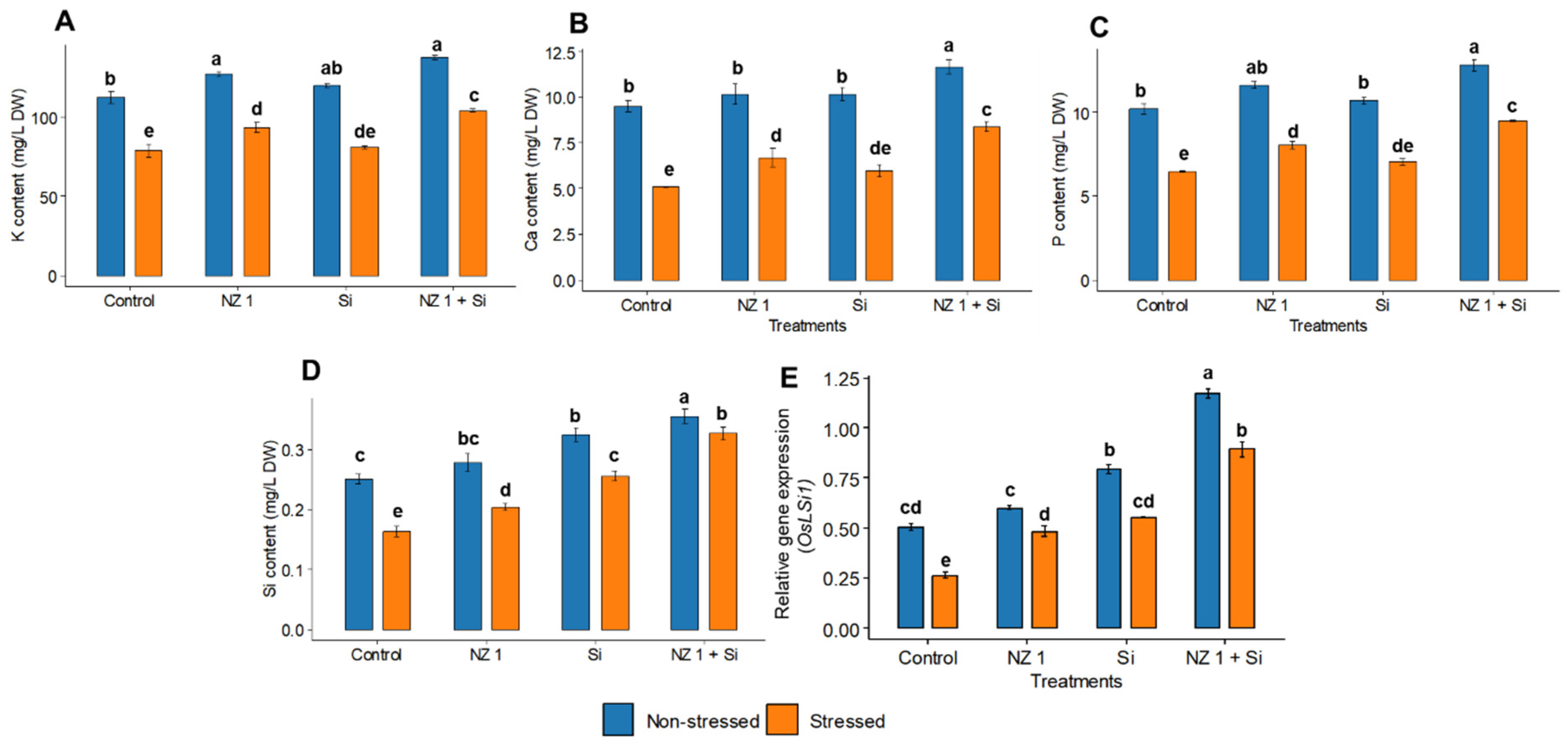
3.8. Potassium, Calcium, Phosphorus, and Si Contents and the Relative Gene Expression of the Si Transporter OsLSi1
3.9. Expression of Transcription Factors OsbZIP23 and OsDREB1B and Si Transporter OsLSi1
4. Discussion
5. Conclusions and Future Aspects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ABA | Abscisic Acid |
| JA | Jasmonic Acid |
| PGPR | Plant Growth-Promoting Rhizobacteria |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
References
- FAO. Proactive Approaches to Drought Preparedness—Where Are We Now and Where Do We Go from Here? FAO White Paper; FAO: Rome, Italy, 2019. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.B.; Hasanuzzaman, M.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. Abscisic acid increases hydrogen peroxide in mitochondria to facilitate stomatal closure. Plant Physiol. 2023, 192, 469–487. [Google Scholar] [CrossRef]
- Yao, L.; Hao, X.; Cao, H.; Ding, C.; Yang, Y.; Wang, L.; Wang, X. ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep. 2020, 39, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, H.; Panda, S.K. Drought stress responses and its management in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–200. [Google Scholar]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A. Insight into recent progress and perspectives in improvement of antioxidant machinery upon PGPR augmentation in plants under drought stress: A review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Pande, V.; Samant, M. Bioremediation: An emerging effective approach towards environment restoration. Environ. Sustain. 2020, 3, 91–103. [Google Scholar] [CrossRef]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into plant beneficial microorganism-triggered induced systemic resistance. Plant Stress 2023, 7, 100140. [Google Scholar] [CrossRef]
- Huang, X.-F.; Zhou, D.; Lapsansky, E.R.; Reardon, K.F.; Guo, J.; Andales, M.J.; Vivanco, J.M.; Manter, D.K. Mitsuaria sp. and Burkholderia sp. from Arabidopsis rhizosphere enhance drought tolerance in Arabidopsis thaliana and maize (Zea mays L.). Plant Soil 2017, 419, 523–539. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W.; Liu, X. 1-Aminocyclopropane-1-Carboxylate deaminase-producing plant growth-promoting rhizobacteria improve drought stress tolerance in grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 706990. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; El-Saadony, M.T.; Abdelaziz, S.; Abdou, N.M. Plant growth-promoting rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of rice plants under full and deficit drip irrigation. Rice 2022, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Asaf, S.; Khan, A.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Bhatta, D.; Adhikari, A.; Kang, S.-M.; Kwon, E.-H.; Jan, R.; Kim, K.-M.; Lee, I.-J. Hormones and the antioxidant transduction pathway and gene expression, mediated by Serratia marcescens DB1, lessen the lethality of heavy metals (As, Ni, and Cr) in Oryza sativa L. Ecotoxicol. Environ. Saf. 2023, 263, 115377. [Google Scholar] [CrossRef]
- Arun, K.D.; Sabarinathan, K.G.; Gomathy, M.; Kannan, R.; Balachandar, D. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. J. Basic Microbiol. 2020, 60, 768–786. [Google Scholar] [CrossRef]
- Das, P.; Manna, I.; Sil, P.; Bandyopadhyay, M.; Biswas, A.K. Silicon augments salt tolerance through modulation of polyamine and GABA metabolism in two indica rice (Oryza sativa L.) cultivars. Plant Physiol. Biochem. 2021, 166, 41–52. [Google Scholar] [CrossRef]
- Cassol, J.; Sponchiado, D.; Dornelles, S.; Tabaldi, L.; Barreto, E.; Pivetta, M.; Lopes, S. Silicon as an attenuator of drought stress in plants of Oryza sativa L. treated with dietholate. Braz. J. Biol. 2020, 81, 1061–1072. [Google Scholar] [CrossRef]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y. Silicon as a smart fertilizer for sustainability and crop improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. Soil Sci. Plant Nutr. 2021, 67, 10–17. [Google Scholar] [CrossRef]
- Jia, M.; Li, Y.; Yue, Z.; Nie, M.; Li, L.; Yin, Y.; Zhou, Z.; Wang, X.; Ding, C. Exploring the effects of water management and silicon or phosphorus pretreatment on arsenic accumulation in rice grains. J. Food Compos. Anal. 2025, 142, 107465. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, C.; Liu, J.; Lu, H.; Duan, H.; Du, J.; Wang, W. Silicon alleviation of cadmium toxicity in mangrove (Avicennia marina) in relation to cadmium compartmentation. J. Plant Growth Regul. 2014, 33, 233–242. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Hordiienko, A.; Samchuk, A.; Kurdysh, I. Influence of silicon dioxide and saponite on growth of Bacillus subtilis IMV B-7023. Mikrobiolohichnyi Zhurnal (Kiev Ukr. 1993) 2010, 72, 33–39. [Google Scholar]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [PubMed]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Kumar, N.; Dubey, A.K.; Gautam, A.; Pandey, S.N.; Mallick, S. Diminution of arsenic accumulation in rice seedlings co-cultured with Anabaena sp.: Modulation in the expression of lower silicon transporters, two nitrogen dependent genes and lowering of antioxidants activity. Ecotoxicol. Environ. Saf. 2018, 151, 109–117. [Google Scholar] [CrossRef]
- Jeong, O.-Y.; Park, H.-S.; Baek, M.-K.; Kim, W.-J.; Lee, G.-M.; Lee, C.-M.; Bombay, M.; Ancheta, M.B.; Lee, J.-H. Review of rice in Korea: Current status, future prospects, and comparisons with rice in other countries. J. Crop Sci. Biotechnol. 2021, 24, 1–11. [Google Scholar]
- Mridha, D.; Gorain, P.C.; Joardar, M.; Das, A.; Majumder, S.; De, A.; Chowdhury, N.R.; Lama, U.; Pal, R.; Roychowdhury, T. Rice grain arsenic and nutritional content during post harvesting to cooking: A review on arsenic bioavailability and bioaccessibility in humans. Food Res. Int. 2022, 154, 111042. [Google Scholar]
- Bouman, B.; Tuong, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Ricciardi, V.; Wane, A.; Sidhu, B.S.; Godde, C.; Solomon, D.; McCullough, E.; Diekmann, F.; Porciello, J.; Jain, M.; Randall, N. A scoping review of research funding for small-scale farmers in water scarce regions. Nat. Sustain. 2020, 3, 836–844. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Mishra, S.S.; Behera, P.K.; Panda, D. Genotypic variability for drought tolerance-related morpho-physiological traits among indigenous rice landraces of Jeypore tract of Odisha, India. J. Crop Improv. 2019, 33, 254–278. [Google Scholar] [CrossRef]
- Shaffique, S.; Shah, A.A.; Peter, O.; Injamum-Ul-Hoque, M.; Elansary, H.O.; Kang, S.-M.; Al Azzawi, T.N.I.; Yun, B.-W.; Lee, I.-J. The rhizobacterial Priestia megaterium strain SH-19 mitigates the hazardous effects of heat stress via an endogenous secondary metabolite elucidation network and molecular regulation signalling. BMC Plant Biol. 2024, 24, 827. [Google Scholar]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio. Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, Y.S.; Yuliani, Y.; Asri, M.T. The ability of bacteria from legume plant roots grown on former coal mining soil to produce Indole-3-Acetic Acid (IAA). In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; p. 03003. [Google Scholar]
- Salmon, J.; Ramos, J.; Callis, J. Degradation of the auxin response factor ARF1. Plant J. 2008, 54, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Walia, A.; Chauhan, A.; Kulshrestha, S.; Shirkot, C. Phosphate solubilisation and plant growth promoting potential by stress tolerant Bacillus sp. isolated from rhizosphere of apple orchards in trans Himalayan region of Himachal Pradesh. Ann. Appl. Biol. 2013, 163, 430–443. [Google Scholar]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998, 116, 387–392. [Google Scholar]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar]
- Kang, S.-M.; Khan, A.L.; Hamayun, M.; Shinwari, Z.K.; Kim, Y.-H.; Joo, G.-J.; Lee, I.-J. Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellins and functional biochemicals. Pak. J. Bot. 2012, 44, 365–372. [Google Scholar]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Khan, A.L.; Al-Harrasi, A.; Shahzad, R.; Imran, Q.M.; Yun, B.-W.; Kim, Y.-H.; Kang, S.-M.; Al-Rawahi, A.; Lee, I.-J. Regulation of endogenous phytohormones and essential metabolites in frankincense-producing Boswellia sacra under wounding stress. Acta Physiol. Plant. 2018, 40, 113. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Khan, M.A.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Enhancement of drought-stress tolerance of Brassica oleracea var. italica L. by newly isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500. [Google Scholar]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Sambo, F.; Finotello, F.; Lavezzo, E.; Baruzzo, G.; Masi, G.; Peta, E.; Falda, M.; Toppo, S.; Barzon, L.; Di Camillo, B. Optimizing PCR primers targeting the bacterial 16S ribosomal RNA gene. BMC Bioinform. 2018, 19, 343. [Google Scholar]
- Clifford, R.J.; Milillo, M.; Prestwood, J.; Quintero, R.; Zurawski, D.V.; Kwak, Y.I.; Waterman, P.E.; Lesho, E.P.; Mc Gann, P. Detection of bacterial 16S rRNA and identification of four clinically important bacteria by real-time PCR. PLoS ONE 2012, 7, e48558. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nam, K.-H.; Kim, D.; Moon, Y.; Pack, I.; Jeong, S.-C.; Kim, H.; Kim, C.-G. Performance of hybrids between abiotic stress-tolerant transgenic rice and its weedy relatives under water-stressed conditions. Sci. Rep. 2020, 10, 9319. [Google Scholar]
- Nam, K.-H.; Shin, H.; Pack, I.-S.; Park, J.-H.; Kim, H.; Kim, C.-G. Growth stage-based metabolite profiling of drought-tolerant transgenic rice under well-watered and deficit conditions. Plant Omics. 2015, 8, 587–594. [Google Scholar]
- Nam, K.H.; Shin, H.J.; Pack, I.S.; Park, J.H.; Kim, H.B.; Kim, C.G. Metabolomic changes in grains of well-watered and drought-stressed transgenic rice. J. Sci. Food Agric. 2016, 96, 807–814. [Google Scholar]
- Turk, H.; Erdal, S. Melatonin alleviates cold-induced oxidative damage in maize seedlings by up-regulating mineral elements and enhancing antioxidant activity. J. Plant Nutr. Soil Sci. 2015, 178, 433–439. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ 1974, 351, 309. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [PubMed]
- Halo, B.A.; Khan, A.L.; Waqas, M.; Al-Harrasi, A.; Hussain, J.; Ali, L.; Adnan, M.; Lee, I.-J. Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J. Plant Interact. 2015, 10, 117–125. [Google Scholar]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 190643. [Google Scholar]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Al-Harrasi, A.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 395863. [Google Scholar]
- Shahzad, R.; Bilal, S.; Imran, M.; Khan, A.L.; Alosaimi, A.A.; Al-Shwyeh, H.A.; Almahasheer, H.; Rehman, S.; Lee, I.-J. Amelioration of heavy metal stress by endophytic Bacillus amyloliquefaciens RWL-1 in rice by regulating metabolic changes: Potential for bacterial bioremediation. Biochem. J. 2019, 476, 3385–3400. [Google Scholar] [CrossRef]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.-J. Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Lan, J.; Tang, C. Characterization of sugar contents and sucrose metabolizing enzymes in developing leaves of Hevea brasiliensis. Front. Plant Sci. 2018, 9, 315477. [Google Scholar]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Qi, Q.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef] [PubMed]
- Team Posit. RStudio: Integrated Development Environment for R. Available online: http://www.posit.co/ (accessed on 20 June 2023).
- Surendran, U.; Raja, P.; Jayakumar, M.; Subramoniam, S.R. Use of efficient water saving techniques for production of rice in India under climate change scenario: A critical review. J. Clean. Prod. 2021, 309, 127272. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pablo, C.H.; Mavrodi, O.V.; Weller, D.M.; Thomashow, L.S.; Mavrodi, D.V. Rhizosphere plant-microbe interactions under water stress. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 115, pp. 65–113. [Google Scholar]
- Davey, M.E.; O’toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Shaffique, S.; Imran, M.; Kang, S.-M.; Khan, M.A.; Asaf, S.; Kim, W.-C.; Lee, I.-J. Seed Bio-priming of wheat with a novel bacterial strain to modulate drought stress in Daegu, South Korea. Front. Plant Sci. 2023, 14, 1118941. [Google Scholar] [CrossRef]
- Astorga-Eló, M.; Gonzalez, S.; Acuña, J.J.; Sadowsky, M.J.; Jorquera, M.A. Rhizobacteria from ‘flowering desert’ events contribute to the mitigation of water scarcity stress during tomato seedling germination and growth. Sci. Rep. 2021, 11, 13745. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Song, A.; Li, P.; Fan, F.; Li, Z.; Liang, Y. The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE 2014, 9, e113782. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, S.; Hussain, S.; Kataria, S.; Hajihashemi, S.; Kumari, P.; Yang, X.; Brestic, M. Does silicon really matter for the photosynthetic machinery in plants…? Plant Physiol. Biochem. 2021, 169, 40–48. [Google Scholar] [CrossRef]
- Mishra, S.S.; Behera, P.K.; Kumar, V.; Lenka, S.K.; Panda, D. Physiological characterization and allelic diversity of selected drought tolerant traditional rice (Oryza sativa L.) landraces of Koraput, India. Physiol. Mol. Biol. Plants 2018, 24, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Zhang, D.; Jin, L.; Wang, S.; Yang, X.; Lei, Y.; Meng, X.; Xu, Z.; Sun, J.; Lyu, J. Controlling water deficiency as an abiotic stress factor to improve tomato nutritional and flavour quality. Food Chem. X 2023, 19, 100756. [Google Scholar] [CrossRef]
- Anuj Kumar, A.K.; Supratim Basu, S.B.; Venkategowda Ramegowda, V.R.; Andy Pereira, A.P. Mechanisms of Drought Tolerance in Rice; Burleigh Dodds Science Publishing: Cambridge, UK, 2017. [Google Scholar]
- Aslam, M.M.; Rashid, M.A.R.; Siddiqui, M.A.; Khan, M.T.; Farhat, F.; Yasmeen, S.; Khan, I.A.; Raja, S.; Rasool, F.; Sial, M.A. Recent insights into signaling responses to cope drought stress in rice. Rice Sci. 2022, 29, 105–117. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, L.; Zhang, Y.; Zheng, Z.; Liu, D. Arabidopsis PHL2 and PHR1 act redundantly as the key components of the central regulatory system controlling transcriptional responses to phosphate starvation. Plant Physiol. 2016, 170, 499–514. [Google Scholar] [CrossRef]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050–Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Yagmur, B.; Gunes, A. Evaluation of the effects of plant growth promoting rhizobacteria (PGPR) on yield and quality parameters of tomato plants in organic agriculture by principal component analysis (PCA). Gesunde Pflanz. 2021, 73, 219–228. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Long, C.; Cui, Z.-X. Effect of biodegradable chelators on induced phytoextraction of uranium-and cadmium-contaminated soil by Zebrina pendula Schnizl. Sci. Rep. 2019, 9, 19817. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, P.P.; Gowthamraj, K.; Balasubramaniam, P.; Chandramani, P.; Yuvaraj, M. Status and distribution of plant available silicon in relation to some soil properties and response of rice (Oryza sativa L.) to silicon nutrition in the intensively rice growing soils of Kanyakumari district, Tamil Nadu, India. Silicon 2021, 14, 1519–1529. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Imran, M.; Lee, K.-E.; Kang, S.-M.; Shin, J.Y.; Joo, G.-J.; Khan, M.; Yun, B.-W.; Lee, I.-J. The combined inoculation of Curvularia lunata AR11 and biochar stimulates synthetic silicon and potassium phosphate use efficiency, and mitigates salt and drought stresses in rice. Front. Plant Sci. 2022, 13, 816858. [Google Scholar]
- Kaloterakis, N.; van Delden, S.H.; Hartley, S.; De Deyn, G.B. Silicon application and plant growth promoting rhizobacteria consisting of six pure Bacillus species alleviate salinity stress in cucumber (Cucumis sativus L.). Sci. Hortic. 2021, 288, 110383. [Google Scholar] [CrossRef]
- Shaffique, S.; Imran, M.; Rahim, W.; Alomrani, S.O.; Yun, B.-W.; Lee, I.-J. A newly isolated Bacillus pumilus strain SH-9 modulates response to drought stress in soybean via endogenous phytohormones and gene expression (Daegu, South Korea). Plant Stress 2023, 10, 100279. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.L.; Mun, B.-G.; Bilal, S.; Shaffique, S.; Kwon, E.-H.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Melatonin and nitric oxide: Dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere 2022, 308, 136575. [Google Scholar]
- Chiappero, J.; del Rosario Cappellari, L.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Babaei, K.; Seyed Sharifi, R.; Pirzad, A.; Khalilzadeh, R. Effects of bio fertilizer and nano Zn-Fe oxide on physiological traits, antioxidant enzymes activity and yield of wheat (Triticum aestivum L.) under salinity stress. J. Plant Interact. 2017, 12, 381–389. [Google Scholar]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Calonego, J.C.; Moreira, A.; Garcia, A.; Momesso, L.; Kuramae, E.E.; Hungria, M. Beneficial microbial species and metabolites alleviate soybean oxidative damage and increase grain yield during short dry spells. Eur. J. Agron. 2021, 127, 126293. [Google Scholar]
- Imran, M.; Mpovo, C.L.; Aaqil Khan, M.; Shaffique, S.; Ninson, D.; Bilal, S.; Khan, M.; Kwon, E.-H.; Kang, S.-M.; Yun, B.-W. Synergistic effect of melatonin and Lysinibacillus fusiformis L.(PLT16) to mitigate drought stress via regulation of hormonal, antioxidants system, and physio-molecular responses in soybean plants. Int. J. Mol. Sci. 2023, 24, 8489. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.; Castro, G.; Silva Junior, D.; Pinheiro, H.; Filippi, M.; Silva, G. Response of photosynthesis and chlorophyll a fluorescence in leaf scald-infected rice under influence of rhizobacteria and silicon fertilizer. Plant Pathol. 2017, 66, 1487–1495. [Google Scholar]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 256717. [Google Scholar]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestič, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar]
- Xu, L.; Islam, F.; Ali, B.; Pei, Z.; Li, J.; Ghani, M.A.; Zhou, W. Silicon and water-deficit stress differentially modulate physiology and ultrastructure in wheat (Triticum aestivum L.). 3 Biotech 2017, 7, 273. [Google Scholar]
- Verma, K.K.; Wu, K.-C.; Singh, P.; Malviya, M.K.; Singh, R.K.; Song, X.-P.; Li, Y.-R. The protective role of silicon in sugarcane under water stress: Photosynthesis and antioxidant enzymes. Biomed. J. Sci. Tech. Res. 2019, 15, 1–7. [Google Scholar]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.M.; Sun, J. JAZ proteins modulate seed germination through interaction with ABI 5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A. Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J. Exp. Bot. 2017, 68, 2913–2918. [Google Scholar] [CrossRef]
- Hassan, M.A.; Dahu, N.; Hongning, T.; Qian, Z.; Yueming, Y.; Yiru, L.; Shimei, W. Drought stress in rice: Morpho-physiological and molecular responses and marker-assisted breeding. Front. Plant Sci. 2023, 14, 1215371. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2014, 84, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef]
- Umezawa, T.; Okamoto, M.; Kushiro, T.; Nambara, E.; Oono, Y.; Seki, M.; Kobayashi, M.; Koshiba, T.; Kamiya, Y.; Shinozaki, K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006, 46, 171–182. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Hewage, K.A.H.; Yang, J.F.; Wang, D.; Hao, G.F.; Yang, G.F.; Zhu, J.K. Chemical manipulation of abscisic acid signaling: A new approach to abiotic and biotic stress management in agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Bandurska, H.; Stroiński, A.; Kubiś, J. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Kong, M.; Freeman, A.; Chen, H.; Liu, F. More stories to tell: NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1, a salicylic acid receptor. Plant Cell Environ. 2021, 44, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Myers Jr, R.J.; Fichman, Y.; Zandalinas, S.I.; Mittler, R. Jasmonic acid and salicylic acid modulate systemic reactive oxygen species signaling during stress responses. Plant Physiol. 2023, 191, 862–873. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Finnegan, P.M.; Younis, A.; Nafees, M.; Zorrig, W.; Hamed, K.B. Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, A.L.; Imran, Q.M.; Asaf, S.; Lee, S.-U.; Yun, B.-W.; Hamayun, M.; Kim, T.-H.; Lee, I.-J. Exogenous application of nitric oxide donors regulates short-term flooding stress in soybean. PeerJ 2019, 7, e7741. [Google Scholar] [CrossRef]
- Odongkara, P.; Lee, I.-J.; Imran, M.; Shaffique, S.; Mo, K.S.; Chebitok, F.; Dan-Dan, Z.; Hojun, G.; Hoque, M.I.-U.; Kwon, E.-H.; et al. Combine application of melatonin and Bacillus sp. strain IPR-4 ameliorates drought stress tolerance via hormonal, antioxidant, and physio-molecular signaling in soybean. Front. Plant Sci. 2024, 15, 1274964. [Google Scholar]
- Meldau, D.G.; Long, H.H.; Baldwin, I.T. A native plant growth promoting bacterium, Bacillus sp. B55, rescues growth performance of an ethylene-insensitive plant genotype in nature. Front. Plant Sci. 2012, 3, 27554. [Google Scholar] [CrossRef]
- Iqbal, A.; Hasnain, S. Aeromonas punctata PNS-1: A promising candidate to change the root morphogenesis of Arabidopsis thaliana in MS and sand system. Acta Physiol. Plant. 2013, 35, 657–665. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 342862. [Google Scholar]
- Lang, D.; Fei, P.; Cao, G.; Jia, X.; Li, Y.; Zhang, X. Silicon promotes seedling growth and alters endogenous IAA, GA3 and ABA concentrations in Glycyrrhiza uralensis under 100 mM NaCl stress. J. Hortic. Sci. Biotechnol. 2019, 94, 87–93. [Google Scholar] [CrossRef]




| Reflectance Index | Equation | Reference |
|---|---|---|
| NDVI (normalized difference vegetation index) | [61] | |
| ARI1 (anthocyanin reflectance index 1) | [62] |
| Amino Acid Content (mg/g) † | Isolate | ||||
|---|---|---|---|---|---|
| NZ 1 | NZ 2 | NZ 3 | MIJ 1 | MIJ 2 | |
| Asp | 7.54 ± 0.03 a | 6.23 ± 0.10 c | 7.38 ± 0.12 a | 7.12 ± 0.58 b | 7.10 ± 0.29 b |
| Thr | 2.42 ± 0.02 b | 3.06 ± 0.05 a | 2.24 ± 0.04 c | 2.45 ± 0.05 b | 2.20 ± 0.20 c |
| Ser | 2.25 ± 0.05 b | 2.05 ± 0.03 c | 1.91 ± 0.03 c | 2.18 ± 0.11 b | 4.57 ± 1.76 a |
| Glu | 22.97 ± 0.37 a | 20.54 ± 0.33 b | 23.03 ± 0.37 a | 22.07 ± 1.00 a | 14.46 ± 7.22 c |
| Gly | 3.32 ± 0.04 a | 2.17 ± 0.04 c | 2.09 ± 0.13 c | 2.92 ± 0.55 b | 3.35 ± 1.33 a |
| Ala | 5.11 ± 0.04 a | 2.57 ± 0.04 c | 2.72 ± 0.16 c | 2.15 ± 0.03 c | 4.32 ± 0.88 b |
| Cys | 4.64 ± 0.06 b | 3.30 ± 0.07 c | 4.53 ± 0.27 b | 3.74 ± 0.06 c | 5.41 ± 0.09 a |
| Val | 6.92 ± 0.08 a | 5.50 ± 0.11 b | 4.55 ± 0.27 c | 4.85 ± 0.08 b | 4.15 ± 0.07 c |
| Met | 2.07 ± 0.07 a | 2.00 ± 0.04 a | 1.51 ± 0.09 b | 1.35 ± 0.02 c | 1.06 ± 0.02 c |
| Ile | 5.81 ± 0.08 a | 3.94 ± 0.08 b | 3.28 ± 0.05 b | 3.57 ± 0.21 b | 2.62 ± 0.04 c |
| Leu | 9.35 ± 0.08 a | 5.43 ± 0.11 b | 3.49 ± 0.06 c | 3.76 ± 0.06 c | 1.96 ± 0.03 d |
| Tyr | 4.29 ± 0.08 a | 1.94 ± 0.04 b | 1.41 ± 0.02 b | 1.55 ± 0.09 b | 1.50 ± 0.02 b |
| Phe | 4.95 ± 0.07 a | 1.65 ± 0.03 b | 1.30 ± 0.08 c | 1.63 ± 0.10 b | 5.35 ± 2.96 a |
| Lys | 8.86 ± 0.20 b | 8.53 ± 0.18 b | 9.81 ± 0.59 a | 9.01 ± 0.54 b | 5.15 ± 4.09 c |
| His | 2.89 ± 0.14 b | 2.90 ± 0.02 b | 3.23 ± 0.19 a | 2.94 ± 0.18 b | 2.47 ± 0.77 b |
| Arg | 2.08 ± 0.14 b | 1.35 ± 0.01 b | 1.24 ± 0.07 b | 1.48 ± 0.09 b | 15.91 ± 10.33 a |
| Pro | 18.13 ± 0.33 b | 15.65 ± 0.13 c | 18.60 ± 1.11 b | 16.75 ± 1.00 c | 19.50 ± 0.32 a |
| Treatment | Number of Tillers | Leaves Per Tiller | Tiller Height (cm) | Leaf Area Index (LAI) | Shoot Fresh Weight (g) | Root Length (cm) | Root Fresh Weight (g) | |
|---|---|---|---|---|---|---|---|---|
| Non-stress | Control | 6.00 ± 0.70 a | 3.54 ± 0.29 b | 45.05 ± 0.29 a | 8.373 ± 0.38 a | 3.89 ± 0.35 a | 18.25 ± 1.79 b | 4.26 ± 0.37 b |
| NZ 1 | 5.75 ± 0.43 a | 3.5 ± 0.24 b | 45.50 ± 1.22 a | 8.914 ± 1.65 a | 3.96 ± 0.28 a | 19.50 ± 2.18 a | 4.28 ± 0.31 b | |
| Si | 5.5 ± 0.50 a | 3.4 ± 0.33 b | 44.95 ± 1.51 a | 8.920 ± 0.78 a | 3.88 ± 0.13 a | 18.88 ± 2.46 b | 4.72 ± 0.21 b | |
| NZ 1+Si | 6.00 ± 0.00 a | 4.00 ± 0.14 a | 45.15 ± 0.12 a | 8.885 ± 0.03 a | 3.98 ± 0.17 a | 19.70 ± 2.35 a | 4.39 ± 0.25 b | |
| Drought-stressed | Control | 4.25 ± 0.43 b | 3.45 ± 0.04 b | 27.90 ± 4.57 c | 4.023 ± 0.26 d | 1.78 ± 0.09 d | 13.00 ± 1.41 d | 2.96 ± 0.21 c |
| NZ 1 | 6.00 ± 0.00 a | 3.83 ± 0.14 b | 40.05 ± 2.49 b | 6.241 ± 0.04 c | 2.95 ± 0.33 c | 16.75 ± 1.09 c | 5.18 ± 0.33 a | |
| Si | 5.5 ± 0.5 a | 3.67 ± 0.27 b | 39.40 ± 1.06 b | 6.392 ± 0.13 c | 2.88 ± 0.19 c | 17.23 ± 0.77 b | 5.31 ± 0.27 a | |
| NZ 1+Si | 6.00 ± 0.00 a | 3.50 ± 0.00 b | 43.15 ± 1.84 b | 7.069 ± 0.75 b | 3.31 ± 0.21 b | 18.75 ± 2.49 b | 5.98 ± 0.31 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainurin, N.; Imran, M.; Shaffique, S.; Khan, M.A.; Kang, S.-M.; Injamum-UL-Hoque, M.; Das, A.K.; Yun, B.-W.; Lee, I.-J. New Insights into the Synergistic Interaction Between Pseudomonas qingdaonensis NZ 1 and Silicon to Mitigate Drought Stress in Rice. Microorganisms 2025, 13, 1046. https://doi.org/10.3390/microorganisms13051046
Zainurin N, Imran M, Shaffique S, Khan MA, Kang S-M, Injamum-UL-Hoque M, Das AK, Yun B-W, Lee I-J. New Insights into the Synergistic Interaction Between Pseudomonas qingdaonensis NZ 1 and Silicon to Mitigate Drought Stress in Rice. Microorganisms. 2025; 13(5):1046. https://doi.org/10.3390/microorganisms13051046
Chicago/Turabian StyleZainurin, Nazree, Muhammad Imran, Shifa Shaffique, Muhammad Aaqil Khan, Sang-Mo Kang, Md. Injamum-UL-Hoque, Ashim Kumar Das, Byung-Wook Yun, and In-Jung Lee. 2025. "New Insights into the Synergistic Interaction Between Pseudomonas qingdaonensis NZ 1 and Silicon to Mitigate Drought Stress in Rice" Microorganisms 13, no. 5: 1046. https://doi.org/10.3390/microorganisms13051046
APA StyleZainurin, N., Imran, M., Shaffique, S., Khan, M. A., Kang, S.-M., Injamum-UL-Hoque, M., Das, A. K., Yun, B.-W., & Lee, I.-J. (2025). New Insights into the Synergistic Interaction Between Pseudomonas qingdaonensis NZ 1 and Silicon to Mitigate Drought Stress in Rice. Microorganisms, 13(5), 1046. https://doi.org/10.3390/microorganisms13051046











