Effects of Liquid Bio-Fertilizer on Plant Growth, Antioxidant Activity, and Soil Bacterial Community During Cultivation of Chinese Cabbage (Brassica rapa L. ssp. pekinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Fertilizer
2.2. Plant Growth Survey
2.3. Chinese Cabbage Extract
2.4. Antioxidant Activity Analysis
2.5. Analysis of the Chemical Composition of the Soil
2.6. Soil Microbiome Analysis
2.7. De Novo Transcriptome Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth of Chinese Cabbage Under Different Treatments
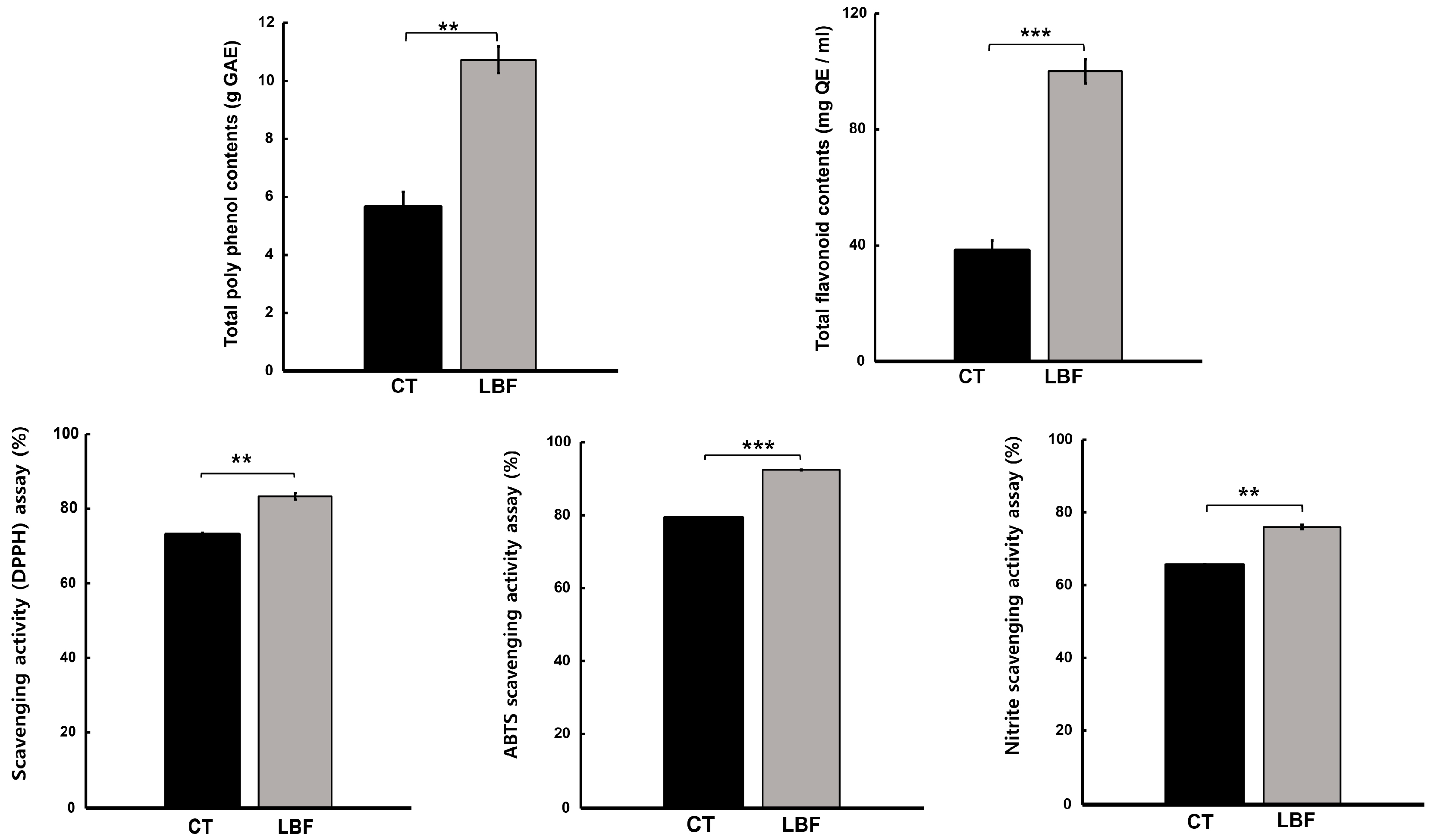
3.2. Antioxidant Activity
3.3. Soil Chemical Composition
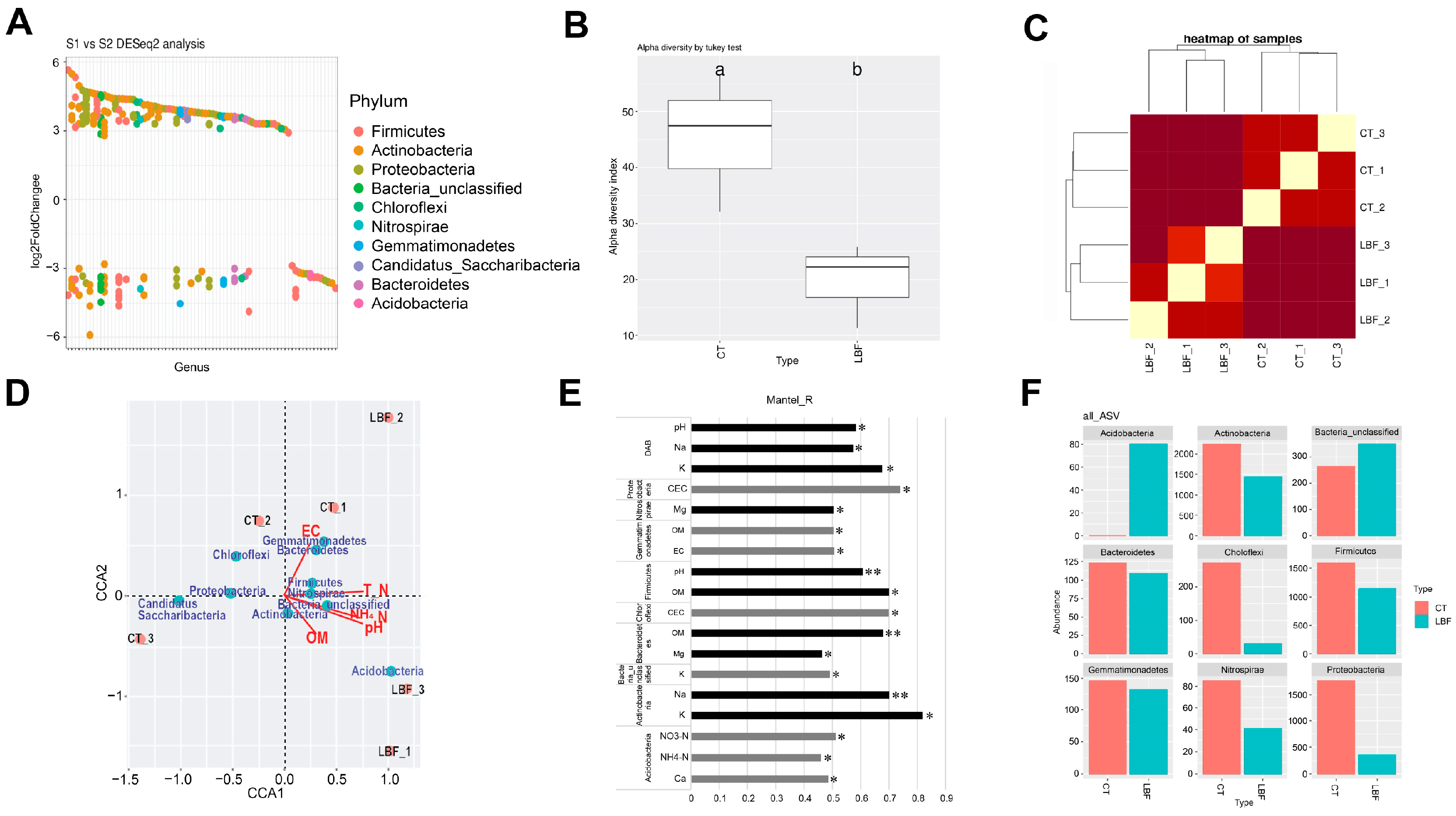
3.4. Soil Microorganism
3.5. De Novo Transcriptome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LBF | Liquid bio-fertilizer |
| CT | Control |
| PGPR | Plant growth-promoting rhizobacteria |
| DAP | Day after planting |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| DPPH | Determining 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| EC | Electrical conductivity |
| T-N | Total nitrogen |
| NH4+-N | Ammonium nitrogen |
| NO3−-N | Nitrate nitrogen |
| P | Phosphorus |
| K | Potassium |
| Na | Sodium |
| Ca | Calcium |
| Mg | Magnesium |
| OM | Organic matter |
| CEC | Cation exchange capacity |
| PCC | Pearson’s correlation coefficients |
| ASVs | Amplicon sequence variants |
| CCA | Canonical correspondence analysis |
| GO | Gene ontology |
| CC | Cellular component |
| BP | Biological process |
| DEGs | Differentially expressed genes |
| DAB | Differentially abundant bacteria |
References
- Maltas, A.; Charles, R.; Jeangros, B.; Sinaj, S. Effect of organic fertilizers and reduced-tillage on soil properties, crop nitrogen response and crop yield: Results of a 12-year experiment in Changins, Switzerland. Soil Tillage Res. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–20. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Mishra, D.; Rajvir, S.; Mishra, U.; Kumar, S.S. Role of bio-fertilizer in organic agriculture: A review. Res. J. Recent Sci. ISSN 2013, 2277, 2502. [Google Scholar]
- Widnyana, I.K.; Ariati, P.E.P.; Sumantra, I.K.; Wijaya, I.M.W.; Suanda, I.W.; Setyobudi, R.H.; Adinurani, P.G.; Ekawati, I.; Purbajanti, E.D.; Anwar, S.; et al. The Effect of Liquid Organic Fertilizer from Plant Waste, Livestock Waste, and Fish Waste on Growth of Marigold. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2023. [Google Scholar]
- Blanco-Canqui, H.; Lal, R. Crop residue removal impacts on soil productivity and environmental quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, W.; Xiong, Y.; Zou, J.; Huang, Q.; Xu, X.; Ren, P.; Huang, G. Impact of short-term organic amendments incorporation on soil structure and hydrology in semiarid agricultural lands. Int. Soil Water Conserv. Res. 2022, 10, 457–469. [Google Scholar] [CrossRef]
- Mujdeci, M.; Simsek, S.; Uygur, V. The effects of organic amendments on soil water retention characteristics under conventional tillage system. Fresenius Environ. Bull. 2017, 26, 4075–4081. [Google Scholar]
- Schreefel, L.; Schulte, R.P.; De Boer, I.J.; Schrijver, A.P.; Van Zanten, H.H. Regenerative agriculture–the soil is the base. Glob. Food Secur. 2020, 26, 100404. [Google Scholar] [CrossRef]
- Bulgari, R.; Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.A.O.L.O.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Sas-Paszt, L.; Frąc, M. New insight into the soil bacterial and fungal microbiome after phosphorus biofertilizer application as an important driver of regenerative agriculture including biodiversity loss reversal and soil health restoration. Appl. Soil Ecol. 2023, 189, 104941. [Google Scholar] [CrossRef]
- Kim, B.S.; Klieber, A. Quality maintenance of minimally processed Chinese cabbage with low temperature and citric acid dip. J. Sci. Food Agric. 1997, 75, 31–36. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT–Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of Flavonoids and Hydroxycinnamic Acids in Pak Choi Varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC–ESI-MS n and NMR and Their Quantification by HPLC–DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.-U.; Hwang, I.-W.; Chung, S.-K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. spp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The effect of heating and fermenting on antioxidant properties of white cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef]
- Lee, J.; Jo, N.Y.; Shim, S.Y.; Le, T.Y.L.; Jeong, W.Y.; Kwak, K.W.; Choi, H.S.; Lee, B.O.; Kim, S.R.; Lee, M.G.; et al. Impact of organic liquid fertilizer on plant growth of Chinese cabbage and soil bacterial communities. Sci. Rep. 2025, 15, 10439. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, Z.; Zhou, R.; Hou, X.; Yu, L.; Cao, Y.; Jiang, F. Nitrogen reduction with bio-organic fertilizer altered soil microorganisms, improved yield and quality of non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino). Agronomy 2022, 12, 1437. [Google Scholar] [CrossRef]
- Li, J.; Xiao, X.; Lyu, J.; Gao, C.; Ali, M.; Zhang, G.; Feng, Z.; Yu, J. Integrating bio-organic fertilization increases twice-yearly cabbage crop production by modulating soil microbial community and biochemical properties in Northwest Plateau. Environ. Technol. Innov. 2024, 35, 103715. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Fan, Y.; Zhang, L.; Wang, H. Using microalgae to reduce the use of conventional fertilizers in hydroponics and soil-based cultivation. Sci. Total Environ. 2024, 912, 169424. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.N.; Lee, M.G.; Kim, S.R.; Hwang, O. Utilization of Thermophilic Aerobic Oxidation and Electrocoagulation to Improve Fertilizer Quality from Mixed Manure Influent. Agronomy 2022, 12, 1417. [Google Scholar] [CrossRef]
- Lee, S. Case Studies on Practical Application of Chlorella Farming Technique. Ph.D. Thesis, Kongju University, Gongju, Republic of Korea, 2017; pp. 55–73. [Google Scholar]
- Jo, N.Y.; Lee, J.; Byeon, J.E.; Park, H.J.; Ryoo, J.W.; Hwang, S.G. Elevated CO2 concentration induces changes in plant growth, transcriptome, and antioxidant activity in fennel (Foeniculum vulgare Mill.). Front. Plant Sci. 2022, 13, 1067713. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-T.; Lu, M.-J.; Chen, C. Preliminary analyses of phenolic compounds and antioxidant activities in tea pollen extracts. J. Food Drug Anal. 2011, 19, 3. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleyman, H.; Karagoz, Y.; Halici, M.; Bayir, Y. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jo, N.Y.; Shim, S.Y.; Le, T.Y.L.; Kim, S.R.; Lee, M.G.; Hwang, S.G. Effects of Hanwoo (Korean cattle) manure as organic fertilizer on plant growth, feed quality, and soil bacterial community. Front. Plant Sci. 2023, 14, 1135947. [Google Scholar] [CrossRef] [PubMed]
- Amplicon, P.; Clean-Up, P.; Index, P. 16s Metagenomic Sequencing Library Preparation; Illumina: San Diego, CA, USA, 2013; p. 21. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Foster, Z.S.; Chamberlain, S.; Grünwald, N.J. Taxa: An R package implementing data standards and methods for taxonomic data. F1000Research 2018, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package; R package version 2.3-2. 2015. Available online: https://www.researchgate.net/publication/282247686_Vegan_Community_Ecology_Package_R_package_version_20-2 (accessed on 26 April 2025).
- de Mendiburu, F.; de Mendiburu, M.F. Package ‘agricolae’. R Package Version 2019, 1, 1143–1149. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. ggplot2: Create elegant data visualisations using the grammar of graphics. R Package Version 2016, 2. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 26 April 2025).
- Kolde, R.; Kolde, M. Package ‘Pheatmap’. R Package, Vol. 1. 790. 2015. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 26 April 2025).
- Neuwirth, E. RColorBrewer: Colorbrewer Palettes. 2014. Available online: https://cran.r-project.org/web/packages/RColorBrewer/index.html (accessed on 26 April 2025).
- Foster, Z.S.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Slowikowski, K.; Schep, A.; Hughes, S.; Dang, T.K.; Lukauskas, S.; Irisson, J.O.; Kamvar, Z.N.; Ryan, T.; Dervieux, C.; Yutani, H.; et al. Package Ggrepel. Automatically Position Non-Overlapping Text Labels with ‘ggplot2. 2018. Available online: https://ggrepel.slowkow.com/ (accessed on 26 April 2025).
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Krueger, F. Trim Galore; Babraham Bioinformatics: Cambridge, UK, 2012. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar]
- Carlson, M.; Falcon, S.; Pages, H.; Li, N. org. At. tair. db: Genome Wide Annotation for Arabidopsis. Bioconduct. Version 2019, 3. Available online: https://bioconductor.org/packages/release/data/annotation/html/org.At.tair.db.html (accessed on 26 April 2025).
- Islam, M.D.S.; Ayesha Ahmed, A.A.; Shahin Mahmud, S.M.; Tusher, T.R.; Khanom, S. Effects of organic fertilizer on the growth and yield of lettuce (Lactuca sativa L.) used as vegetables. Int. J. Agric. Sci. Res. 2012, 2, 116–128. [Google Scholar]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, B.; Shi, D.; Chen, A.; Liu, C. How does bio-organic fertilizer combined with biochar affect Chinese small cabbage’s growth and quality on newly reclaimed land? Plants 2024, 13, 598. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shaffique, S.; Kim, L.-R.; Kwon, E.-H.; Kim, S.-H.; Lee, Y.-H.; Kalsoom, K.; Khan, M.A.; Lee, I.-J. Effects of organic fertilizer mixed with food waste dry powder on the growth of Chinese cabbage seedlings. Environments 2021, 8, 86. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J. Crushed cotton gin compost on soil biological properties and rice yield. Eur. J. Agron. 2006, 25, 22–29. [Google Scholar] [CrossRef]
- Mardani-Talaee, M.; Nouri-Ganblani, G.; Razmjou, J.; Hassanpour, M.; Naseri, B.; Asgharzadeh, A. Effects of chemical, organic and bio-fertilizers on some secondary metabolites in the leaves of bell pepper (Capsicum annuum) and their impact on life table parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Hassani, D.; Bilal, M.; Liao, J.; Huang, D. Elevation of secondary metabolites synthesis in Brassica campestris ssp. chinensis L. via exogenous inoculation of Piriformospora indica with appropriate fertilizer. PLoS ONE 2017, 12, e0177185. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Pereira, D.M.; Pereira, J.A.; Bento, A.; Rodrigues, M.A.; Dopico-García, S.; Valentão, P.; Lopes, G.; Ferreres, F.; Seabra, R.M.; et al. Multivariate analysis of tronchuda cabbage (Brassica oleracea L. var. costata DC) phenolics: Influence of fertilizers. J. Agric. Food Chem. 2008, 56, 2231–2239. [Google Scholar] [CrossRef]
- Bonilla, N.; Gutiérrez-Barranquero, J.A.; de Vicente, A.; Cazorla, F.M. Enhancing soil quality and plant health through suppressive organic amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017, 58, 35. [Google Scholar] [CrossRef]
- Moradzadeh, S.; Siavash Moghaddam, S.; Rahimi, A.; Pourakbar, L.; Sayyed, R.Z. Combined bio-chemical fertilizers ameliorate agro-biochemical attributes of black cumin (Nigella sativa L.). Sci. Rep. 2021, 11, 11399. [Google Scholar] [CrossRef]
- Mponda, S.Y.; Kim, J.K. Sustainable, quality biofertilizer produced from Nile perch (Lates niloticus) wastewater using three Bacillus species: Demonstration in barley hydroponics. J. Environ. Chem. Eng. 2023, 11, 110037. [Google Scholar]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Ochoa Pacheco, A.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Isaac Aleman, E.; et al. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Han, H.-S.; Lee, K. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130. [Google Scholar] [CrossRef]
- Kour, D.; Wali, V.K.; Bakshi, P.; Bhat, D.J.; Sharma, B.C.; Sharma, V.; Sinha, B.K. Effect of integrated nutrient management strategies on nutrient status and soil microbial population in Aonla (Emblica officinalis Gaertn.) Cv. Na-7. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1272–1281. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, M.; Wang, X.; Huang, Q.; Nie, J.; Li, Z.; Li, S.; Hwang, S.W.; Lee, K.B. Effects of organic amendments on soil carbon sequestration in paddy fields of subtropical China. J. Soils Sediments 2012, 12, 457–470. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson: London, UK, 2017; Volume 1104. [Google Scholar]
- Wang, J.; Liu, L.; Gao, X.; Hao, J.; Wang, M. Elucidating the effect of biofertilizers on bacterial diversity in maize rhizosphere soil. PLoS ONE 2021, 16, e0249834. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Degrange, V.; Prosser, J.I.; Poly, F.; Commeaux, C.; Guillaumaud, N.; Le Roux, X. Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ. Microbiol. 2007, 9, 2211–2219. [Google Scholar] [CrossRef]
- Aloo, B.N.; Makumb, B.; Mbega, E. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Mthiyane, P.; Aycan, M.; Mitsui, T. Integrating Biofertilizers with Organic Fertilizers Enhances Photosynthetic Efficiency and Upregulates Chlorophyll-Related Gene Expression in Rice. Sustainability 2024, 16, 9297. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Baxter, C.J.; Redestig, H.; Schauer, N.; Repsilber, D.; Patil, K.R.; Nielsen, J.; Selbig, J.; Liu, J.; Fernie, A.R.; Sweetlove, L.J. The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol. 2007, 143, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, W.W.; Alba, R.; Yu, Y.S.; Bordeaux, J.M.; Simões, M.; Dean, J.F. Microarray analysis and scale-free gene networks identify candidate regulators in drought-stressed roots of loblolly pine (P. taeda L.). BMC Genom. 2011, 12, 1–17. [Google Scholar] [CrossRef]
- Brouwer, B.; Ziolkowska, A.; Bagard, M.; Keech, O.; Gardeström, P. The impact of light intensity on leaf and embryo transcriptomes in Arabidopsis thaliana. Plant Cell Environ. 2014, 37, 450–462. [Google Scholar]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2015, 7, plv002. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, C.H.; Zhu, J.K. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr. Opin. Plant Biol. 2012, 15, 303–308. [Google Scholar] [CrossRef]


| Unit | CT | LBF | p-Value |
|---|---|---|---|
| pH [1:5] | 5.44 | 5.83 | 0.011 * |
| EC [1:1] (dS/m) | 3.60 | 3.82 | 0.225 |
| OM (%) | 4.53 | 5.64 | 0.031 * |
| T-N(mg/kg) | 2171.28 | 2470.56 | 0.036 * |
| NH4+-N (mg/kg) | 78.75 | 196.78 | 0.002 ** |
| NO3−-N (mg/kg) | 260.88 | 383.58 | 0.017 ** |
| Available P (mg/kg) | 2110.30 | 2644.75 | 0.002 ** |
| Exchangeable K (cmol+/kg) | 0.70 | 0.89 | 0.057 |
| Exchangeable Ca (cmol+/kg) | 6.90 | 8.97 | 0.014 * |
| Exchangeable Mg (cmol+/kg) | 1.50 | 1.86 | 0.036 * |
| Exchangeable Na (cmol+/kg) | 0.15 | 0.30 | 0.004 ** |
| CEC (cmol+/kg) | 14.74 | 15.50 | 0.051 |
| pH | EC | OM | T-N | NH4+-N | NO3−-N | Available P | Exchangeable K | Exchangeable Ca | Exchangeable Mg | Exchangeable Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 0.069 | ||||||||||
| OM | 0.766 | 0.232 | |||||||||
| T-N | 0.675 | 0.655 | 0.74 | ||||||||
| NH4+-N | 0.85 * | 0.437 | 0.9 * | 0.938 ** | |||||||
| NO3−-N | 0.737 | 0.583 | 0.833 * | 0.967 ** | 0.973 | ||||||
| Available P | 0.794 | 0.364 | 0.889 * | 0.817 * | 0.934 ** | 0.936 ** | |||||
| Exchangeable K | 0.832 * | −0.083 | 0.476 | 0.463 | 0.63 | 0.581 | 0.72 | ||||
| Exchangeable Ca | 0.783 | 0.417 | 0.894 * | 0.945 ** | 0.986 *** | 0.965 ** | 0.903 * | 0.556 | |||
| Exchangeable Mg | 0.668 | 0.335 | 0.938 ** | 0.84 * | 0.922 ** | 0.914 * | 0.922 ** | 0.483 | 0.951 ** | ||
| Exchangeable Na | 0.878 * | 0.228 | 0.685 | 0.674 | 0.816 * | 0.797 | 0.9 * | 0.925 ** | 0.735 | 0.68 | |
| CEC | 0.718 | 0.532 | 0.428 | 0.821 * | 0.766 | 0.805 | 0.706 | 0.743 | 0.714 | 0.531 | 0.818 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.Y.L.; Lee, J.; Shim, S.-Y.; Jung, J.; Kim, S.-R.; Hong, S.-H.; Lee, M.-G.; Hwang, S.-G. Effects of Liquid Bio-Fertilizer on Plant Growth, Antioxidant Activity, and Soil Bacterial Community During Cultivation of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Microorganisms 2025, 13, 1036. https://doi.org/10.3390/microorganisms13051036
Le TYL, Lee J, Shim S-Y, Jung J, Kim S-R, Hong S-H, Lee M-G, Hwang S-G. Effects of Liquid Bio-Fertilizer on Plant Growth, Antioxidant Activity, and Soil Bacterial Community During Cultivation of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Microorganisms. 2025; 13(5):1036. https://doi.org/10.3390/microorganisms13051036
Chicago/Turabian StyleLe, Tran Yen Linh, Junkyung Lee, Su-Yeon Shim, Jiwon Jung, Soo-Ryang Kim, Sung-Ha Hong, Myung-Gyu Lee, and Sun-Goo Hwang. 2025. "Effects of Liquid Bio-Fertilizer on Plant Growth, Antioxidant Activity, and Soil Bacterial Community During Cultivation of Chinese Cabbage (Brassica rapa L. ssp. pekinensis)" Microorganisms 13, no. 5: 1036. https://doi.org/10.3390/microorganisms13051036
APA StyleLe, T. Y. L., Lee, J., Shim, S.-Y., Jung, J., Kim, S.-R., Hong, S.-H., Lee, M.-G., & Hwang, S.-G. (2025). Effects of Liquid Bio-Fertilizer on Plant Growth, Antioxidant Activity, and Soil Bacterial Community During Cultivation of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Microorganisms, 13(5), 1036. https://doi.org/10.3390/microorganisms13051036





