Preliminary Data on the Antiviral Activity of Helleborus bocconei subsp. intermedius Root Extracts Against Animal Herpesviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, Extraction and Purification Procedures
2.2. Cell Lines
2.3. Viruses
2.4. Cytotoxicity Assays
2.5. Antiviral Assays
2.5.1. Cell Culture Pretreatment with H. bocconei Extracts (Method A)
2.5.2. Treatment with H. bocconei Estracts Post Virus Infection (Method B)
2.5.3. Simultaneous Viral Infection and Treatment of Cell Cultures with H. bocconei Extracts (Method C)
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of BE
3.2. Cytotoxicity of H. bocconei Extracts
3.3. Antiviral Assays
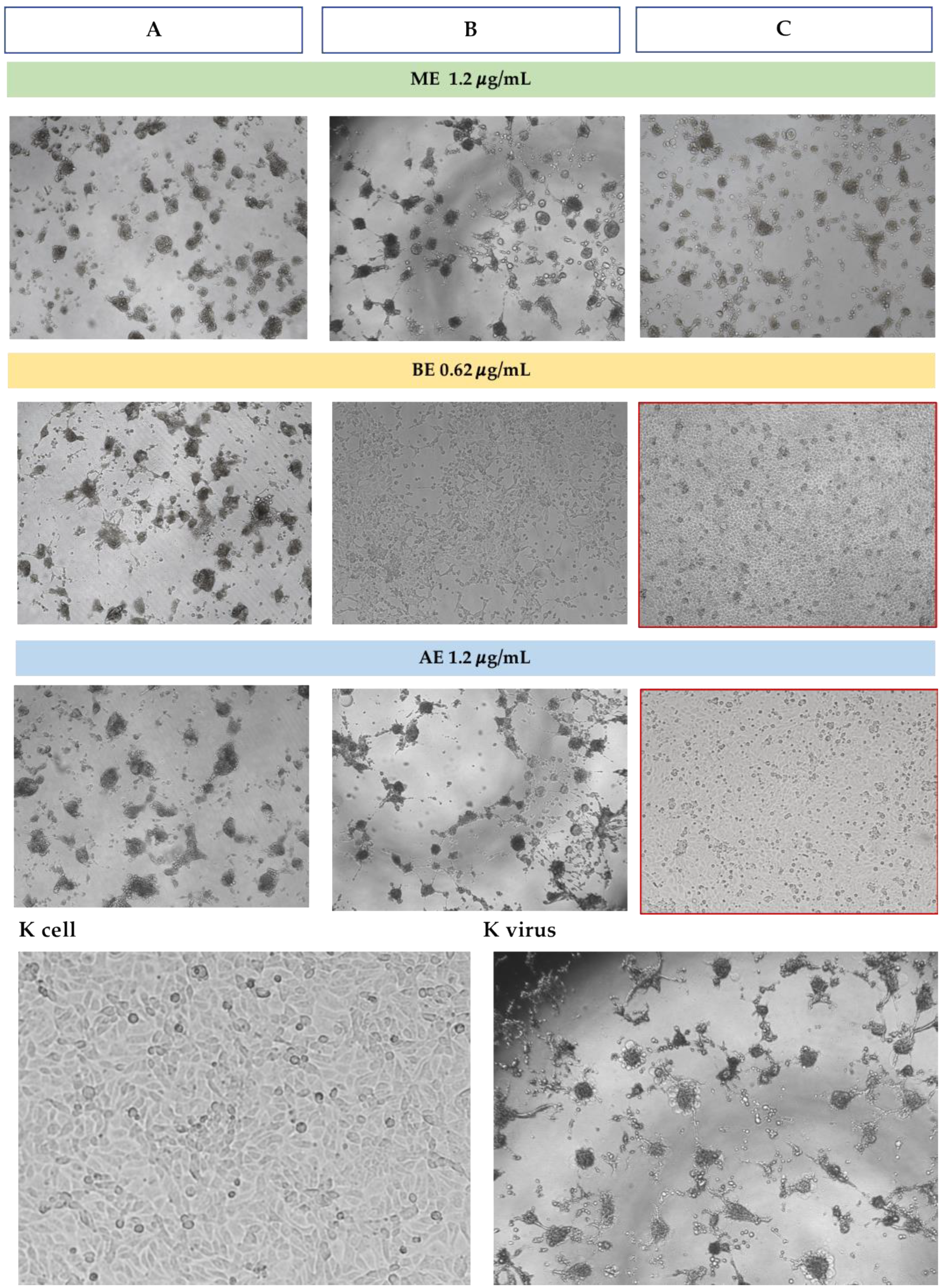
3.3.1. Antiviral Assays Against BoAHV1
3.3.2. Antiviral Assays Against CpAHV1
3.3.3. Antiviral Assays Against EqAHV1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ME | MeOH extract |
| BE | n-BuOH extract |
| AE | Aqueous extract |
References
- Azab, W.; Dayaram, A.; Greenwood, A.D.; Osterrieder, N. How Host Specific Are Herpesviruses? Lessons from Herpesviruses Infecting Wild and Endangered Mammals. Annu. Rev. Virol. 2018, 5, 53–68. [Google Scholar] [CrossRef]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant Alphaherpesviruses Related to Bovine Herpesvirus 1. Vet. Res. 2006, 37, 169–190. [Google Scholar] [CrossRef]
- Patel, J.R.; Didlick, S. Epidemiology, Disease and Control of Infections in Ruminants by Herpesviruses—An Overview. J. S. Afr. Vet. Assoc. 2008, 79, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Can, M.F.; Ataseven, V.S.; Yalçın, C. Estimation of Production and Reproductive Performance Losses in Dairy Cattle Due to Bovine Herpesvirus 1 (BoHV-1) Infection. Vet. Arh. 2016, 86, 499–513. [Google Scholar]
- Smith, K.C. Herpesviral abortion in domestic animals. Vet J. 1997, 153, 253–268. [Google Scholar] [CrossRef]
- Parameswaran, T.; Senthil, N.R. Analysis of Economic Loss Due to Equine Herpes Viral Infection. Int. J. Adv. Vet. Sci. Technol. 2014, 3, 134–139. [Google Scholar] [CrossRef]
- Vandenberghe, E.; Boshuizen, B.; Delesalle, C.J.G.; Goehring, L.S.; Groome, K.A.; Van Maanen, K.; De Bruijn, C.M. New Insights into the Management of an EHV-1 (Equine Hospital) Outbreak. Viruses 2021, 13, 1429. [Google Scholar] [CrossRef]
- Tikoo, S.K.; Campos, M.; Popowych, Y.I.; van Drunen Littel-van den Hurk, S.; Babiuk, L.A. Lymphocyte Proliferative Responses to Recombinant Bovine Herpes Virus Type 1 (BHV-1) Glycoprotein gD (gIV) in Immune Cattle: Identification of a T Cell Epitope. Viral Immunol. 1995, 8, 19–25. [Google Scholar] [CrossRef]
- Tempesta, M.; Pratelli, A.; Corrente, M.; Buonavoglia, C. A Preliminary Study on the Pathogenicity of a Strain of Caprine Herpesvirus-1. Comp. Immunol. Microbiol. Infect. Dis. 1999, 22, 137–143. [Google Scholar] [CrossRef]
- Suavet, F.; Champion, J.-L.; Bartolini, L.; Bernou, M.; Alzieu, J.-P.; Brugidou, R.; Darnatigues, S.; Reynaud, G.; Perrin, C.; Adam, G.; et al. First Description of Infection of Caprine Herpesvirus 1 (CpHV-1) in Goats in Mainland France. Pathogens 2016, 5, 17. [Google Scholar] [CrossRef]
- Nugent, J.; Birch-Machin, I.; Smith, K.C.; Mumford, J.A.; Swann, Z.; Newton, J.R.; Bowden, R.J.; Allen, G.P.; Davis-Poynter, N. Analysis of Equid Herpesvirus 1 Strain Variation Reveals a Point Mutation of the DNA Polymerase Strongly Associated with Neuropathogenic versus Nonneuropathogenic Disease Outbreaks. J. Virol. 2006, 80, 4047–4060. [Google Scholar] [CrossRef]
- Allen, G.P.; Bolin, D.C.; Bryant, U.; Carter, C.N.; Giles, R.C.; Harrison, L.R.; Hong, C.B.; Jackson, C.B.; Poonacha, K.; Wharton, R.; et al. Prevalence of Latent, Neuropathogenic Equine Herpesvirus-1 in the Thoroughbred Broodmare Population of Central Kentucky. Equine Vet. J. 2008, 40, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.S. Equid Herpesvirus–Associated Myeloencephalopathy. In Robinson’s Current Therapy in Equine Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 387–390. ISBN 978-1-4557-4555-5. [Google Scholar]
- Villa, T.G.; Feijoo-Siota, L.; Rama, J.L.R.; Ageitos, J.M. Antivirals against Animal Viruses. Biochem. Pharmacol. 2017, 133, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Thomasy, S.M.; Maggs, D.J. A Review of Antiviral Drugs and Other Compounds with Activity against Feline Herpesvirus Type 1. Vet. Ophthalmol. 2016, 19, 119–130. [Google Scholar] [CrossRef]
- Jones, C. Herpes Simplex Virus Type 1 and Bovine Herpesvirus 1 Latency. Clin. Microbiol. Rev. 2003, 16, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Ostler, J.B.; Jones, C. The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry. Viruses 2023, 15, 552. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Sirakov, I.; Jacquet, R.; Quideau, S.; Galabov, A.S. Antiviral activities of ellagitannins against bovine herpesvirus-1, suid alphaherpesvirus-1 and caprine herpesvirus-1. J. Vet. Med. Anim. Health 2020, 12, 139–143. [Google Scholar] [CrossRef]
- Bule, M.; Khan, F.; Niaz, K. Antivirals: Past, Present and Future. In Recent Advances in Animal Virology; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer: Singapore, 2019; pp. 425–446. ISBN 9789811390722. [Google Scholar]
- Dal Pozzo, F.; Thiry, E. Antiviral Chemotherapy in Veterinary Medicine: Current Applications and Perspectives. Rev. Sci. Tech. OIE 2014, 33, 791–797. [Google Scholar] [CrossRef]
- de Armas, E.; Scagliarini, A.; Battilani, M.; Alfonso, P. In Vitro Antiviral Activity of Rhizophora Mangle L. Aqueous Bark Extract and the Butanolic Fraction against Canine Distemper Virus and Bovine Herpes Virus Type. Rev. Salud Anim. 2018, 40, 1–10. [Google Scholar]
- Gabryszewska, E. Propagation in Vitro of Hellebores (Helleborus L.). Acta Sci. Pol. Hortorum Cultus 2017, 16, 61–72. [Google Scholar]
- Maior, M.; Dobrotă, C. Natural Compounds with Important Medical Potential Found in Helleborus sp. Open Life Sci. 2013, 8, 272–285. [Google Scholar] [CrossRef]
- Balázs, V.L.; Filep, R.; Ambrus, T.; Kocsis, M.; Farkas, Á.; Stranczinger, S.; Papp, N. Ethnobotanical, Historical and Histological Evaluation of Helleborus L. Genetic Resources Used in Veterinary and Human Ethnomedicine. Genet. Resour. Crop Evol. 2020, 67, 781–797. [Google Scholar] [CrossRef]
- Challinor, V.L.; Piacente, S.; De Voss, J.J. NMR Assignment of the Absolute Configuration of C-25 in Furostanol Steroidal Saponins. Steroids 2012, 77, 602–608. [Google Scholar] [CrossRef]
- Brillatz, T.; Jacmin, M.; Vougogiannopoulou, K.; Petrakis, E.A.; Kalpoutzakis, E.; Houriet, J.; Pellissier, L.; Rutz, A.; Marcourt, L.; Queiroz, E.F.; et al. Antiseizure Potential of the Ancient Greek Medicinal Plant Helleborus Odorus Subsp. Cyclophyllus and Identification of Its Main Active Principles. J. Ethnopharmacol. 2020, 259, 112954. [Google Scholar] [CrossRef]
- Horstmann, B.; Zinser, E.; Turza, N.; Kerek, F.; Steinkasserer, A. MCS-18, a Novel Natural Product Isolated from Helleborus Purpurascens, Inhibits Dendritic Cell Activation and Prevents Autoimmunity as Shown in Vivo Using the EAE Model. Immunobiology 2007, 212, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Littmann, L.; Rössner, S.; Kerek, F.; Steinkasserer, A.; Zinser, E. Modulation of Murine Bone Marrow-Derived Dendritic Cells and B-Cells by MCS-18 a Natural Product Isolated from Helleborus Purpurascens. Immunobiology 2008, 213, 871–878. [Google Scholar] [CrossRef]
- Neacsu, C.; Ciobanu, C.; Barbu, I.; Toader, O.; Szegli, G.; Kerek, F.; Babes, A. Substance MCS-18 Isolated from Helleborus Purpurascens Is a Potent Antagonist of the Capsaicin Receptor, TRPV1, in Rat Cultured Sensory Neurons. Physiol. Res. 2010, 59, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Cakar, J.; Parić, A.; Vidic, D.; Haverić, A.; Haverić, S.; Maksimović, M.; Bajrović, K. Antioxidant and Antiproliferative Activities of Helleborus Odorus Waldst. & Kit, H. Multifidus Vis. and H. Hercegovinus Martinis. Nat. Prod. Res. 2011, 25, 1969–1974. [Google Scholar] [CrossRef]
- Vochita, G.; Mihai, C.T.; Gherghel, D.; Iurea, D.; Roman, G.; Radu, G.L.; Rotinberg, P. New Potential Antitumoral Agents of Polyphenolic Nature Obtained from Helleborus Purpurascens by Membranary Micro- and Ultrafiltration Techniques. Analele Stiintifice Ale Univ. Alexandru Ioan Cuza Sect. Genet. Biol. Mol. 2011, 12, 41–51. [Google Scholar]
- Puglisi, S.; Speciale, A.; Acquaviva, R.; Ferlito, G.; Ragusa, S.; De Pasquale, R.; Iauk, L. Antibacterial Activity of Helleborus Bocconei Ten. Subsp. Siculus Root Extracts. J. Ethnopharmacol. 2009, 125, 175–177. [Google Scholar] [CrossRef]
- Roman, G.P.; Neagu, E.; Radu, G.L. Membrane Processes for the Purification and Concentration of Helleborus Purpurascens Extracts and Evaluation of Antioxidant Activity. Rev. Chim. 2010, 61, 877–881. [Google Scholar]
- Apetrei, N.S.; Călugăru, A.; Kerek, F.; Panteli, M.; Rasit, I.; Cremer, L.; Szegli, G.; Lupu, A.-R. A Highly Purified Vegetal Fraction Able to Modulate HMGB1 and to Attenuate Septic Shock in Mice. Roum. Arch. Microbiol. Immunol. 2011, 70, 114–123. [Google Scholar] [PubMed]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional Plant Use in the Areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional Uses of Plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Raimondo, F.; Lentini, F. Indagini Etnobotaniche in Sicilia. I. Le Piante Della Flora Locale Nella Tradizione Popolare Delle Madonie (Palermo). Nat. Sicil. 1990, 14, 77–99. [Google Scholar]
- Rosselli, S.; Maggio, A.; Bruno, M.; Spadaro, V.; Formisano, C.; Irace, C.; Maffettone, C.; Mascolo, N. Furostanol Saponins and Ecdysones with Cytotoxic Activity from Helleborus bocconei ssp. intermedius. Phytother. Res. PTR 2009, 23, 1243–1249. [Google Scholar] [CrossRef]
- Viol, D.I.; Chagonda, L.S.; Moyo, S.R.; Mericli, A.H. Toxicity and Antiviral Activities of Some Medicinal Plants Used by Traditional Medical Practitioners in Zimbabwe. Am. J. Plant Sci. 2016, 7, 1538–1544. [Google Scholar] [CrossRef]
- Enan, G.; Abdallah, F.M.; Sobhy, H. Effect of Acyclovir on Bovine Herpesvirus Type 1 Infection in in vitro Cultured Cells. Int. J. Virol. 2012, 8, 307–312. [Google Scholar] [CrossRef]
- Boubaker-Elandalousi, R.; Mekni-Toujani, M.; Kaabi, B.; Larbi, I.; Diouani, M.F.; Gharbi, M.; Akkari, H.; B’chir, F.; Ghram, A. Non-cytotoxic Thymus capitata extracts prevent Bovine herpesvirus-1 infection in cell cultures. BMC Vet. Res. 2014, 10, 231. [Google Scholar] [CrossRef]
- Girault, J.P.; Lafont, R. The Complete 1H-NMR Assignment of Ecdysone and 20-Hydroxyecdysone. J. Insect Physiol. 1988, 34, 701–706. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Vleggaar, R. A Revised 13C NMR Spectral Assignment of Hellebrigenin. Magn. Reson. Chem. 1988, 26, 464–467. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.-F.; Yang, F.-Y.; Gao, X.-M. New Minor Spirostanol Glycosides from Helleborus Thibetanus. Nat. Prod. Res. 2017, 31, 925–931. [Google Scholar] [CrossRef] [PubMed]
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral Effect of Polyphenol Rich Plant Extracts on Herpes Simplex Virus Type 1. Food Sci. Hum. Wellness 2018, 7, 91–101. [Google Scholar] [CrossRef]
- Borozan, A.B.; Popescu, S.; Moldovan, C.; Sărac, I.; Bordean, D. Helleborus-phytochemistry and antimicrobial properties. A review. J. Hortic. For. Biotechnol. 2022, 26, 69–75. [Google Scholar]
- Grigore, A.; Bubueanu, C.; Pirvu, L.; Neagu, G.; Bejanaru, I.; Vulturescu, V.; Panteli, M.; Rasit, I. Immunomodulatory Effect of Helleborus purpurascens Waldst. & Kit. Plants 2021, 10, 1990. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Liu, L.T.; Shieh, D.E.; Lin, C.C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Medica 2003, 69, 705–709. [Google Scholar] [CrossRef]
- Lin, L.T.; Chung, C.Y.; Hsu, W.C.; Chang, S.P.; Hung, T.C.; Shields, J.; Russell, R.S.; Lin, C.C.; Li, C.F.; Yen, M.H.; et al. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J. Hepatol. 2015, 62, 541–548. [Google Scholar] [CrossRef]
- Wijayasinghe, Y.S.; Bhansali, P.; Viola, R.E.; Kamal, M.A.; Poddar, N.K. Natural Products: A Rich Source of Antiviral Drug Lead Candidates for the Management of COVID-19. Curr. Pharm. Des. 2021, 27, 3526–3550. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, X.Q.; Dong, X.F.; Guo, P.; Wang, S.B.; Qin, Z.H. Saikosaponin B2, Punicalin, and Punicalagin in Vitro Block Cellular Entry of Feline Herpesvirus-1. Viruses 2024, 16, 231. [Google Scholar] [CrossRef]
- Atanasiu, D.; Saw, W.T.; Gallagher, J.R.; Hannah, B.P.; Matsuda, Z.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cellfusion induced by HSV entry glycoproteins. J. Virol. 2013, 87, 11332–11345. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2021, 9, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Feng, H.; Zhuo, Y.; Li, M.; Zhu, X.; Huang, L.; Zhang, X.; Zhou, Z.; Zheng, C.; Jiang, Y.; et al. Bufalin inhibits hepatitis B virus-associated hepatocellular carcinoma development through androgen receptor dephosphorylation and cell cycle-related kinase degradation. Cell. Oncol. 2020, 43, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
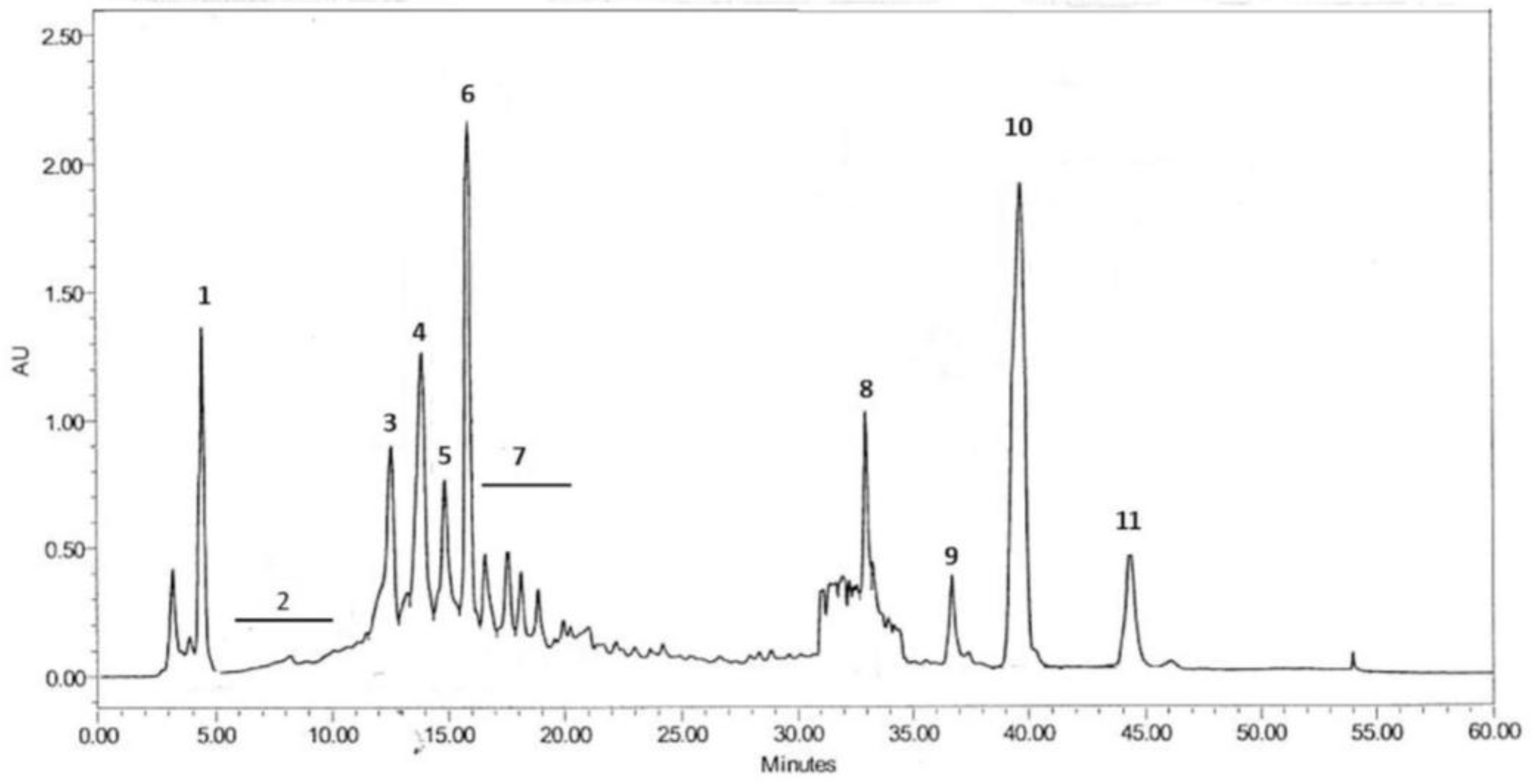





| Cell Line | Concentration (µg/mL) | Mean Cell Survival Percentage (±SD) | ||
|---|---|---|---|---|
| ME | BE | AE | ||
| MDBK | 0.31 | 101 (±0.1) | 100 (±0.05) | 100 (±1.03) |
| 0.62 | 100 (±0.13) | 95 (±0.1) | 100 (±0.9) | |
| 1.25 | 97.68 (±0.12) | 72.04 (±0.1) | 99.63 (±0.14) | |
| 2.5 | 59.79 (±0.15) | 54.46 (±0.13) | 77.12 (±0.12) | |
| 25 | 38.14 (±0.05) | 34.40 (±0.09) | 44.62 (±0.05) | |
| RK-13 | 0.31 | 100 (±0.06) | 100 (±0.03) | 100 (±0.02) |
| 0.62 | 100 (±0.01) | 100 (±0.1) | 100 (±0.06) | |
| 1.25 | 100 (±0.04) | 45.24 (±0.08) | 100 (±0.08) | |
| 2.5 | 98.23 (±0.09) | 40 (±0.05) | 100 (±0.1) | |
| 25 | 60 (±0.08) | 35.9 (±0.07) | 50 (±0.1) | |
| Cell Line | Extract | CC50 (µg/mL) |
|---|---|---|
| MDBK | ME | 12.67 |
| BE | 7.32 | |
| AE | 21.27 | |
| RK-13 | ME | >25 |
| BE | 1.19 | |
| AE | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, P.; Di Bella, S.; Migliore, S.; Raimondi, M.V.; Bivacqua, R.; Borgonovo, G.; Princiotto, S.; Girgenti, A.; Palumbo, L.; Dara, S.; et al. Preliminary Data on the Antiviral Activity of Helleborus bocconei subsp. intermedius Root Extracts Against Animal Herpesviruses. Microorganisms 2025, 13, 891. https://doi.org/10.3390/microorganisms13040891
Galluzzo P, Di Bella S, Migliore S, Raimondi MV, Bivacqua R, Borgonovo G, Princiotto S, Girgenti A, Palumbo L, Dara S, et al. Preliminary Data on the Antiviral Activity of Helleborus bocconei subsp. intermedius Root Extracts Against Animal Herpesviruses. Microorganisms. 2025; 13(4):891. https://doi.org/10.3390/microorganisms13040891
Chicago/Turabian StyleGalluzzo, Paola, Santina Di Bella, Sergio Migliore, Maria Valeria Raimondi, Roberta Bivacqua, Gigliola Borgonovo, Salvatore Princiotto, Antonella Girgenti, Laura Palumbo, Salvatore Dara, and et al. 2025. "Preliminary Data on the Antiviral Activity of Helleborus bocconei subsp. intermedius Root Extracts Against Animal Herpesviruses" Microorganisms 13, no. 4: 891. https://doi.org/10.3390/microorganisms13040891
APA StyleGalluzzo, P., Di Bella, S., Migliore, S., Raimondi, M. V., Bivacqua, R., Borgonovo, G., Princiotto, S., Girgenti, A., Palumbo, L., Dara, S., Guercio, A., Alduina, R., Loria, G. R., & Cannella, V. (2025). Preliminary Data on the Antiviral Activity of Helleborus bocconei subsp. intermedius Root Extracts Against Animal Herpesviruses. Microorganisms, 13(4), 891. https://doi.org/10.3390/microorganisms13040891











