Phyllanthus emblica: Phytochemistry, Antimicrobial Potential with Antibiotic Enhancement, and Toxicity Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sources
2.2. Extract Preparation
2.3. Antibiotics and Bacterial Strains
2.4. Antibacterial Susceptibility Screening
2.5. Minimum Inhibitory Concentration (MIC)
2.6. Assessment of Fractional Inhibitory Concentration (FIC)
2.7. Toxicity Evaluation
2.8. Non-Targeted Headspace Quantitative Analysis Using LC-MS
3. Results
3.1. Antibacterial Assays
3.2. Combinatorial Studies: Fractional Inhibitory Concentration Determinations
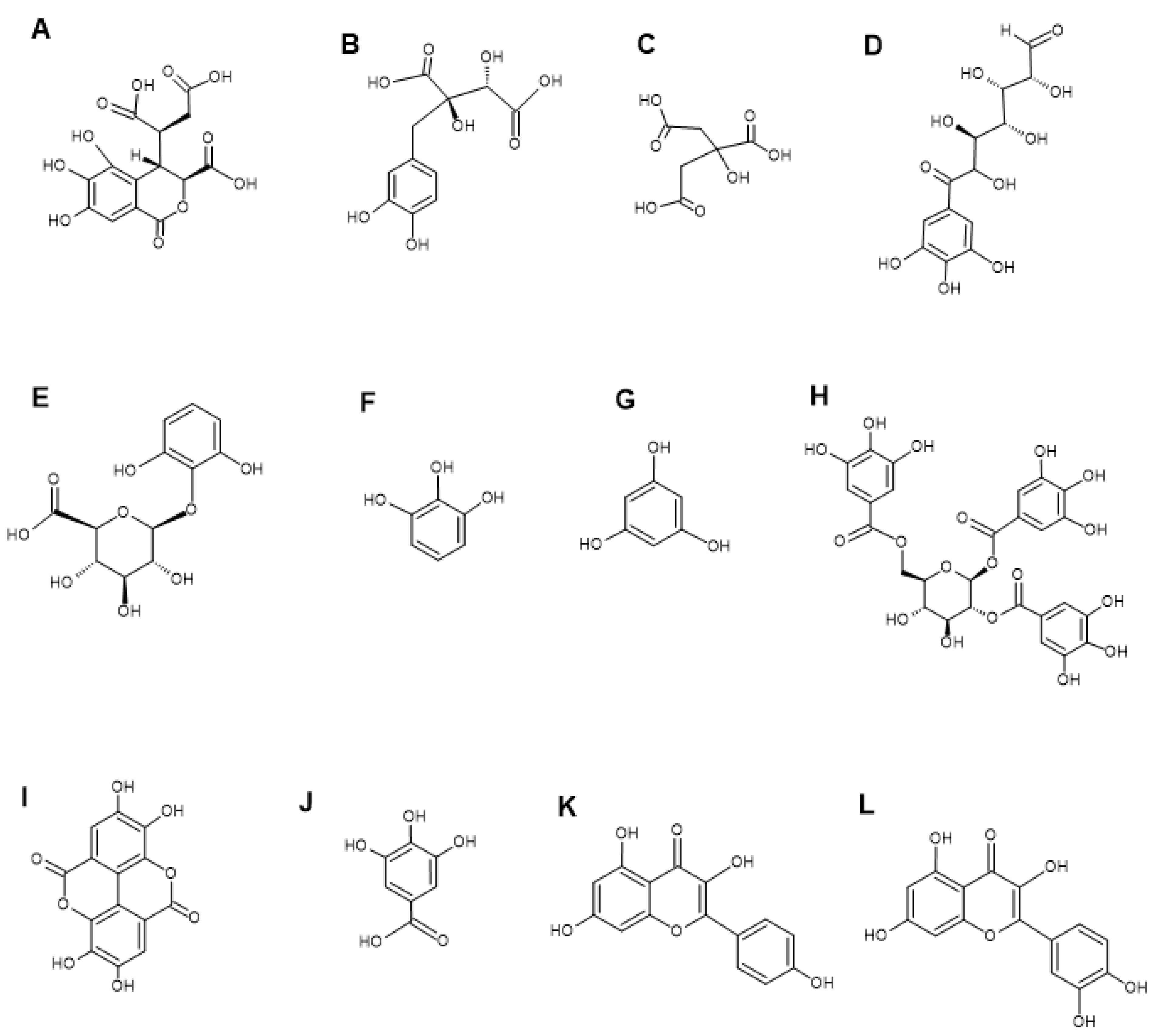
3.3. LC-MS Metabolomic Analysis and Compound Characterisation
3.4. Toxicity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 31 January 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. A review of ayurvedic principles and the use of ayurvedic plants to control diarrhoea and gastrointestinal infections. Pharmacogn. Commun. 2023, 13, 152–162. [Google Scholar] [CrossRef]
- Mundy, L.; Pendry, B.; Rahman, M. Antimicrobial resistance and synergy in herbal medicine. J. Herb. Med. 2016, 6, 53–58. [Google Scholar] [CrossRef]
- De Oliveira, S.M.S.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Costa, M.J.C.; Diniz, M.F.F.M. Modulation of drug resistance in Staphylococcus aureus by extract of mango (Mangifera indica L., anacardiaceae) peel. Rev. Bras. Farmacogn. 2011, 21, 190–193. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, V.; Sourirajan, A.; Dev, K. Fruit extract and phenolic compounds of Phyllanthus emblica fruits as bioactivity enhancer of chloramphenicol against bacterial species. Plant Foods Hum. Nutr. 2024, 79, 656–661. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, V.; Patel, C.N.; Sourirajan, A.; Dev, K. Synergistic antibacterial activity of Phyllanthus emblica fruits and its phytocompounds with ampicillin: A computational and experimental study. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 397, 857–871. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of control: The need for standardised solvent approaches and data reporting in antibiofilm assays incorporating dimethyl-sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar] [CrossRef]
- Jahan, N.; Akter, S. Assessment of the antimicrobial activity of the ethanolic extract of Phyllanthus emblica in combination with different classes of antibiotics against single and multi-drug resistant strains. J. Pharmacogn. Phytochem. 2015, 4, 142–155. [Google Scholar]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus niruri Linn.: Antibacterial activity, phytochemistry, and enhanced antibiotic combinatorial strategies. Antibiotics 2024, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Vitko, N.P.; Richardson, A.R. Laboratory maintenance of methicillin-resistant Staphylococcus aureus (MRSA). Curr. Protoc. Microbiol. 2013, 28, 9C-2. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective efficacy of the antioxidants vitamin E and Trolox against Microcystis aeruginosa and Microcystin-LR in Artemia franciscana Nauplii. J. Toxicol. Environ. Health A 2009, 72, 1567–1575. [Google Scholar] [CrossRef]
- Cajka, T.; Hricko, J.; Rudl Kulhava, L.; Paucova, M.; Novakova, M.; Kuda, O. Optimization of mobile phase modifiers for fast LC-MS-based untargeted metabolomics and lipidomics. Int. J. Mol. Sci. 2023, 24, 31987. [Google Scholar] [CrossRef]
- Angelini, P. Plant-derived antimicrobials and their crucial role in combating antimicrobial resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; White, A.; Cheesman, M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020, 260, 112937. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Steck, T.R. The relationship between agar thickness and antimicrobial susceptibility testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 2000, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Mwangi, M.; Chung, M.; Milheirco, C.; De Lencastre, H.; Tomasz, A. The mechanism of heterogeneous beta-lactam resistance in MRSA: Key role of the stringent stress response. PLoS ONE 2013, 8, e82814. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Cheesman, M.; Ilanko, A.; Blonk, B.; Cock, I. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [CrossRef]
- Cai, X.; Javor, S.; Gan, B.H.; Köhler, T.; Reymond, J.L. The antibacterial activity of peptide dendrimers and polymyxin B increases sharply above pH 7.4. Chem. Commun. 2021, 57, 5654–5657. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Subedi, L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2014, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, H.; Yasin, K.A.; Choudhary, M.A.; Khaliq, N.; Ur-Rahman, A.; Choudhary, M.I.; Malik, S. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 2007, 21, 775–781. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Grewal, A.S.; Guarve, K. Pharmacological properties of chebulinic acid and related ellagitannins from nature: An emerging contemporary bioactive entity. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100163. [Google Scholar] [CrossRef]
- Yang, Z.N.; Su, B.J.; Wang, Y.Q.; Liao, H.B.; Chen, Z.F.; Liang, D. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid derivatives from Euphorbia hirta. J. Nat. Prod. 2020, 83, 985–995. [Google Scholar] [CrossRef]
- Ayşe, E.; Eliuz, E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2021, 72, 332–340. [Google Scholar] [CrossRef]
- Li, X.-S.; Xue, J.-Z.; Qi, Y.; Muhammad, I.; Wang, H.; Li, X.-Y.; Luo, Y.-J.; Zhu, D.-M.; Gao, Y.-H.; Kong, L.-C.; et al. Citric acid confers broad antibiotic tolerance through alteration of bacterial metabolism and oxidative stress. Int. J. Mol. Sci. 2023, 24, 9089. [Google Scholar] [CrossRef]
- Ahmad, B.; Hafeez, N.; Rauf, A.; Bashir, S.; Linfang, H.; Rehman, M.U.; Mubarak, M.S.; Uddin, M.S.; Bawazeer, S.; Shariati, M.A.; et al. Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S. Afr. J. Bot. 2021, 138, 278–310. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Efflux-pump inhibitory activity of a gallotannin from Terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Synergistic antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from Terminalia chebula fruit in combination with gentamicin and trimethoprim against multidrug-resistant uropathogenic Escherichia coli biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Anek, P.; Kumpangcum, S.; Roytrakul, S.; Khanongnuch, C.; Saenjum, C.; Phannachet, K. Antibacterial activities of phenolic compounds in Miang extract: Growth inhibition and change in protein expression of extensively drug-resistant Klebsiella pneumoniae. Antibiotics 2024, 13, 536. [Google Scholar] [CrossRef]
- Jenic, D.; Waller, H.; Collins, H.; Erridge, C. Reversal of tetracycline resistance by cepharanthine, cinchonidine, ellagic acid and propyl gallate in a multidrug-resistant Escherichia coli. Nat. Prod. Bioprospect. 2021, 11, 345–355. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The antibacterial activity of kaempferol combined with colistin against colistin-resistant Gram-negative bacteria. Microbiol. Spectr. 2022, 10, e02265-22. [Google Scholar] [CrossRef]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Alnour, T.M.S.; Ahmed-Abakur, E.H.; Elssaig, E.H.; Abuduhier, F.M.; Ullah, M.F. Antimicrobial synergistic effects of dietary flavonoids rutin and quercetin in combination with antibiotics gentamicin and ceftriaxone against E. coli (MDR) and P. mirabilis (XDR) strains isolated from human infections: Implications for food–medicine interactions. Ital. J. Food Sci. 2022, 34, 34–42. [Google Scholar] [CrossRef]
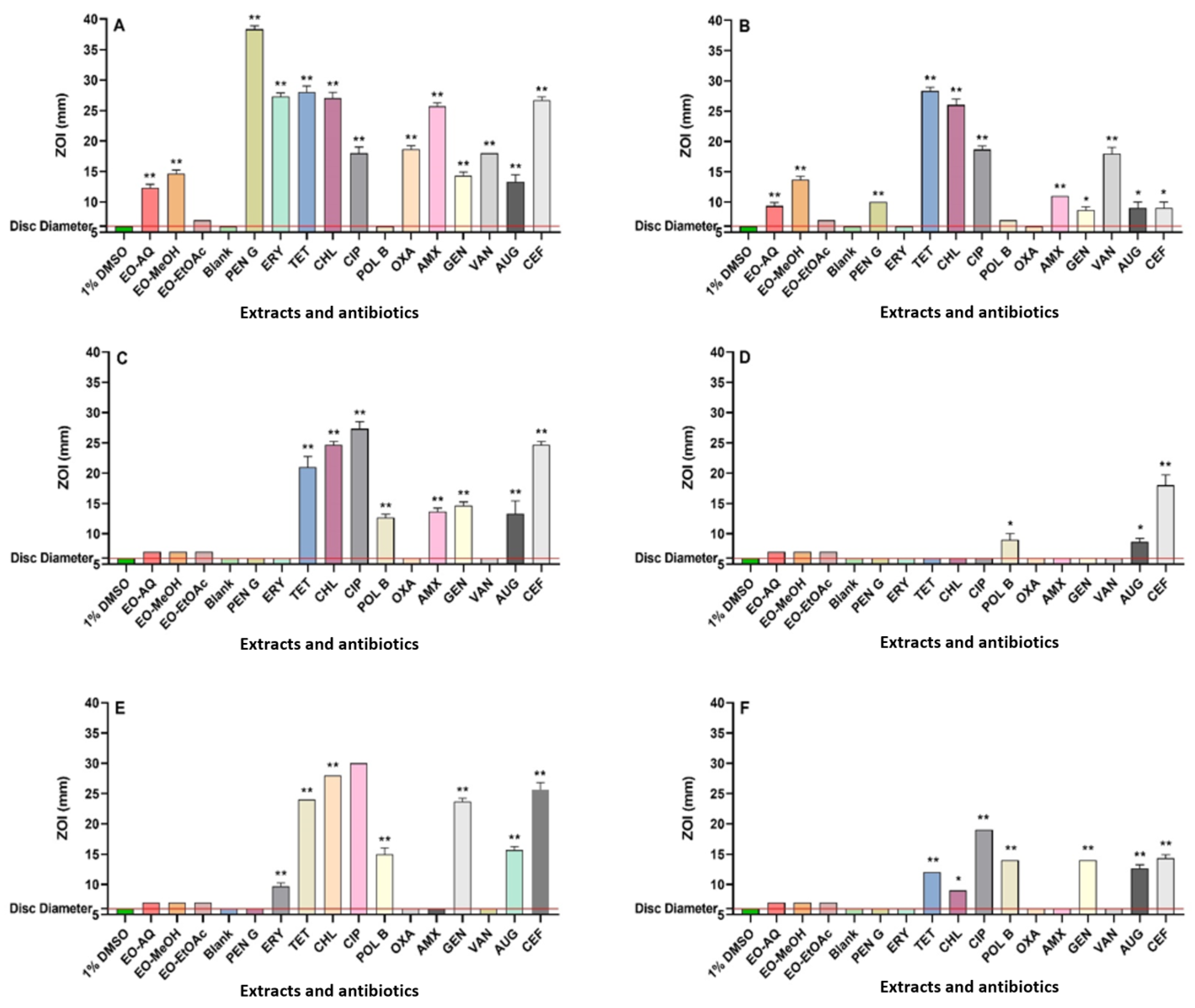
| Extract Type or Antibiotic | Bacterial Species and MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | MRSA | E. coli | ESBL E. coli | K. pneumoniae | ESBL K. pneumoniae | |
| EO-AQ | 316 | 158 | 5063 | 5063 | 633 | 10,125 |
| EO-MeOH | 185 | 185 | 2956 | 1478 | 2956 | 2956 |
| EO-EtOAc | 863 | 1725 | >10,000 | >10,000 | >10,000 | >10,000 |
| PENG | 1.25 | - | - | - | - | - |
| ERY | 0.31 | - | - | - | - | - |
| TET | 0.16 | 0.04 | 0.31 | - | 0.625 | - |
| CHL | - | - | 2.5 | - | 2.5 | - |
| CIP | 0.16 | 0.625 | 0.02 | - | 0.02 | 0.16 |
| POLB | - | - | 0.02 | 0.02 | 0.02 | 0.04 |
| OXA | 0.16 | - | - | - | - | - |
| AMX | 0.625 | - | - | - | - | - |
| GEN | - | - | 0.625 | - | 0.625 | - |
| VAN | 1.25 | 1.25 | - | - | - | - |
| Bacteria | Extracts | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PENG | ERY | TET | CHL | CIP | POLB | OXA | AMX | GEN | VAN | ||
| S. aureus | EO-AQ | 0.53 | 1.25 | 0.75 | - | 1.50 | - | 1.50 | 1.13 | - | 1.06 |
| EO-MeOH | 0.53 | 1.13 | 1.25 | - | 1.25 | - | 1.25 | 1.06 | - | 1.03 | |
| EO-EtOAc | 1.00 | 1.25 | 1.13 | - | 2.15 | - | 2.25 | 1.50 | - | 2.00 | |
| MRSA | EO-AQ | - | - | 1.00 | - | 1.06 | - | - | - | - | 1.03 |
| EO-MeOH | - | - | 1.00 | - | 1.06 | - | - | - | - | 1.03 | |
| EO-EtOAc | - | - | 1.02 | - | 0.63 | - | - | - | - | 0.75 | |
| E. coli | EO-AQ | - | - | 1.25 | 1.50 | 8.31 | 63.50 | - | - | 3.00 | - |
| EO-MeOH | - | - | 1.50 | 1.25 | 2.25 | 64.50 | - | - | 2.00 | - | |
| ESBL E. coli | EO-AQ | - | - | - | - | - | 63.50 | - | - | - | - |
| EO-MeOH | - | - | - | - | - | 16.50 | - | - | - | - | |
| K. pneumoniae | EO-AQ | - | - | 1.25 | 2.12 | 3.00 | 17.50 | - | - | 2.50 | - |
| EO-MeOH | - | - | 1.00 | 1.25 | 2.50 | 32.25 | - | - | 2.00 | - | |
| ESBL K. pneumoniae | EO-AQ | - | - | - | - | 2.13 | 31.75 | - | - | - | - |
| EO-MeOH | - | - | - | - | 2.50 | 8.25 | - | - | - | - | |
| Retention Time (min) | Molecular Mass | Empirical Formula | Putative Compounds | % Relative Abundance | ||
|---|---|---|---|---|---|---|
| AQ | MeOH | EtOAc | ||||
| 1.191 | 148.037 | C5 H8 O5 | δ-Ribono-1,4-lactone | - | 1.51% | 1.68% |
| 1.251 | 160.0369 | C6 H8 O5 | Cortalcerone | - | 1.14% | - |
| 1.269 | 116.0108 | C4 H4 O4 | Maleic acid | 0.75% | 0.97% | - |
| 1.371 | 194.0789 | C7 H14 O6 | Methyl β-D-glucopyranoside | - | 0.09% | - |
| 1.373 | 356.0377 | C14 H12 O11 | (+)-Chebulic acid | 0.15% | 0.95% | 2.55% |
| 1.377 | 158.02104 | C6 H6 O5 | 2-Methylene-4-oxopentanedioic acid | - | - | 2.37% |
| 1.41 | 100.0523 | C5 H8 O2 | Tiglic acid | - | 0.50% | - |
| 1.454 | 137.0475 | C7 H7 N O2 | Trigonelline | 2.53% | 0.03% | - |
| 1.457 | 130.02623 | C5 H6 O4 | Mesaconic acid | 0.48% | - | - |
| 1.464 | 104.01077 | C3 H4 O4 | Malonic acid | 0.23% | - | - |
| 1.589 | 206.0424 | C7 H10 O7 | 2-Methylcitric acid | - | 0.06% | - |
| 1.593 | 96.02098 | C5 H4 O2 | 2-Furancarboxaldehyde | 0.06% | 0.07% | 0.38% |
| 1.597 | 272.05292 | C11 H12 O8 | Fukiic acid | - | - | 15.32% |
| 1.619 | 85.089 | C5 H11 N | Piperidine | 0.06% | 0.34% | 0.12% |
| 1.752 | 210.03735 | C6 H10 O8 | D-Saccharic acid | 0.97% | - | 8.83% |
| 1.769 | 192.0269 | C6 H8 O7 | Isocitric acid | 15.42% | 17.26% | 1.61% |
| 1.814 | 192.02685 | C6 H8 O7 | Citric acid | 5.62% | - | 2.61% |
| 1.933 | 370.0534 | C15 H14 O11 | 2-O-Caffeoylhydroxycitric acid | - | 0.16% | - |
| 1.947 | 206.02141 | C10 H6 O5 | Flaviolin | - | - | 1.08% |
| 1.968 | 316.0794 | C13 H16 O9 | Ginnalin B | - | 0.03% | - |
| 2.066 | 134.0215 | C4 H6 O5 | D-(+)-Malic acid | 0.38% | 2.96% | 2.41% |
| 2.067 | 148.03702 | C5 H8 O5 | Ribonolactone | 0.09% | - | - |
| 2.084 | 634.08037 | C27 H22 O18 | Corilagin | 0.30% | - | - |
| 2.165 | 222.05273 | C11 H10 O5 | Isofraxidin | 0.01% | - | - |
| 2.186 | 178.02655 | C9 H6 O4 | Aesculetin | 0.01% | - | 0.03% |
| 2.303 | 332.0742 | C13 H16 O10 | 6-Galloylglucose | 19.79% | 2.80% | 7.03% |
| 2.375 | 204.00925 | C7 H8 O5 S | O-methoxy catechol-O-sulphate | 0.01% | - | - |
| 2.631 | 240.06348 | C11 H12 O6 | Lignicol | 0.04% | - | - |
| 2.786 | 346.0902 | C14 H18 O10 | Methyl 6-O-galloyl-β-D-glucopyranoside | 0.01% | 0.01% | - |
| 3.272 | 342.09498 | C15 H18 O9 | Glucocaffeic acid | - | - | 2.02% |
| 3.418 | 302.0638 | C12 H14 O9 | Pyrogallol-2-O-glucuronide | 10.94% | 4.52% | 1.03% |
| 3.48 | 296.05274 | C13 H12 O8 | Caffeoylmalic acid | 0.01% | - | - |
| 3.914 | 484.0849 | C20 H20 O14 | Hamamelitannin | 0.35% | 0.74% | 0.11% |
| 3.919 | 126.0316 | C6 H6 O3 | Phloroglucinol | 1.55% | 0.76% | 0.79% |
| 4.151 | 232.00061 | C12 H8 O S2 | Arctinal | - | - | 0.01% |
| 4.236 | 348.08412 | C17 H16 O8 | 3,5,7,3′,4′,5′-Hexahydroxy-6,8-dimethylflavanone | - | - | 1.01% |
| 4.585 | 312.0479 | C13 H12 O9 | Caftaric acid | - | 1.69% | - |
| 4.679 | 484.0857 | C20 H20 O14 | 1,6-bis-O-(3,4,5-trihydroxybenzoyl) hexopyranose | 1.59% | 7.35% | 3.59% |
| 6.312 | 264.027 | C13 H12 O2 S2 | Arctinol | T | 0.01% | - |
| 8.821 | 634.0809 | C27 H22 O18 | Sanguiin H4 | - | 0.03% | 1.77% |
| 9.699 | 126.0317 | C6 H6 O3 | Pyrogallol | - | 9.18% | 6.74% |
| 9.898 | 498.101 | C21 H22 O14 | Methyl 4,6-di-O-galloyl-β-D-glucopyranoside | - | 0.01% | - |
| 10.264 | 314.0635 | C13 H14 O9 | β-D-glucopyranuronic acid | 0.99% | 2.18% | - |
| 10.44 | 636.0962 | C27 H24 O18 | 1,2,6-trigalloyl-β-D-glucopyranose | - | 1.30% | - |
| 10.872 | 434.04837 | C19 H14 O12 | Ellagic acid arabinoside | - | - | 1.05% |
| 10.925 | 176.0319 | C6 H8 O6 | Ascorbic acid | - | 0.11% | - |
| 10.932 | 636.09613 | C27 H24 O18 | 1,3,4-trigalloyl-β-D-glucopyranose | 0.02% | - | 0.16% |
| 11.195 | 170.0214 | C7 H6 O5 | Gallic acid | 1.19% | 0.38% | 1.42% |
| 11.221 | 442.09013 | C22 H18 O10 | Robinetinidol 3-O-gallate | - | - | 0.01% |
| 11.422 | 464.0953 | C21 H20 O12 | Myricitrin | 0.14% | 0.21% | - |
| 11.608 | 180.09368 | C14 H12 | Stilbene | - | - | 2.04% |
| 11.616 | 172.0735 | C8 H12 O4 | (-)-Corey lactone | 0.03% | 0.06% | - |
| 11.641 | 448.0641 | C20 H16 O12 | Ellagic acid 2-rhamnoside | 0.01% | 0.01% | 1.06% |
| 11.663 | 478.11122 | C22 H22 O12 | 6-Methoxyluteolin 7-glucoside | 0.01% | - | - |
| 11.731 | 600.1113 | C28 H24 O15 | Isoorientin 2″-O-gallate | 0.01% | 0.01% | - |
| 11.746 | 162.06799 | C10 H10 O2 | 4,5-dihydro-1-benzoxepin-3(2H)-one | - | - | 0.01% |
| 11.916 | 448.1005 | C21 H20 O11 | Trifolin | 0.59% | 0.55% | 0.31% |
| 11.917 | 286.0476 | C15 H10 O6 | Kaempferol | - | 0.46% | 0.14% |
| 11.963 | 184.037 | C8 H8 O5 | Methyl gallate | 0.24% | 0.90% | - |
| 11.965 | 170.0214 | C7 H6 O5 | 2,4,6-trihydroxybenzoic acid | 0.24% | 1.69% | 1.27% |
| 11.978 | 310.1049 | C15 H18 O7 | (E)-1-O-cinnamoyl-β-D-glucose | - | 3.86% | 2.01% |
| 11.99 | 302.0062 | C14 H6 O8 | Ellagic acid | 12.37% | 7.84% | 9.17% |
| 11.991 | 302.0423 | C15 H10 O7 | Quercetin | 0.18% | 0.27% | - |
| 12.033 | 594.101 | C29 H22 O14 | Epicatechin 3,5-di-O-gallate | - | T | - |
| 12.179 | 272.0683 | C15 H12 O5 | Naringeninchalcone | 0.04% | 0.06% | - |
| 12.292 | 492.0908 | C22 H20 O13 | 6-Methoxyluteolin 7-glucuronide | 0.01% | 0.03% | 1.01% |
| 12.307 | 286.04732 | C15 H10 O6 | Fisetin | 0.31% | - | - |
| 12.324 | 148.0524 | C9 H8 O2 | Cinnamic acid | 1.01% | 1.26% | 1.45% |
| 12.437 | 349.19973 | C18 H27 N3 O4 | Coutaric acid | 0.02% | - | - |
| 12.464 | 474.07948 | C22 H18 O12 | Chicoric acid | 0.01% | - | - |
| 12.48 | 292.09429 | C15 H16 O6 | (S)-Angelicain | 0.01% | - | - |
| 12.545 | 432.1059 | C21 H20 O10 | Afzelin | - | 0.03% | - |
| 12.674 | 550.1689 | C26 H30 O13 | Licuroside | - | 0.01% | 0.01% |
| 12.757 | 150.06792 | C9 H10 O2 | Hydrocinnamic acid | - | - | 0.04% |
| 12.919 | 185.14141 | C10 H19 N O2 | 1-Methylpiperidin-4-yl butanoate | - | - | 0.09% |
| 12.937 | 304.0581 | C15 H12 O7 | Nigrescin | 0.02% | 0.02% | - |
| 12.983 | 572.1901 | C29 H32 O12 | Amorphigenin O-glucoside | 0.02% | 0.02% | - |
| 12.996 | 262.01149 | C13 H10 O2 S2 | Arctinone A | 0.01% | - | - |
| 13.009 | 262.0477 | C13 H10 O6 | Maclurin | - | T | - |
| 13.026 | 444.1054 | C22 H20 O10 | 3′-O-Methylderhamnosylmaysin | 0.08% | 0.06% | 0.01% |
| 13.043 | 524.15321 | C24 H28 O13 | Barbatoflavan | - | - | 1.01% |
| 13.046 | 538.16926 | C25 H30 O13 | Lippioside I | T | - | - |
| 13.058 | 218.05794 | C12 H10 O4 | Liqcoumarin | T | - | - |
| 13.159 | 506.1062 | C23 H22 O13 | Quercetin 3- (6″-ethylglucuronide) | - | 0.04% | - |
| 13.188 | 504.34447 | C30 H48 O6 | Protobassic acid | - | - | 1.02% |
| 13.188 | 486.334 | C30 H46 O5 | Bassic acid | - | - | 1.01% |
| 13.561 | 400.1155 | C21 H20 O8 | Torosaflavone A | 0.02% | 0.03% | - |
| 13.647 | 462.1162 | C22 H22 O11 | Leptosin | T | 0.03% | 0.01% |
| 13.722 | 274.08384 | C15 H14 O5 | Phloretin | 0.02% | - | - |
| 13.874 | 614.1273 | C29 H26 O15 | 2-Cinnamoyl-1,6-digalloyl-β-D-glucopyranose | - | 0.01% | - |
| 13.92 | 260.15199 | C15 H20 N2 O2 | Baptifoline | T | - | - |
| 14.278 | 148.0523 | C9 H8 O2 | p-Coumaraldehyde | 0.18% | 0.26% | - |
| 14.472 | 290.0788 | C15 H14 O6 | Marshrin | - | 0.04% | - |
| 14.812 | 286.0477 | C15 H10 O6 | Maritimetin | - | 0.08% | - |
| 14.829 | 580.1582 | C30 H28 O12 | Naringenin 7- (2-p-Coumaroylglucoside) | 0.08% | - | - |
| 16.15 | 316.13099 | C18 H20 O5 | 4,2′,6′-Trihydroxy-4′-methoxy-3′,5′-dimethyldihydrochalcone | - | - | 1.01% |
| 17.041 | 184.09993 | C12 H12 N2 | Harmalan | - | - | 0.02% |
| 17.512 | 286.12056 | C17 H18 O4 | 4-Hydroxy-2′,4′-dimethoxydihydrochalcone | T | - | - |
| 17.969 | 256.07342 | C15 H12 O4 | Isoliquiritigenin | - | - | 1.01% |
| 18.036 | 314.11518 | C18 H18 O5 | 4′-Hydroxyenterolactone | - | - | 0.06% |
| 18.608 | 242.09407 | C15 H14 O3 | 2′,4′-Dihydroxydihydrochalcone | - | - | 0.03% |
| 18.835 | 388.20942 | C19 H32 O8 | 5a,6a-Epoxy-7E-megastigmene-3a,9e-diol 3-glucoside | - | - | 0.08% |
| 19.447 | 226.099 | C15 H14 O2 | 7-Hydroxyflavan | - | 0.03% | 0.02% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus emblica: Phytochemistry, Antimicrobial Potential with Antibiotic Enhancement, and Toxicity Insights. Microorganisms 2025, 13, 611. https://doi.org/10.3390/microorganisms13030611
Tiwana G, Cock IE, Cheesman MJ. Phyllanthus emblica: Phytochemistry, Antimicrobial Potential with Antibiotic Enhancement, and Toxicity Insights. Microorganisms. 2025; 13(3):611. https://doi.org/10.3390/microorganisms13030611
Chicago/Turabian StyleTiwana, Gagan, Ian Edwin Cock, and Matthew James Cheesman. 2025. "Phyllanthus emblica: Phytochemistry, Antimicrobial Potential with Antibiotic Enhancement, and Toxicity Insights" Microorganisms 13, no. 3: 611. https://doi.org/10.3390/microorganisms13030611
APA StyleTiwana, G., Cock, I. E., & Cheesman, M. J. (2025). Phyllanthus emblica: Phytochemistry, Antimicrobial Potential with Antibiotic Enhancement, and Toxicity Insights. Microorganisms, 13(3), 611. https://doi.org/10.3390/microorganisms13030611








