The Role of Nitric Oxide in the Growth and Development of Schizophyllum commune Under Anaerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Media
2.2. Mycelial, Basidiospore, and Fruiting Body Cultures of S. commune 20R-7-F01 Under Anaerobic Conditions
2.3. Basidiospore Collection from Fruiting Bodies
2.4. Qualitative Detection of NO Concentration in Mycelia and Basidiospores
2.5. Quantification of NO Content
2.6. Gene Expression Analysis Using RT-qPCR
2.7. Statistical Analysis
3. Results
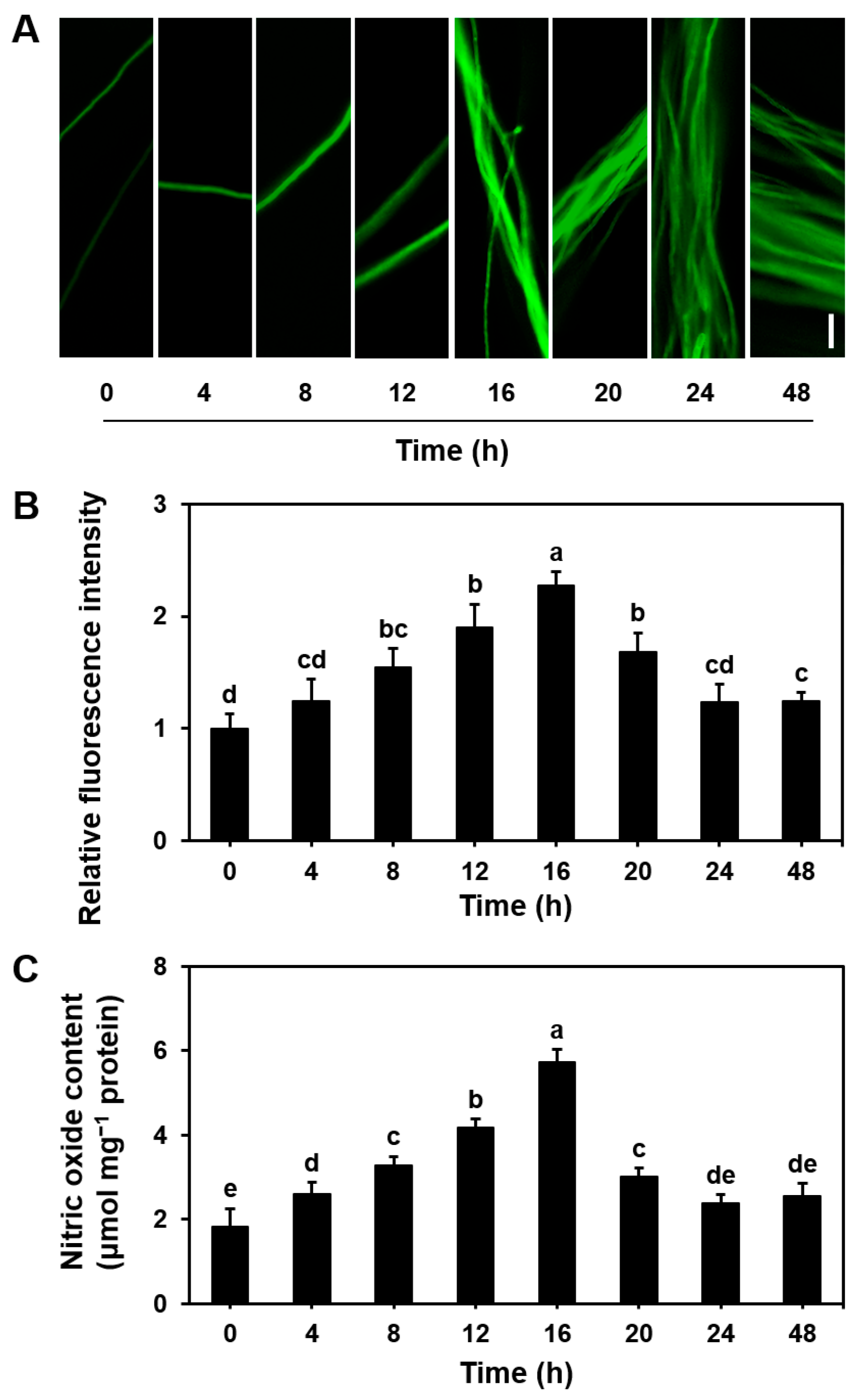
3.1. Dynamics of NO Levels During the Growth of S. commune Mycelium
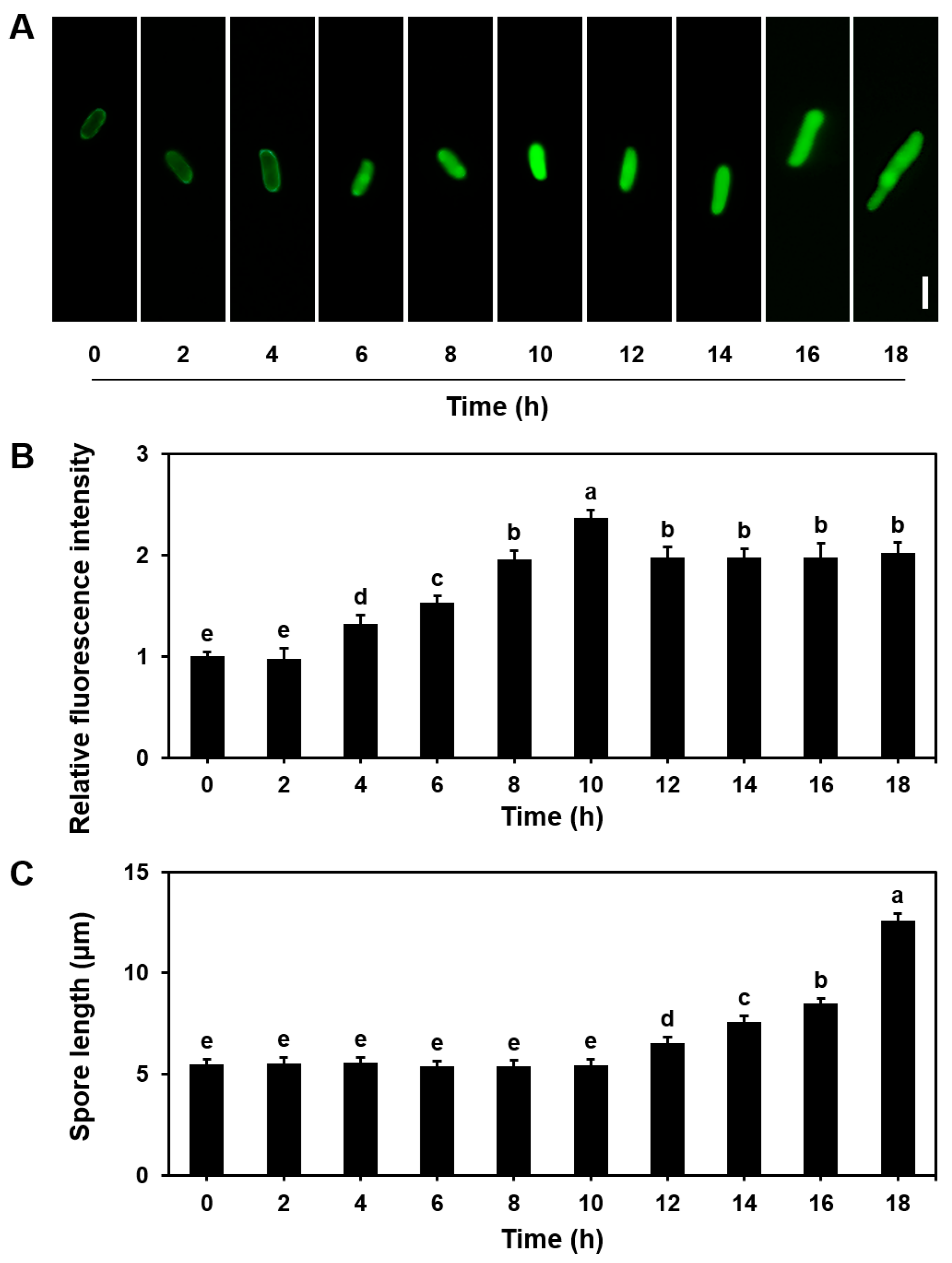
3.2. NO Dynamics During Basidiospore Germination in S. commune
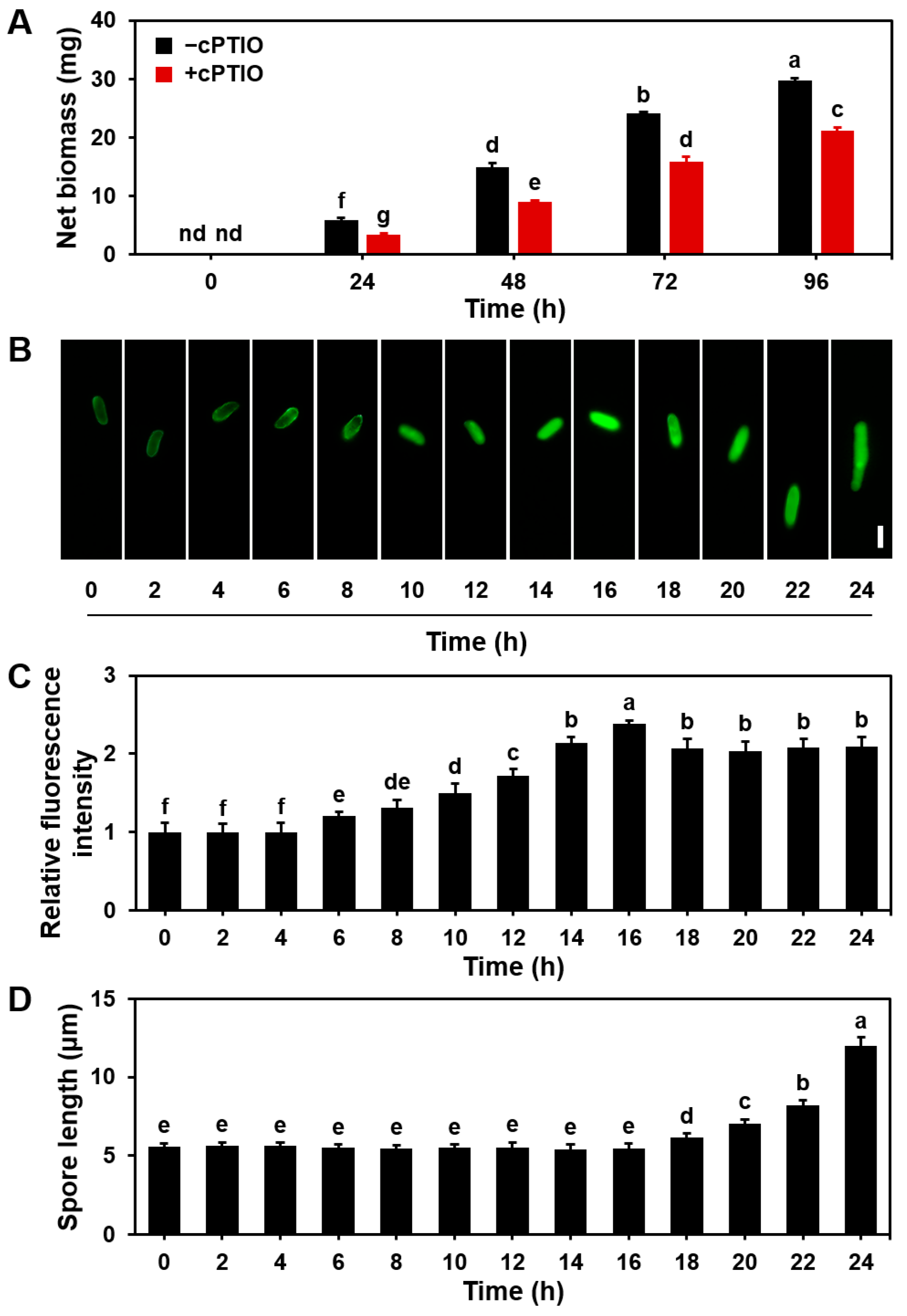
3.3. Effect of the NO Scavenger cPTIO on Mycelial Growth and Basidiospore Germination in S. commune
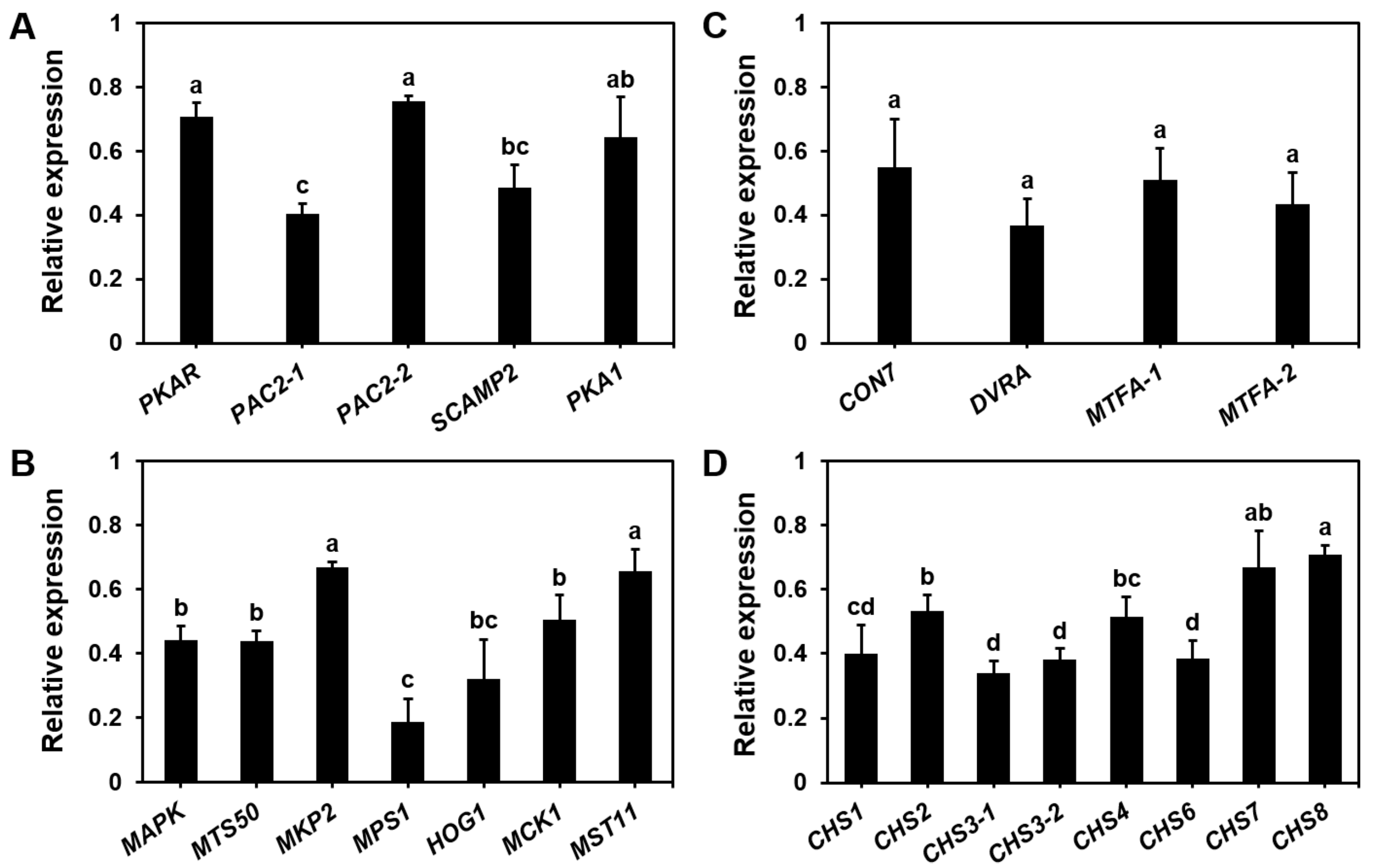
3.4. Endogenous NO Regulation of Basidiospore Germination Genes in S. commune
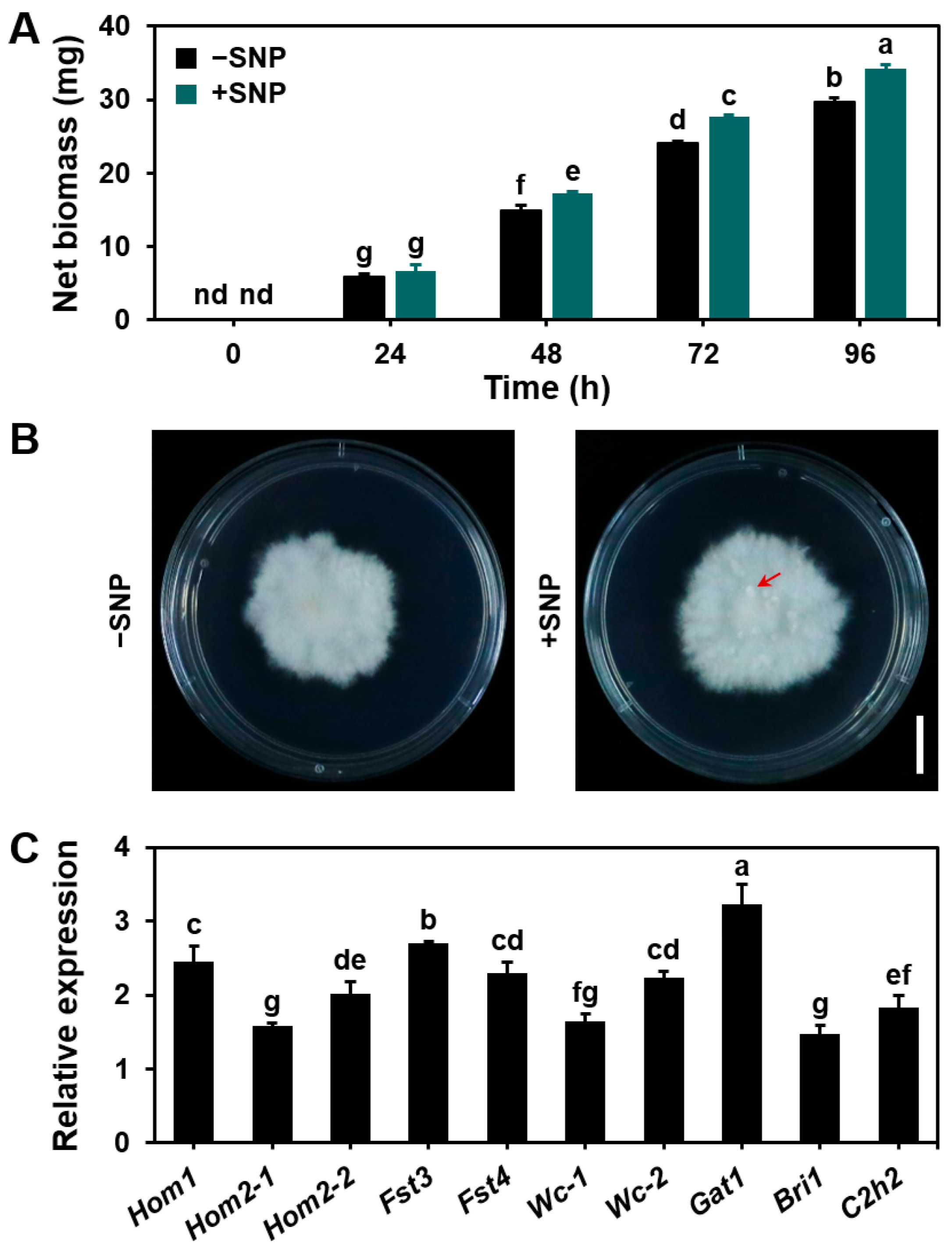
3.5. Influence of Exogenous NO on Mycelial Growth and Sexual Reproduction in S. commune
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Huang, X.; Chu, C.; Xu, H.; Wang, L.; Xue, Y.; Muhammad, Z.U.A.; Inagaki, F.; Liu, C. Genome, genetic evolution, and environmental adaptation mechanisms of Schizophyllum commune in deep subseafloor coal-bearing sediments. Iscience 2022, 25, 104417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, D.; Liu, J.; Fang, J.; Liu, C. Pressure-tolerant survival mechanism of Schizophyllum commune 20R-7-F01 isolated from deep sediments 2 kilometers below the seafloor. Front. Mar. Sci. 2024, 11, 1471465. [Google Scholar] [CrossRef]
- Li, J.; Hou, R.; Zhang, F. A new Schizophyllum commune strain as a potential biocontrol agent against blueberry root rot. Arch. Microbiol. 2024, 206, 235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bharti, A.K.; Bezie, Y. Schizophyllum commune: A fungal cell-factory for production of valuable metabolites and enzymes. BioResources 2022, 17, 5420–5436. [Google Scholar] [CrossRef]
- Marian, I.M.; Vonk, P.J.; Valdes, I.D.; Barry, K.; Bostock, B.; Carver, A.; Daum, C.; Lerner, H.; Lipzen, A.; Park, H. The transcription factor Roc1 is a key regulator of cellulose degradation in the wood-decaying mushroom Schizophyllum commune. mBio 2022, 13, e00628-22. [Google Scholar] [CrossRef]
- Saghebi, M.; Khalilvandi-Behroozyar, H.; Pirmohammadi, R.; Donyadoust, M. The effect of wheat straw processing using liquid-and solid-state culture of Schizophyllum commune on chemical composition, in vitro digestibility and fermentation parameters and rumen degradability. J. Anim. Sci. Res. 2023, 33, 47–62. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon monoxide and nitric oxide as examples of the youngest class of transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef]
- Signori, D.; Magliocca, A.; Hayashida, K.; Graw, J.A.; Malhotra, R.; Bellani, G.; Berra, L.; Rezoagli, E. Inhaled nitric oxide: Role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med. Exp. 2022, 10, 28. [Google Scholar] [CrossRef]
- Pawar, A.; Pardasani, K.R. Effects of disorders in interdependent calcium and IP3 dynamics on nitric oxide production in a neuron cell. Eur. Phys. J. Plus. 2022, 137, 543. [Google Scholar] [CrossRef]
- Okda, M.; Spina, S.; Fakhr, B.S.; Carroll, R.W. The Antimicrobial Effects of Nitric Oxide: A Narrative Review. Nitric Oxide 2025, 155, 20–40. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.; Bi, Y.; Li, J.; Bao, G.; Liu, J.; Yu, X. Effects of nitric oxide on growth of Fusarium sulphureum and its virulence to potato tubers. Eur. Food Res. Technol. 2014, 238, 1007–1014. [Google Scholar] [CrossRef]
- Hou, L.; Huang, C.; Wu, X.; Zhang, J.; Zhao, M. Nitric oxide negatively regulates the rapid formation of Pleurotus ostreatus primordia by inhibiting the mitochondrial aco gene. J. Fungi 2022, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.T.; Shinyashiki, M.; Switzer, C.H.; Valentine, J.S.; Gralla, E.B.; Thiele, D.J.; Fukuto, J.M. Effects of nitric oxide on the copper-responsive transcription factor Ace1 in Saccharomyces cerevisiae: Cytotoxic and cytoprotective actions of nitric oxide. Arch. Biochem. Biophys. 2000, 377, 296–303. [Google Scholar] [CrossRef]
- Yu, N.-N.; Park, G. Nitric Oxide in Fungi: Production and Function. J. Fungi 2024, 10, 155. [Google Scholar] [CrossRef]
- Ninnemann, H.; Maier, J. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem. Photobiol. 1996, 64, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Fu, Y.; Jiang, D.; Li, G.; Yi, X.; Peng, Y. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet. Biol. 2007, 44, 1368–1379. [Google Scholar] [CrossRef]
- Li, B.; Fu, Y.; Jiang, D.; Xie, J.; Cheng, J.; Li, G.; Hamid, M.I.; Yi, X. Cyclic GMP as a second messenger in the nitric oxide-mediated conidiation of the mycoparasite Coniothyrium minitans. Appl. Environ. Microbiol. 2010, 76, 2830–2836. [Google Scholar] [CrossRef]
- Wang, J.; Higgins, V.J. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet. Biol. 2005, 42, 284–292. [Google Scholar] [CrossRef]
- Maier, J.; Hecker, R.; Rockel, P.; Ninnemann, H. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol. 2001, 126, 1323–1330. [Google Scholar] [CrossRef]
- Kig, C.; Temizkan, G. Nitric oxide as a signaling molecule in the fission yeast Schizosaccharomyces pombe. Protoplasma 2009, 238, 59–66. [Google Scholar] [CrossRef]
- Vieira, A.L.; Linares, E.; Augusto, O.; Gomes, S.L. Evidence of a Ca2+-NO-cGMP signaling pathway controlling zoospore biogenesis in the aquatic fungus Blastocladiella emersonii. Fungal Genet. Biol. 2009, 46, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Gao, Z.; Wang, C.; Huang, L.; Kang, Z.; Zhang, H. Nitric oxide and reactive oxygen species coordinately regulate the germination of Puccinia striiformis f. sp. tritici urediniospores. Front. Microbiol. 2016, 7, 178. [Google Scholar] [CrossRef]
- Arifeen, M.Z.U.; Ma, Z.J.; Wu, S.; Liu, J.Z.; Xue, Y.R.; Liu, C.H. Effect of oxygen concentrations and branched-chain amino acids on the growth and development of sub-seafloor fungus, Schizophyllum commune 20R-7-F01. Environ. Microbiol. 2021, 23, 6940–6952. [Google Scholar] [CrossRef] [PubMed]
- Arifeen, M.Z.U.; Chu, C.; Yang, X.; Liu, J.; Huang, X.; Ma, Y.; Liu, X.; Xue, Y.; Liu, C. The anaerobic survival mechanism of Schizophyllum commune 20R-7-F01, isolated from deep sediment 2 km below the seafloor. Environ. Microbiol. 2021, 23, 1174–1185. [Google Scholar] [CrossRef]
- Zhao, M.; Li, D.; Liu, J.; Fang, J.; Li, C.u. Fungal methane production under high hydrostatic pressure in deep subseafloor sediments. Microorganisms 2024, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Huang, X.; Xie, T.N.; Duan, N.; Xue, Y.R.; Zhao, T.X.; Lever, M.A.; Hinrichs, K.U.; Inagaki, F. Exploration of cultivable fungal communities in deep coal-bearing sediments from ∼1.3 to 2.5 km below the ocean floor. Environ. Microbiol. 2017, 19, 803–818. [Google Scholar] [CrossRef]
- Whitaker, D. Studies in the biochemistry of cellulolytic microorganisms: I. Carbon balances of wood-rotting fungi in surface culture. Can. J. Bot. 1951, 29, 159–175. [Google Scholar] [CrossRef]
- Pengkit, A.; Jeon, S.S.; Son, S.J.; Shin, J.H.; Baik, K.Y.; Choi, E.H.; Park, G. Identification and functional analysis of endogenous nitric oxide in a filamentous fungus. Sci. Rep. 2016, 6, 30037. [Google Scholar] [CrossRef][Green Version]
- Catalá, M.; Gasulla, F.; del Real, A.E.P.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiol. 2010, 10, 297. [Google Scholar] [CrossRef]
- Li, D.; Chu, C.; Zhao, M.; Hou, S.; Ji, R.; Liu, C. Nitric oxide-mediated regulation of chitinase activity and cadmium sequestration in the response of Schizophyllum commune to cadmium stress. Microorganisms 2025, 13, 470. [Google Scholar] [CrossRef]
- Santiago-Martínez, M.G.; Ferry, J.G. Methods for culturing anaerobic microorganisms. In Oxygen Sensing: Methods and Protocols; Weinert, E.E., Ed.; CRC Press: Humana, NY, USA, 2023; Volume 2648, pp. 231–238. [Google Scholar]
- Govender, R.; Mabaso, N.; Abbai, N.S. Investigating links between Trichomonas vaginalis, T. vaginalis virus, Mycoplasma hominis, and metronidazole resistance. J. Infect. Dev. Ctries. 2024, 18, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Lu, C.; Li, X.; Zhou, J.; Wang, J. Lanthanum: A novel inducer for enhancement of fungal laccase production by Shiraia bambusicola. J. Rare Earths 2022, 40, 508–516. [Google Scholar] [CrossRef]
- Karimi, F.; Hamidian, Y.; Behrouzifar, F.; Mostafazadeh, R.; Ghorbani-HasanSaraei, A.; Alizadeh, M.; Mortazavi, S.-M.; Janbazi, M.; Asrami, P.N. An applicable method for extraction of whole seeds protein and its determination through Bradford’s method. Food Chem. Toxicol. 2022, 164, 113053. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ferraro, A.; Siciliano, A.; Spampinato, M.; Morello, R.; Trancone, G.; Race, M.; Guida, M.; Fabbricino, M.; Spasiano, D.; Fratino, U. A multi-disciplinary approach based on chemical characterization of foreshore sediments, ecotoxicity assessment and statistical analyses for environmental monitoring of marine-coastal areas. Mar. Environ. Res. 2024, 202, 106780. [Google Scholar] [CrossRef]
- d’Enfert, C. Fungal Spore Germination: Insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 1997, 21, 163–172. [Google Scholar] [CrossRef]
- Yu, N.-N.; Veerana, M.; Ketya, W.; Sun, H.-N.; Park, G. RNA-seq-based transcriptome analysis of nitric oxide scavenging response in Neurospora crassa. J. Fungi 2023, 9, 985. [Google Scholar] [CrossRef]
- Allen, P.J. Metabolic aspects of spore germination in fungi. Annu. Rev. Phytopathol. 1965, 3, 313–342. [Google Scholar] [CrossRef]
- Šubíková, V.; Šubík, J. Energetic aspects of spore germination in filamentous fungi. Folia Microbiol. 1974, 19, 367–372. [Google Scholar] [CrossRef]
- Lazar, E.; Wills, R.; Ho, B.; Harris, A.; Spohr, L. Antifungal effect of gaseous nitric oxide on mycelium growth, sporulation and spore germination of the postharvest horticulture pathogens, Aspergillus niger, Monilinia fructicola and Penicillium italicum. Lett. Appl. Microbiol. 2008, 46, 688–692. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Chu, C.; Zhao, M.; Hou, S.; Liu, C. The Role of Nitric Oxide in the Growth and Development of Schizophyllum commune Under Anaerobic Conditions. Microorganisms 2025, 13, 887. https://doi.org/10.3390/microorganisms13040887
Li D, Chu C, Zhao M, Hou S, Liu C. The Role of Nitric Oxide in the Growth and Development of Schizophyllum commune Under Anaerobic Conditions. Microorganisms. 2025; 13(4):887. https://doi.org/10.3390/microorganisms13040887
Chicago/Turabian StyleLi, Dongxu, Chen Chu, Mengshi Zhao, Suying Hou, and Changhong Liu. 2025. "The Role of Nitric Oxide in the Growth and Development of Schizophyllum commune Under Anaerobic Conditions" Microorganisms 13, no. 4: 887. https://doi.org/10.3390/microorganisms13040887
APA StyleLi, D., Chu, C., Zhao, M., Hou, S., & Liu, C. (2025). The Role of Nitric Oxide in the Growth and Development of Schizophyllum commune Under Anaerobic Conditions. Microorganisms, 13(4), 887. https://doi.org/10.3390/microorganisms13040887






