In Vitro Ferrophilic Responses of Photobacterium damselae subsp. piscicida EKL1 and Characterization of the Fe(III)-Piscibactin Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Condition
2.2. Growth and Biofilm Formation Assays
Anti-Biofilm Effect of Deferoxamine (DFO)
2.3. Evaluation of Siderophore Production
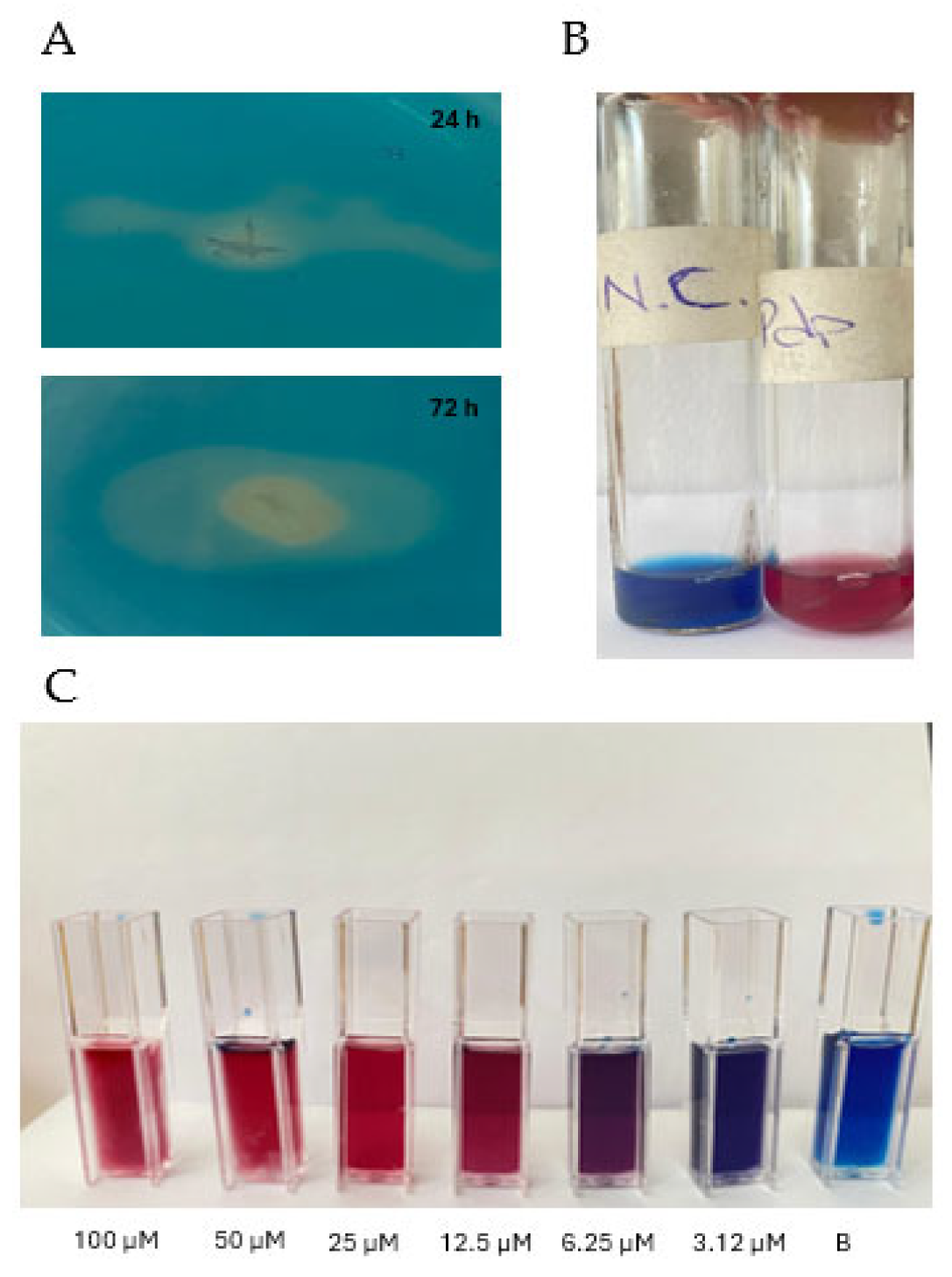
2.3.1. Qualitative Assay
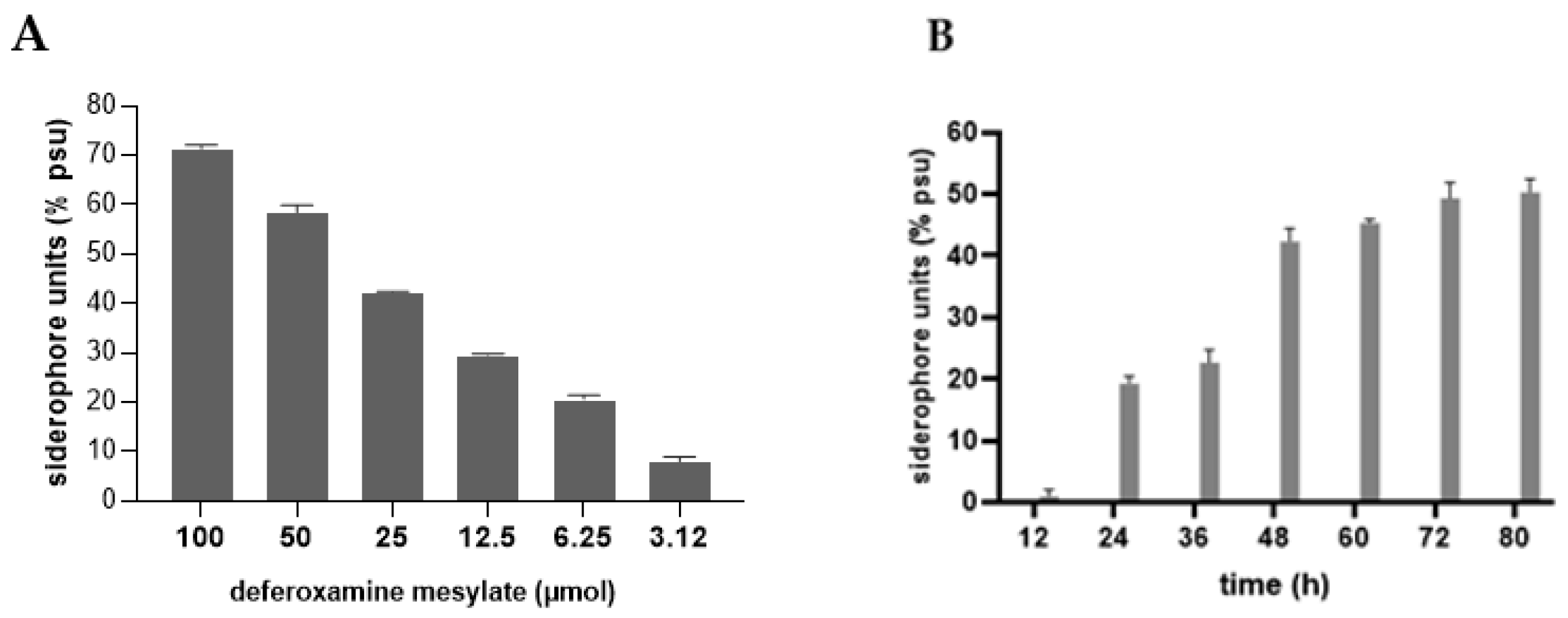
2.3.2. Quantitative Assay
2.4. Production and Characterization of Piscibactin
Nuclear Magnetic Resonance (NMR) Spectroscopy
3. Results
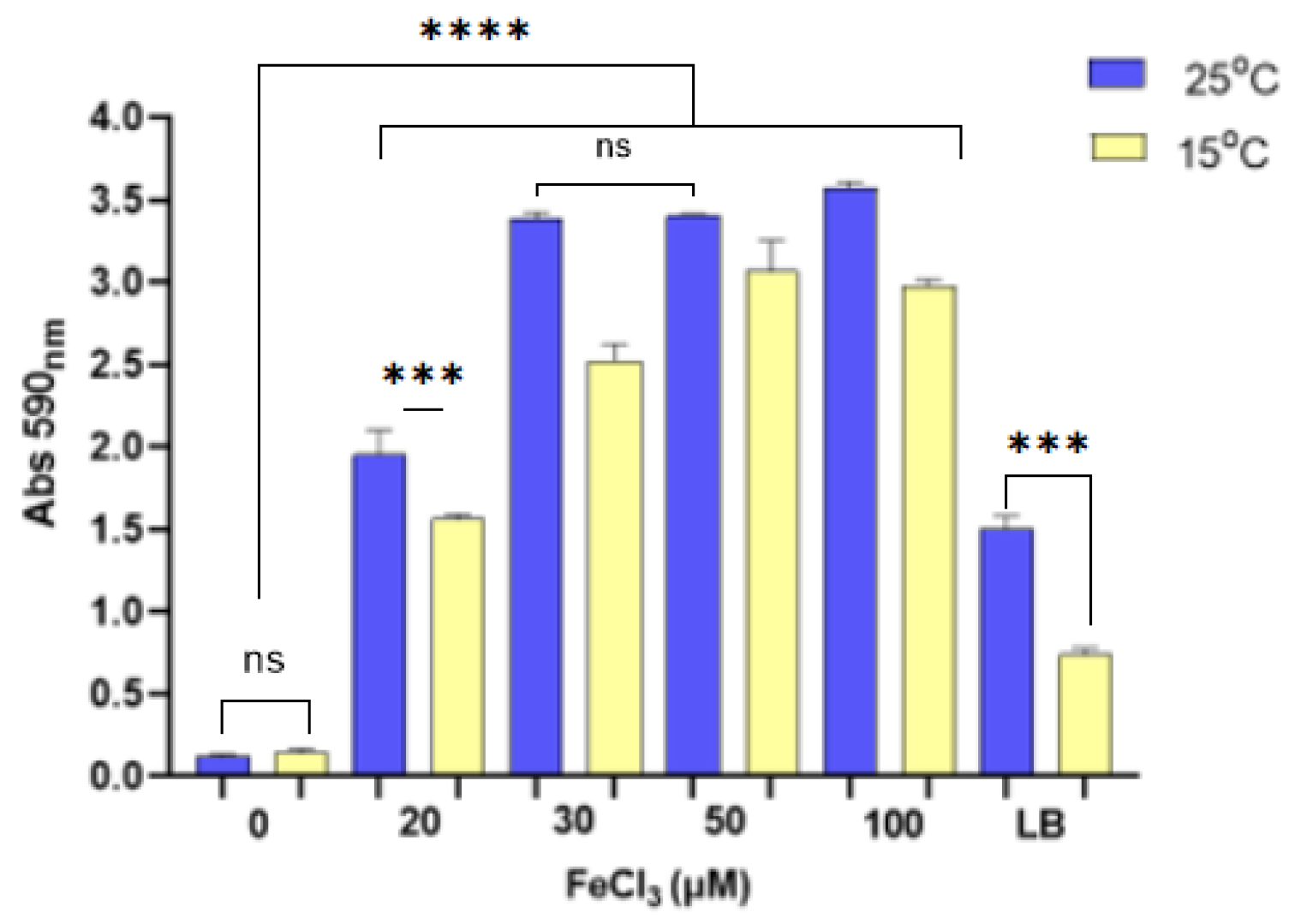
3.1. The Role of Ferric Iron in the Biofilm Production and Growth
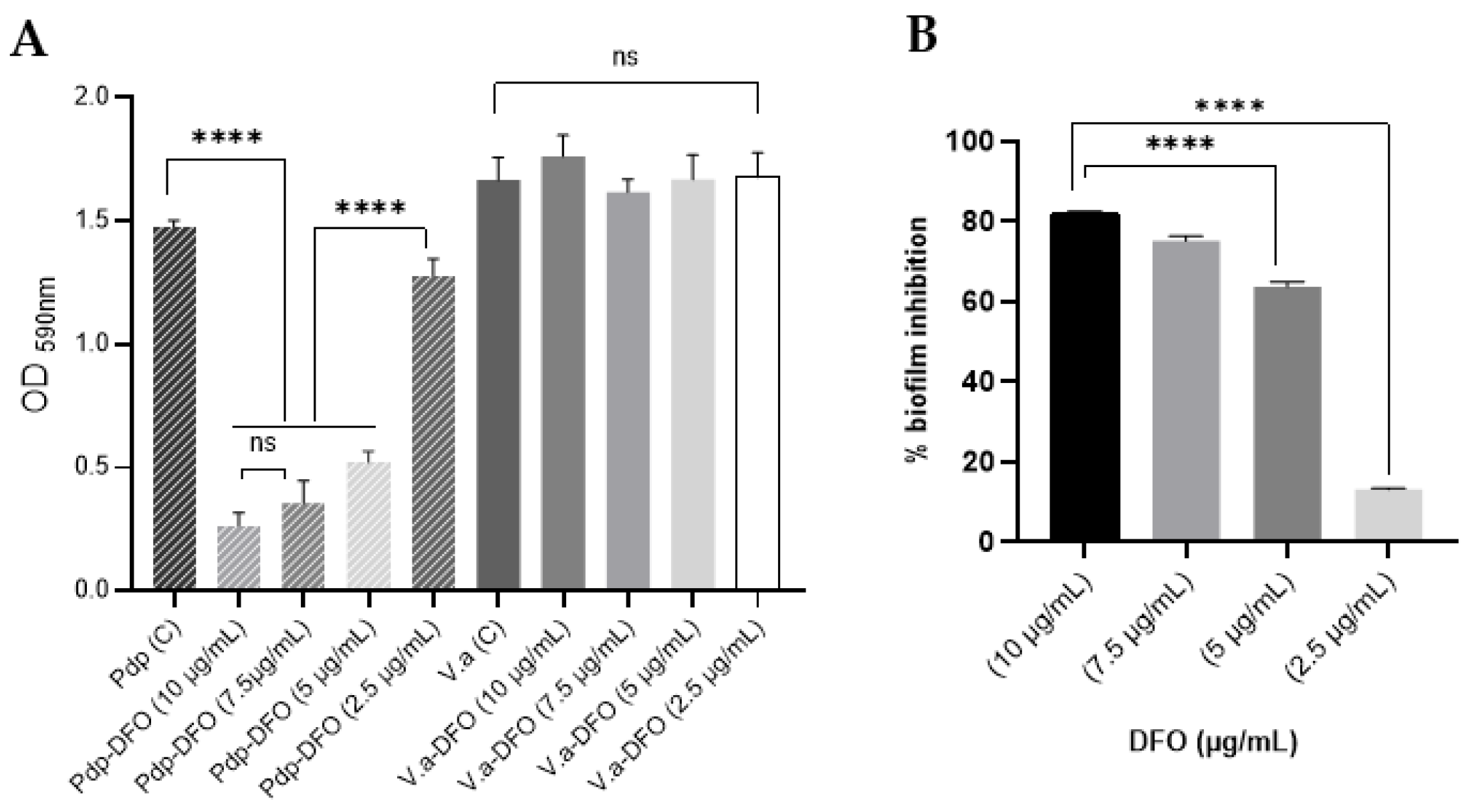
3.2. Anti-Biofilm Effect of Deferoxamine Mesylate (DFO)
3.3. Siderophore Production
3.4. Purification of Pdp Siderophore
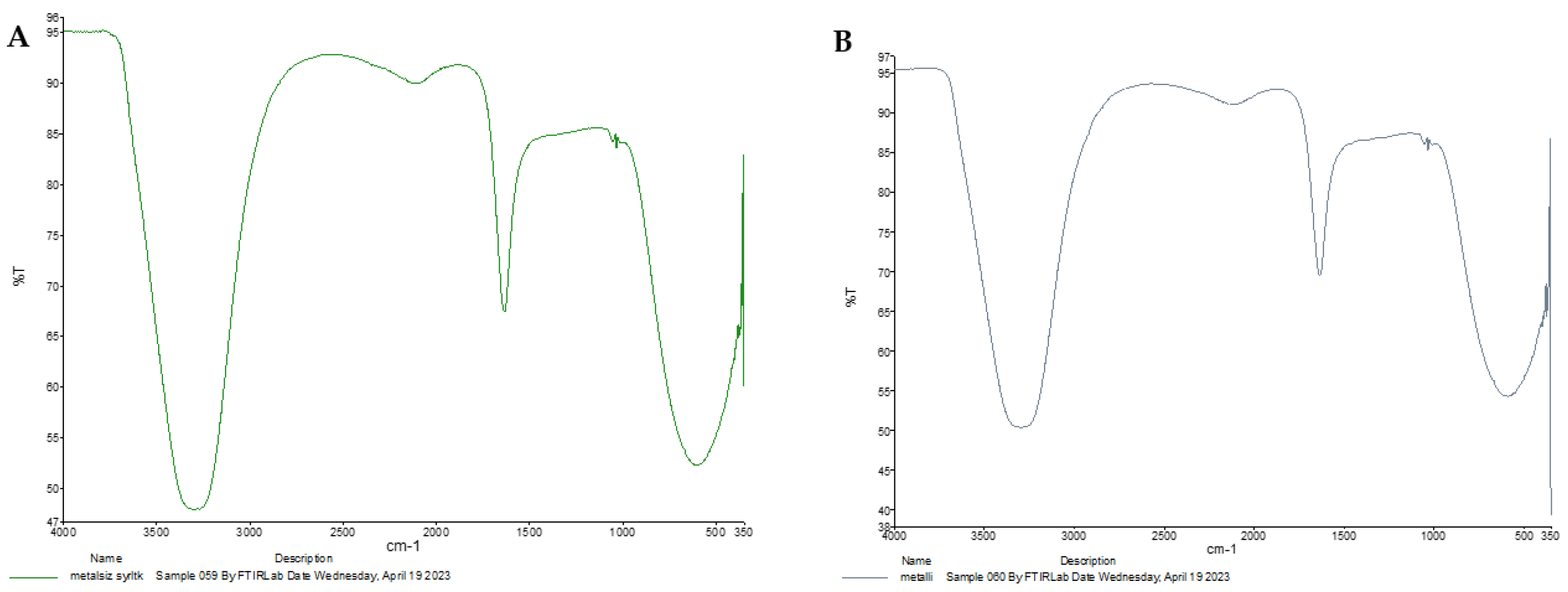
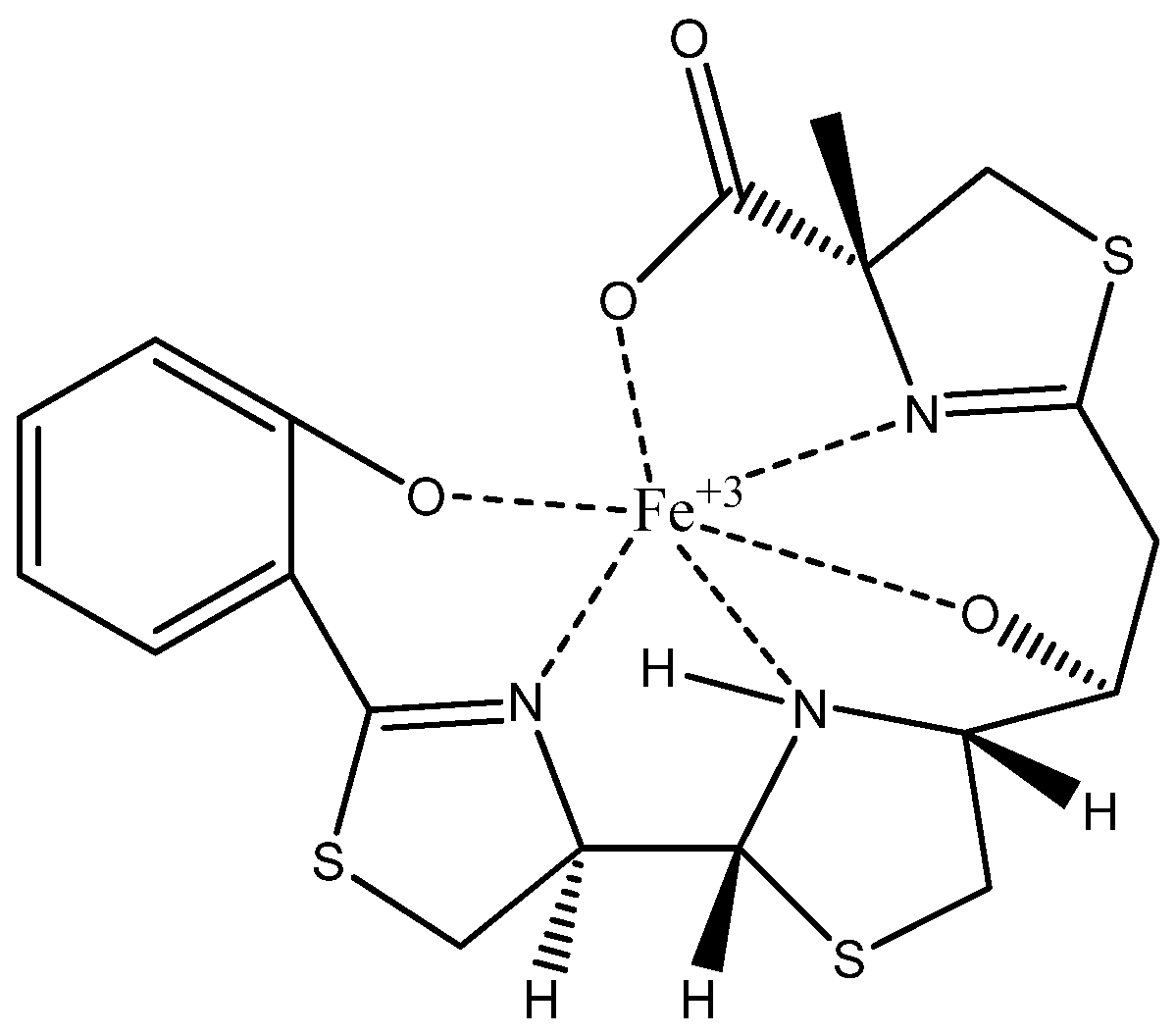
3.5. Determination of the Structure of Siderophore by Spectroscopic Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snieszko, S.F.; Bullock, G.L.; Hollis, E.; Boone, J.G. Pasteurella sp. from an epizootic of white perch (Roccus americanus) in Chesapeake Bay tidewater areas. J. Bacteriol. 1964, 88, 1814–1815. [Google Scholar] [CrossRef] [PubMed]
- Håstein, T.; Gudding, R.; Evensen, O. Bacterial vaccines for fish—An update of the current situation worldwide. Dev. Biol. 2005, 121, 55–74. [Google Scholar]
- Essam, H.M.; Abdellrazeq, G.S.; Tayel, S.I.; Torky, H.A.; Fadel, A.H. Pathogenesis of Photobacterium damselae subspecies infections in sea bass and sea bream. Microb. Pathog. 2016, 99, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, G.; Cammà, C.; Curini, V.; Mazzariol, S.; Proietto, U.; Di Francesco, C.E.; Ferri, N.; Di Provvido, A.; Di Guardo, G. Coinfection by Ureaplasma spp., Photobacterium damselae and an Actinomyces-like microorganism in a bottlenose dolphin (Tursiops truncatus) with pleuropneumonia stranded along the Adriatic coast of Italy. Res. Vet. Sci. 2016, 105, 111–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahoney, J.C.; Gerding, M.J.; Jones, S.H.; Whistler, C.A. Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Appl. Environ. Microbiol. 2010, 76, 7459–7465. [Google Scholar] [CrossRef]
- Lages, M.A.; Balado, M.; Lemos, M.L. The expression of virulence factors in Vibrio anguillarum is dually regulated by iron levels and temperature. Front. Microbiol. 2019, 10, 2335. [Google Scholar] [CrossRef]
- Do Vale, A.; Pereira, C.; Osorio, C.R.; dos Santos, N.M.S. The apoptogenic toxin AIP56 is secreted by the type II secretion system of Photobacterium damselae subsp. piscicida. Toxins 2017, 9, 368. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Elsholz, A.K.; Muth, C.; Girguis, P.R.; Kolter, R.; Losick, R. Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev. 2013, 27, 887–899. [Google Scholar] [CrossRef]
- Grandchamp, G.M. Microbial Interactions Affecting Sporulation and Specialized Metabolite Production. Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2019. [Google Scholar]
- Osorio, C.R.; Rivas, A.J.; Balado, M.; Fuentes-Monteverde, J.C.; Rodríguez, J.; Jiménez, C.; Lemos, M.L.; Waldor, M.K. A Transmissible Plasmid-Borne Pathogenicity Island Confers Piscibactin Biosynthesis in the Fish Pathogen Photobacterium damselae subsp. piscicida. Appl. Environ. Microbiol. 2020, 81, 5867–5879. [Google Scholar] [CrossRef]
- do Vale, A.; Magariños, B.; Romalde, J.L.; Lemos, M.L.; Ellis, A.E.; Toranzo, A.E. Binding of haemin by the fish pathogen Photobacterium damselae subsp. piscicida. Dis. Aquat. Org. 2002, 48, 109–115. [Google Scholar] [CrossRef]
- Hogle, S.L.; Barbeau, K.A.; Gledhill, M. Heme in the marine environment: From cells to the iron cycle. Metallomics 2014, 6, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Manck, L.E.; Coale, T.H.; Stephens, B.M.; Forsch, K.O.; Aluwihare, L.I.; Dupont, C.L.; Allen, A.E.; Barbeau, K.A. Iron limitation of heterotrophic bacteria in the California Current System tracks relative availability of organic carbon and iron. ISME J. 2024, 18, wrae061. [Google Scholar] [CrossRef] [PubMed]
- Thode, S.K.; Rojek, E.; Kozlowski, M.; Ahmad, R.; Haugen, P. Distribution of siderophore gene systems on a Vibrionaceae phylogeny: Database searches, phylogenetic analyses and evolutionary perspectives. PLoS ONE 2018, 13, e0191860. [Google Scholar] [CrossRef]
- Souto, A.; Montaos, M.A.; Rivas, A.J.; Balado, M.; Osorio, C.R.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Structure and biosynthetic assembly of piscibactin, a siderophore from Photobacterium damselae subsp. piscicida, predicted from genome analysis. Eur. J. Org. Chem. 2012, 2012, 5693–5700. [Google Scholar] [CrossRef]
- De la Fuente, M.C.; Segade, Y.; Valderrama, K.; Rodríguez, J.; Jiménez, C. Convergent Total Synthesis of the Siderophore Piscibactin as Its Ga3+ Complex. Org. Lett. 2021, 23, 340–345. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.C.; Ageitos, L.; Lages, M.A.; Martínez-Matamoros, D.; Forero, A.M.; Balado, M.; Lemos, M.L.; Rodríguez, J.; Jiménez, C. Structural Requirements for Ga3+ Coordination in Synthetic Analogues of the Siderophore Piscibactin Deduced by Chemical Synthesis and Density Functional Theory Calculations. Inorg. Chem. 2023, 62, 7503–7514. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, 2437. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Raaska, L.; Viikari, L.; Mattila-Sandholm, T. Detection of siderophores in growing cultures of Pseudomonas spp. J. Ind. Microbiol. Biotechnol. 1993, 11, 181–186. [Google Scholar] [CrossRef]
- Miller, A.L.; Li, S.; Eichhorn, C.D.; Zheng, Y.; Du, L. Identification and biosynthetic study of the siderophore lysochelin in the biocontrol agent Lysobacter enzymogenes. J. Agric. Food Chem. 2023, 71, 7418–7426. [Google Scholar] [CrossRef]
- Singh, A.; Bhattacharjee, S.; Bhardwaj, A.; Singh, S.S.; Mishra, A.K. Elucidating the structure of novel cyanobacterial siderophore produced by Anabaena oryzae and its implication in removal of cadmium. J. Appl. Phycol. 2024, 36, 3393–3408. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Macrocyclic polyketides with siderophore mode of action from marine heterotrophic Shewanella algae: Prospective anti-infective leads attenuate drug-resistant pathogens. J. Appl. Microbiol. 2021, 130, 1552–1570. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Stephen, J.; Lekshmi, M.; Ammini, P.; Kumar, S.H.; Varela, M.F. Membrane efflux pumps of pathogenic vibrio species: Role in antimicrobial resistance and virulence. Microorganisms 2022, 10, 382. [Google Scholar] [CrossRef]
- Faleye, O.S.; Sathiyamoorthi, E.; Lee, J.H.; Lee, J. Inhibitory effects of cinnamaldehyde derivatives on biofilm formation and virulence factors in Vibrio species. Pharmaceutics 2021, 13, 2176. [Google Scholar] [CrossRef]
- Pruzzo, C.; Huq, A.; Colwell, R.R.; Donelli, G. Pathogenic Vibrio species in the marine and estuarine environment. In Oceans and Health: Pathogens in the Marine Environment; Springer: Boston, MA, USA, 2005; pp. 217–252. [Google Scholar] [CrossRef]
- Magariños, B.; Toranzo, A.E.; Barja, J.L.; Romalde, J.L. Existence of Two Geographically-Linked Clonal Lineages in the Bacterial Fish Pathogen Photobacterium damselae subsp. piscicida Evidenced by Random Amplified Polymorphic DNA Analysis. Epidemiol. Infect. 2000, 125, 213–219. [Google Scholar] [CrossRef]
- Balado, M.; Benzekri, H.; Labella, A.M.; Claros, M.G.; Manchado, M.; Borrego, J.J.; Osorio, C.R.; Lemos, M.L. Genomic Analysis of the Marine Fish Pathogen Photobacterium damselae subsp. piscicida: Insertion Sequence Proliferation Is Associated with Chromosomal Reorganizations and Rampant Gene Decay. Infect. Genet. Evol. 2017, 54, 221–229. [Google Scholar] [CrossRef]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef]
- Gentile, V.; Frangipani, E.; Bonchi, C.; Minandri, F.; Runci, F.; Visca, P. Iron and Acinetobacter baumannii biofilm formation. Pathogens 2014, 3, 704–719. [Google Scholar] [CrossRef]
- Norfolk, W.A.; Shue, C.; Henderson, W.M.; Glinski, D.A.; Lipp, E.K. Vibrio alginolyticus growth kinetics and the metabolic effects of iron. Microbiol. Spectr. 2023, 11, e02680-23. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Boiteau, R.M.; McLean, C.; Babcock-Adams, L.; McIlvin, M.R.; Moran, D.M.; Repeta, D.J.; Saito, M.A. Dynamic proteome response of a marine Vibrio to a gradient of iron and ferrioxamine bioavailability. Mar. Chem. 2021, 229, 103913. [Google Scholar] [CrossRef]
- Eickhoff, M.J. Regulation of Vibrio Quorum Sensing in Natural and Competitive Environments. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2021. [Google Scholar]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Neupane, G.P.; Kim, D.M. Comparison of the effects of deferasirox, deferiprone, and deferoxamine on the growth and virulence of Vibrio vulnificus. Transfusion 2009, 49, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Erinmez, M.; Zer, Y. In vitro effects of deferoxamine on antibiotic susceptibility in Gram-negative bacteria. Adv. Clin. Exp. Med. 2024, 33, 491–497. [Google Scholar] [CrossRef]
- Choon-Mee, K.; Yong-Jin, P.; Sung-Heui, S. A widespread deferoxamine-mediated iron-uptake system in Vibrio vulnificus. J. Infect. Dis. 2007, 196, 1537–1545. [Google Scholar] [CrossRef]
- Arifin, A.J.; Hannauer, M.; Welch, I.; Heinrichs, D.E. Deferoxamine mesylate enhances virulence of community-associated methicillin resistant Staphylococcus aureus. Microbes Infect. 2014, 16, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Melchiorsen, J.; Spanggaard, B.; Huber, I.; Nielsen, T.F. Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl. Environ. Microbiol. 1999, 65, 969–973. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, W.; Li, C. Inhibition of marine Vibrio sp. by pyoverdine from Pseudomonas aeruginosa PA1. J. Hazard. Mater. 2016, 302, 217–224. [Google Scholar] [CrossRef]
- Lemos, M.L.; Balado, M.; Osorio, C.R. Anguibactin-versus vanchrobactin-mediated iron uptake in Vibrio anguillarum: Evolution and ecology of a fish pathogen. Environ. Microbiol. Rep. 2010, 2, 19–26. [Google Scholar] [CrossRef]
- Lages, M.A.; Lemos, M.L.; Balado, M. The Temperature-Dependent Expression of the High-Pathogenicity Island Encoding Piscibactin in Vibrionaceae Results From the Combined Effect of the AraC-Like Transcriptional Activator PbtA and Regulatory Factors From the Recipient Genome. Front. Microbiol. 2021, 12, 748147. [Google Scholar] [CrossRef]
- Himpsl, S.D.; Pearson, M.M.; Arewång, C.J.; Nusca, T.D.; Sherman, D.H.; Mobley, H.L. Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis. Mol. Microbiol. 2010, 78, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.E.; McIntyre, D.D.; Mouck, M.; Sokol, P.A. Physical and structural characterization of yersiniophore, a siderophore produced by clinical isolates of Yersinia enterocolitica. Biometals 1996, 9, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.R.; Juiz-Rio, S.; Lemos, M.L. A siderophore biosynthesis gene cluster from the fish pathogen Photobacterium damselae subsp. piscicida is structurally and functionally related to the Yersinia high-pathogenicity island. Microbiology 2006, 152, 3327–3341. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Oyedele, A.Q.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular modeling in drug discovery. Inform. Med. Unlocked 2022, 29, 100880. [Google Scholar] [CrossRef]
- Schalk, I.J. Bacterial siderophores: Diversity, uptake pathways and applications. Nat. Rev. Microbiol. 2024, 23, 24–40. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eren Eroğlu, A.E.; Toklu, K.; Sarıkahya, N.; Yaşa, İ. In Vitro Ferrophilic Responses of Photobacterium damselae subsp. piscicida EKL1 and Characterization of the Fe(III)-Piscibactin Complex. Microorganisms 2025, 13, 858. https://doi.org/10.3390/microorganisms13040858
Eren Eroğlu AE, Toklu K, Sarıkahya N, Yaşa İ. In Vitro Ferrophilic Responses of Photobacterium damselae subsp. piscicida EKL1 and Characterization of the Fe(III)-Piscibactin Complex. Microorganisms. 2025; 13(4):858. https://doi.org/10.3390/microorganisms13040858
Chicago/Turabian StyleEren Eroğlu, Asiye Esra, Kadriye Toklu, Nazlı Sarıkahya, and İhsan Yaşa. 2025. "In Vitro Ferrophilic Responses of Photobacterium damselae subsp. piscicida EKL1 and Characterization of the Fe(III)-Piscibactin Complex" Microorganisms 13, no. 4: 858. https://doi.org/10.3390/microorganisms13040858
APA StyleEren Eroğlu, A. E., Toklu, K., Sarıkahya, N., & Yaşa, İ. (2025). In Vitro Ferrophilic Responses of Photobacterium damselae subsp. piscicida EKL1 and Characterization of the Fe(III)-Piscibactin Complex. Microorganisms, 13(4), 858. https://doi.org/10.3390/microorganisms13040858





