Expression and Antagonistic Activity Against Plant Pathogens of the Phage Tail-like Protein from Burkholderia multivorans WS-FJ9
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Plant Pathogens
2.2. In Vitro Antifungal Activity
2.3. Bioinformatics Analysis of the Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
2.4. Gene Cloning of the Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
2.5. qRT-PCR
2.6. Construction of a Prokaryotic Expression Vector with the Phage Tail-like Protein Bm_67459 Gene
2.7. Induced Expression and Purification of the Phage Tail-like Protein Bm_67459 of B. Multivorans WS-FJ9
2.8. Western Blot Analysis of the Bm_67459 Fusion Protein of B. multivorans WS-FJ9
2.9. Antibacterial Effect of Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
2.10. Statistical Analysis of the Data
3. Results
3.1. Antagonistic Effect of B. multivorans WS-FJ9 on Forest Pathogens
3.2. Effect of B. multivorans WS-FJ9 on Hyphae of Tree Pathogens by Electron Microscopy
3.3. Analysis and Cloning of Phage Tail-like Protein Genes from B. multivorans WS-FJ9
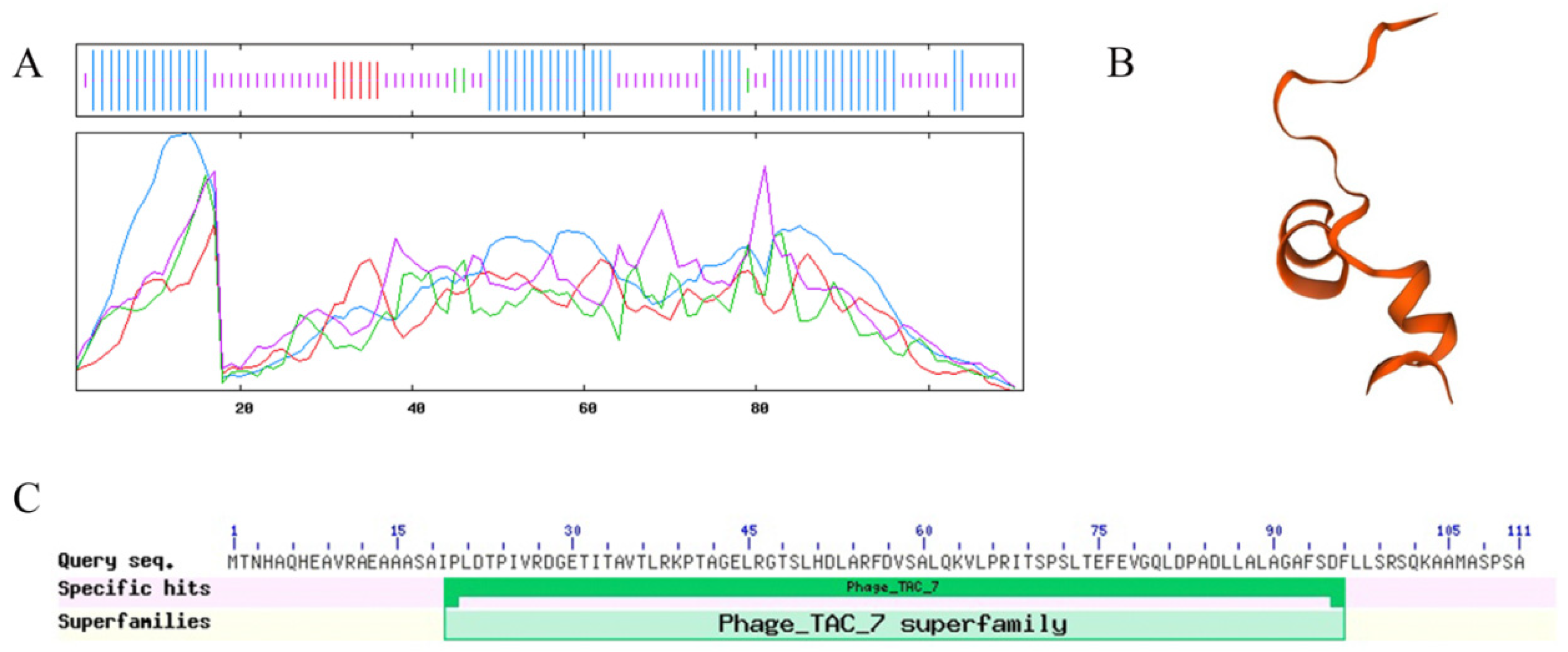
3.4. Physicochemical Properties and Structural Analysis of the Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
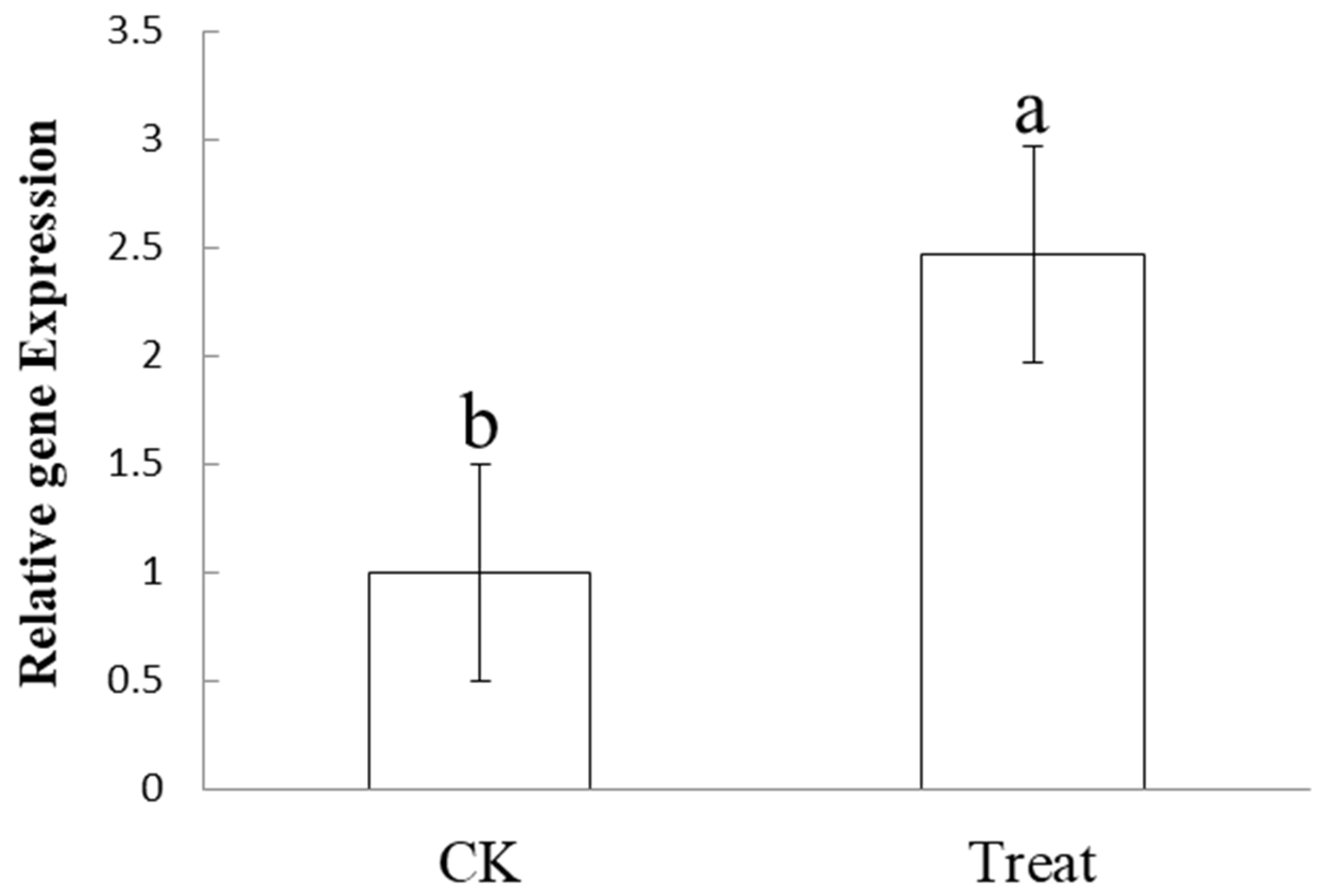
3.5. Differential Expression Analysis of the Phage Tail-like Protein Bm_67459 Gene of B. multivorans WS-FJ9
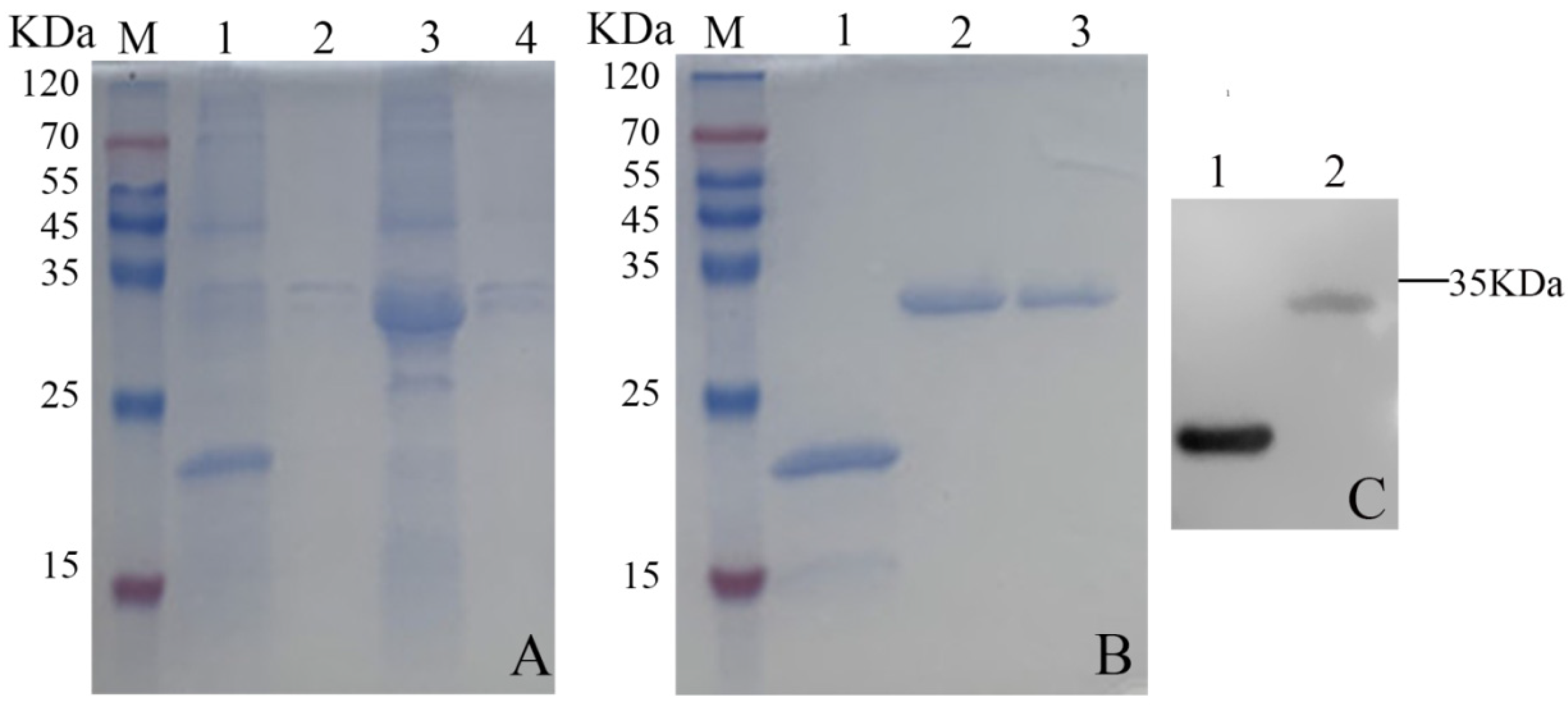
3.6. Induced Expression and Purification of the Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
3.7. Antibacterial Activity of the Phage Tail-like Protein Bm_67459 of B. multivorans WS-FJ9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holland, R.A.; Crossthwaite, A. Alkylsulfones: Novel chemical scaffolds targeting the vesicular acetylcholine transporter usher in a new generation of insecticides. Pest. Manag. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Vitenberg, T.; Khatib, S.; Opatovsky, I. Effect of entomopathogenic fungus Beauveria bassiana on the growth characteristics and metabolism of black soldier fly larvae. Pestic. Biochem. Physiol. 2023, 197, 105684. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Santhanam, R.; Menezes, R.C.; Grabe, V.; Li, D.; Baldwin, I.T.; Groten, K. A suite of complementary biocontrol traits allows a native consortium of root-associated bacteria to protect their host plant from a fungal sudden-wilt disease. Mol. Ecol. 2019, 28, 1154–1169. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Woudstra, C.; Sørensen, A.N.; Sørensen MC, H.; Brøndsted, L. Strategies for developing phages into novel antimicrobial tailocins. Trends Microbiol. 2024, 32, 996–1006. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; Dillen, Y.R.; Lambrichts, V.; Proost, P.; Wattiez, R.; De Mo, R. Different Ancestries of R Tailocins in Rhizospheric Pseudomonas Isolates. Genome Biol. Evol. 2015, 7, 2810–2828. [Google Scholar] [CrossRef]
- Scholl, D. Phage Tail-Like Bacteriocins. Annu. Rev. Virol. 2017, 4, 453–467. [Google Scholar] [CrossRef]
- Nakayama, K.; Takashima, K.; Ishihara, H.; Shinomiya, T.; Kageyama, M.; Kanaya, S.; Ohnishi, M.; Murata, T.; Mori, H.; Hayashi, T. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 2000, 38, 213–231. [Google Scholar] [CrossRef]
- Canchaya, C.; Fournous, G.; Brüssow, H. The impact of prophages on bacterial chromosomes. Mol. Microbiol. 2004, 53, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Godino, A.; Príncipe, A.; Fischer, S. A ptsP deficiency in PGPR Pseudomonas fluorescens SF39a affects bacteriocin production and bacterial fitness in the wheat rhizosphere. Res. Microbiol. 2016, 167, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Godino, A.; Quesada, J.M.; Cordero, P.; Jofré, E.; Mori, G.; Espinosa-Urgel, M. Characterization of a phage-like pyocin from the plant growth-promoting rhizobacterium Pseudomonas fluorescens SF4c. Microbiology 2012, 158, 1493–1503. [Google Scholar] [CrossRef]
- Fernandez, M.; Godino, A.; Príncipe, A.; Morales, G.M.; Fischer, S. Effect of a Pseudomonas fluorescens tailocin against phytopathogenic Xanthomonas observed by atomic force microscopy. J. Biotechnol. 2017, 256, 13–20. [Google Scholar] [CrossRef]
- Príncipe, A.; Fernandez, M.; Torasso, M.; Godino, A.; Fischer, S. Effectiveness of tailocins produced by Pseudomonas fluorescens SF4c in controlling the bacterial-spot disease in tomatoes caused by Xanthomonas vesicatoria. Microbiol. Res. 2018, 212–213, 94–102. [Google Scholar] [CrossRef]
- Yao, G.W.; Duarte, I.; Le, T.T.; Carmody, L.; LiPuma, J.J.; Young, R.; Gonzalez, C.F. A Broad-Host-Range Tailocin from Burkholderia cenocepacia. Appl. Environ. Microb. 2017, 83, e03414-16. [Google Scholar] [CrossRef]
- Morse, S.A.; Jones, B.V.; Lysko, P.G. Pyocin inhibition of Neisseria gonorrhoeae: Mechanism of action. Antimicrob. Agents Chemother. 1980, 18, 416–423. [Google Scholar] [CrossRef]
- Swain, D.M.; Yadav, S.K.; Tyagi, I.; Kumar, R.; Kumar, R.; Ghosh, S.; Das, J.; Jha, G. A prophage tail-like protein is deployed by Burkholderia bacteria to feed on fungi. Nat. Commun. 2017, 8, 404. [Google Scholar] [CrossRef]
- Hou, L. Studies on Screening of Efficient Phosphate-Solubilizing Bacteria in the Rhizosphere of Pine Trees and on Their Characteristics; Nanjing Forestry University: Nanjing, China, 2012. [Google Scholar]
- Zeng, Q.; Wu, X.; Wang, J. Burkholderia multivorans Phosphate Solubilization and Gene Expression of Phosphate-Solubilizing Bacterium WS-FJ9 under Different Levels of Soluble Phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Ye, J.; Yang, H. Characteristics of Organic Acid Secretion Associated with the Interaction between Burkholderia multivorans WS-FJ9 and Poplar Root System. Biomed. Res. Int. 2018, 2018, 9619724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Kong, W.; Liu, W.; Xie, X.; Wu, X. Identification, cloning and expression patterns of the genes related to phosphate solubilization in Burkholderia multivorans WS-FJ9 under different soluble phosphate levels. Amb Express 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Li, M.; Yang, L.; Chen, X. Cloning, phylogenetic research, and prokaryotic expression study of the metabolic detoxification gene EoGSTs1 in Empoasca onukii Matsuda. PEERJ 2019, 7, e7641. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Ye, J. Effects of Burkholderia multivorans WS-FJ9 on Nutrient Metabolism and Root Activity of Poplar. Acta Agric. Univ. Jiangxiensis 2014, 36, 531–535. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microb. Biot. 2011, 28, 1327–1350. [Google Scholar] [CrossRef]
- Dorosky, R.J.; Yu, J.M.; Pierson, L.S., III; Pierson, E.A. Pseudomonas chlororaphis Produces Two Distinct R-Tailocins That Contribute to Bacterial Competition in Biofilms and on Roots. Appl. Environ. Microb. 2017, 83, e00706-17. [Google Scholar] [CrossRef]
- Matsui, H.; Sano, Y.; Ishihara, H.; Shinomiya, T. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J. Bacteriol. 1993, 175, 1257–1263. [Google Scholar] [CrossRef]
- Lloubes, R.; Bernadac, A.; Houot, L.; Pommier, S. Non classical secretion systems. Res. Microbiol. 2013, 164, 655–663. [Google Scholar] [CrossRef]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Yin, M.; Xu, Y.; Liang, X.; Huang, Y.P. Characterization of maltocin S16, a phage tail-like bacteriocin with antibacterial activity against Stenotrophomonas maltophilia and Escherichia coli. J. Appl. Microbiol. 2019, 127, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, P.; Zheng, C.Y.; Huang, Y.P. Characterization of Maltocin P28, a Novel Phage Tail-Like Bacteriocin from Stenotrophomonas maltophilia. Appl. Environ. Microb. 2013, 79, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Ross, R.P.; Hill, C. Bacteriocins and bacteriophage; a narrow-minded approach to food and gut microbiology. Fems Microbiol. Rev. 2017, 41, S129–S153. [Google Scholar] [CrossRef]
- Hansen, E. Phytophthora in North American forests. In Sudden Oak Death Online Symposium; American Phytopathological Society: St Paul, MN, USA, 2003. [Google Scholar]
- Wang, M.S.; Zhang, S.H.; Chen, X.D. Research Progress on Biological Characteristics and Control Techniques of Phytophthora cinnamomi. J. Anhui Agric. Sci. 2018, 46, 21–24. [Google Scholar]
- Méndez-Bravo, A.; Cortazar-Murillo, E.M.; Guevara-Avendaño, E.; Ceballos-Luna, O.; Rodríguez-Haas, B.; Kiel-Martínez, A.L.; Hernández-Cristóbal, O.; Guerrero-Analco, J.A.; Reverchon, F. Plant growth-promoting rhizobacteria associated with avocado display antagonistic activity against Phytophthora cinnamomi through volatile emissions. PLoS ONE 2018, 13, e194665. [Google Scholar] [CrossRef]
- Kong, W.L.; Zhou, M.; Wu, X.Q. Characteristics of siderophores production by Rahnella aquatilis JZ-GX1 and its antagonism against forest pathogens. Microbiol. China 2019, 46, 3278–3285. [Google Scholar]
- Esmaeel, Q.; Pupin, M.; Kieu, N.P.; Chataigne, G.; Bechet, M.; Deravel, J.; Krier, F.; Hofte, M.; Jacques, P.; Leclere, V. Burkholderia genome mining for nonribosomal peptide synthetases reveals a great potential for novel siderophores and lipopeptides synthesis. Microbiologyopen 2016, 5, 512–526. [Google Scholar] [CrossRef]





| Primers Name | Secquences (5′ to 3′) | Bases Number/bp | Funcion |
|---|---|---|---|
| Bm_67459-F | ATGACGAACCACGCTCAACACG | 22 | Bm_67459 clone |
| Bm_67459-R | CGCAGACGGGGATGCCAT | 18 | |
| qBm_67459-F | ATGACGAACCACGCTCAACACG | 22 | Bm_67459 clone |
| qBm_67459-R | GACCTCGAATTCCGTCAGCGA | 21 | |
| 16s rDNA-F | GGGGAGTACGGTCGCAAGAT | 20 | Reference genes |
| 16s rDNA-R | CATGTCAAGGGTAGGTAAGGTTT | 23 |
| Orf Name | Location | Protein Length | Predicted Function |
|---|---|---|---|
| contig67_457 | 9087–11,729 | 880 | phage tail tape measure protein |
| contig67_458 | 11,726–11,842 | 38 | GpE family phage tail protein |
| contig67_459 | 11,851–12,186 | 111 | phage tail assembly protein |
| contig67_460 | 12,214–12,717 | 167 | phage major tail tube protein |
| contig67_461 | 12,756–13,928 | 390 | phage tail protein |
| contig67_462 | 13,982–14,587 | 201 | phage tail protein |
| contig430_2941 | 25,664–26,125 | 153 | phage tail protein |
| contig430_2944 | 29,164–30,354 | 396 | phage tail protein |
| contig430_2945 | 30,773–31,993 | 406 | phage tail protein |
| contig430_2946 | 32,388–33,608 | 406 | phage tail protein |
| contig430_2947 | 33,679–34,128 | 149 | phage tail protein |
| contig597_4008 | 6070–7005 | 311 | phage tail protein |
| contig1014_6085 | 4909–5319 | 136 | phage tail protein |
| contig1014_6087 | 6935–7384 | 149 | phage tail protein |
| contig1014_6090 | 8856–9344 | 162 | phage tail protein |
| contig1120_6584 | 1388–2494 | 368 | phage tail protein |
| contig1120_6586 | 3847–6261 | 804 | phage tail protein |
| contig1120_6588 | 6748–7125 | 125 | phage tail protein |
| contig1120_6589 | 7155–8576 | 473 | tail sheath protein |
| contig1519_7719 | 106–237 | 43 | phage tail protein |
| contig1519_7720 | 351–806 | 151 | phage tail protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, T.-Y.; Xie, X.-L.; Kong, W.-L.; Wu, X.-Q. Expression and Antagonistic Activity Against Plant Pathogens of the Phage Tail-like Protein from Burkholderia multivorans WS-FJ9. Microorganisms 2025, 13, 853. https://doi.org/10.3390/microorganisms13040853
Wen T-Y, Xie X-L, Kong W-L, Wu X-Q. Expression and Antagonistic Activity Against Plant Pathogens of the Phage Tail-like Protein from Burkholderia multivorans WS-FJ9. Microorganisms. 2025; 13(4):853. https://doi.org/10.3390/microorganisms13040853
Chicago/Turabian StyleWen, Tong-Yue, Xing-Li Xie, Wei-Liang Kong, and Xiao-Qin Wu. 2025. "Expression and Antagonistic Activity Against Plant Pathogens of the Phage Tail-like Protein from Burkholderia multivorans WS-FJ9" Microorganisms 13, no. 4: 853. https://doi.org/10.3390/microorganisms13040853
APA StyleWen, T.-Y., Xie, X.-L., Kong, W.-L., & Wu, X.-Q. (2025). Expression and Antagonistic Activity Against Plant Pathogens of the Phage Tail-like Protein from Burkholderia multivorans WS-FJ9. Microorganisms, 13(4), 853. https://doi.org/10.3390/microorganisms13040853






