Zirconium Dental Implants as Potential Optical Waveguides in Photodynamic Inactivation of Bacterial Biofilms—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
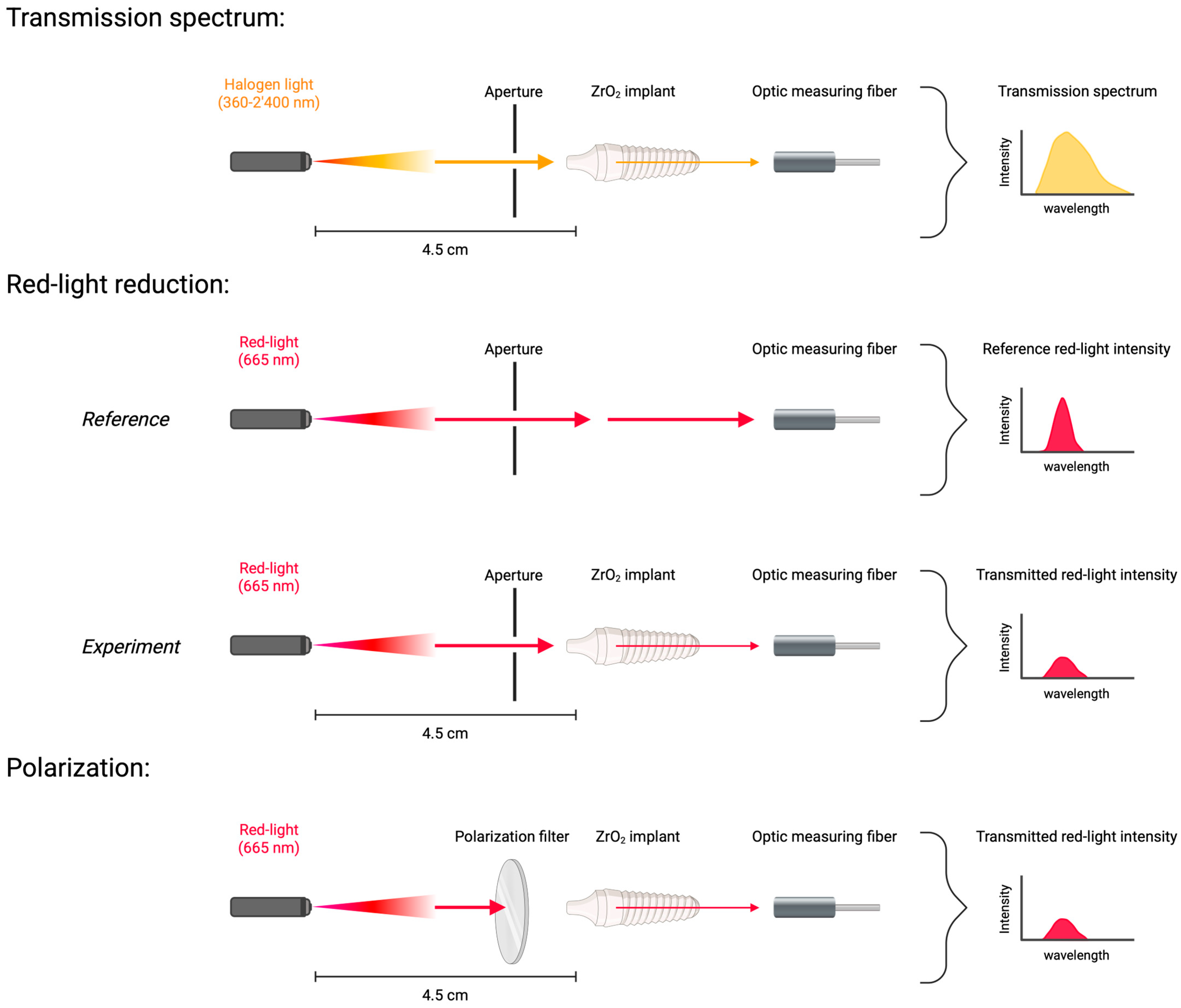
2.1. Optical Experiments on Zirconium Dental Implants
2.2. Photodynamic Inactivation (PDI) of Bacterial Biofilm
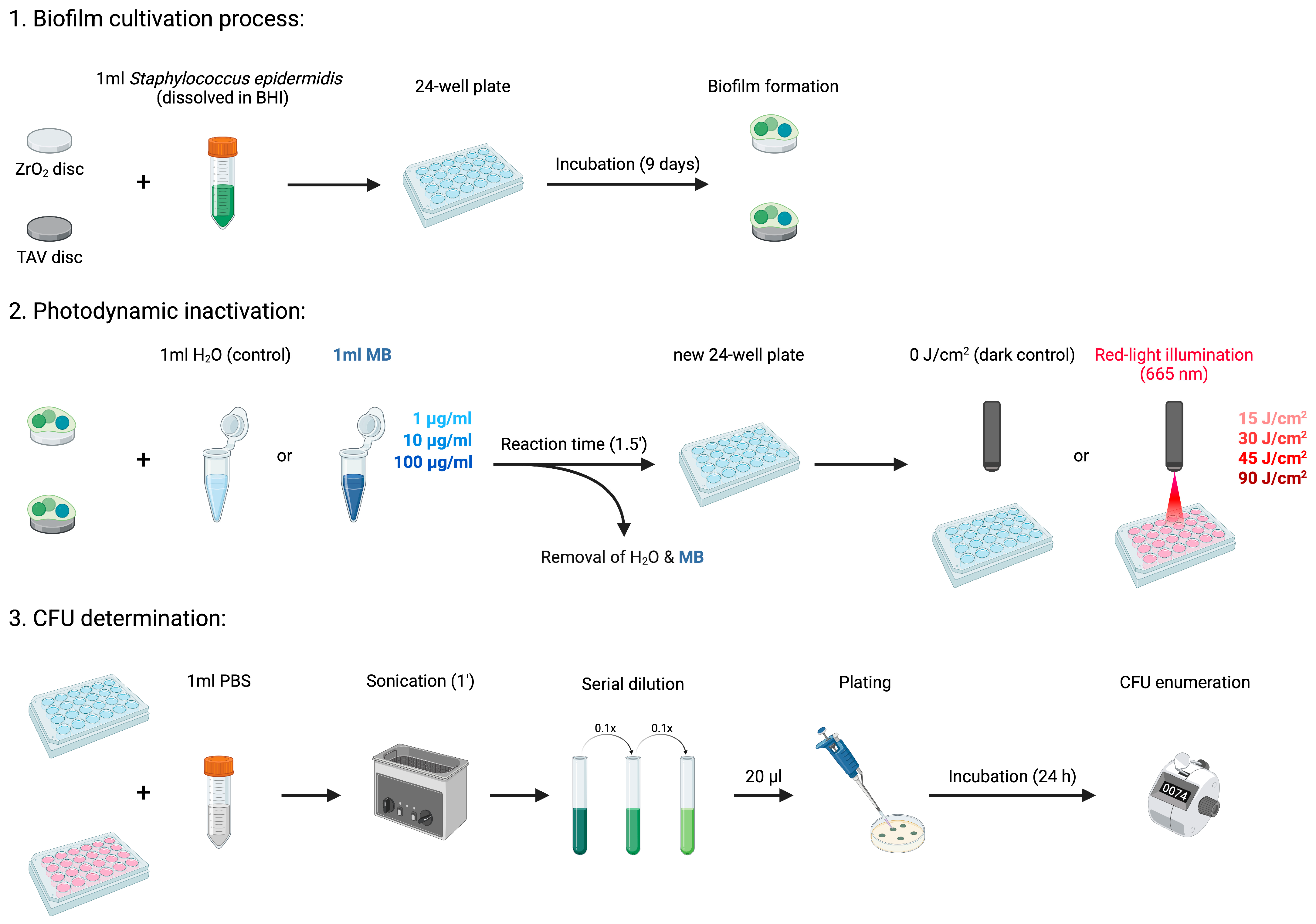
2.2.1. Biofilm Cultivation Process
2.2.2. In Vitro Photodynamic Inactivation Process
2.3. Statistical Analysis
3. Results
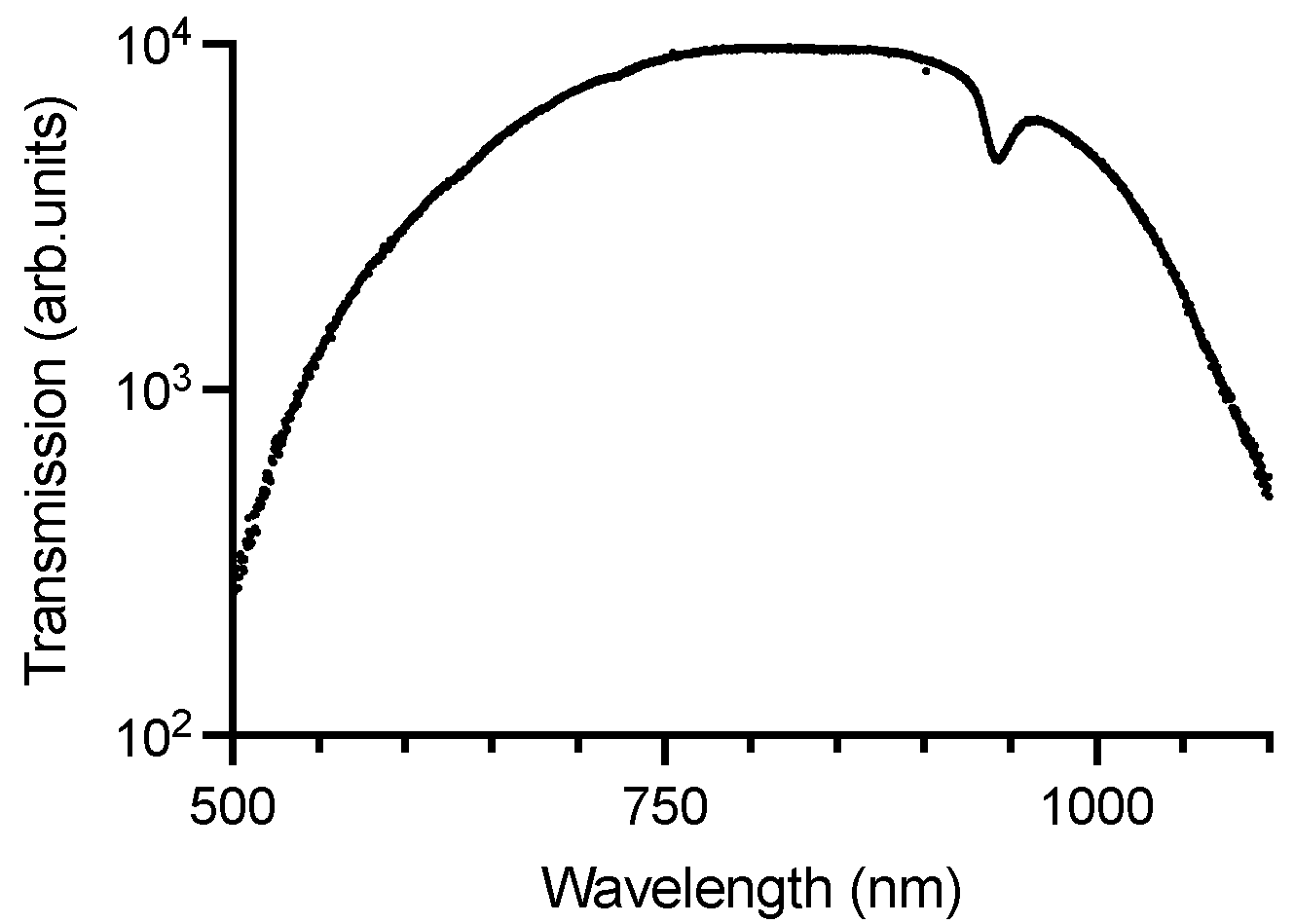
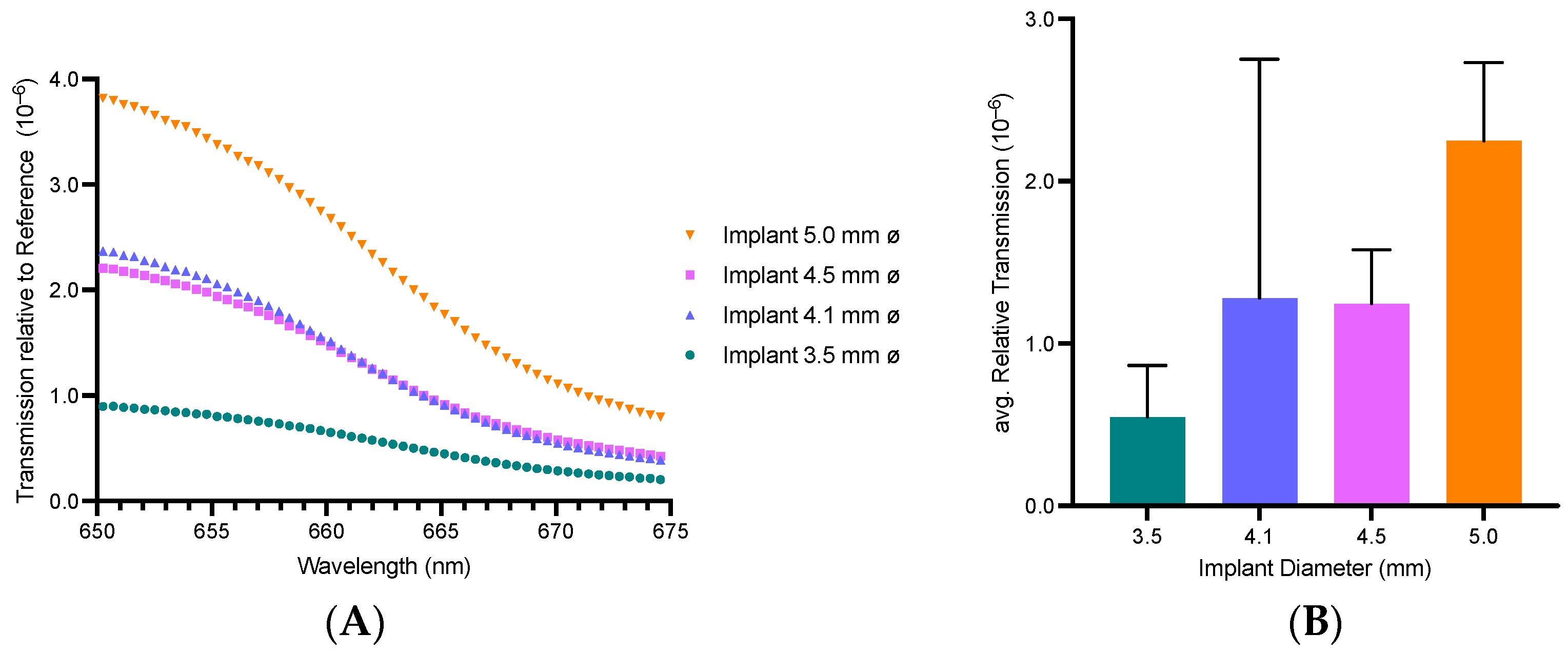
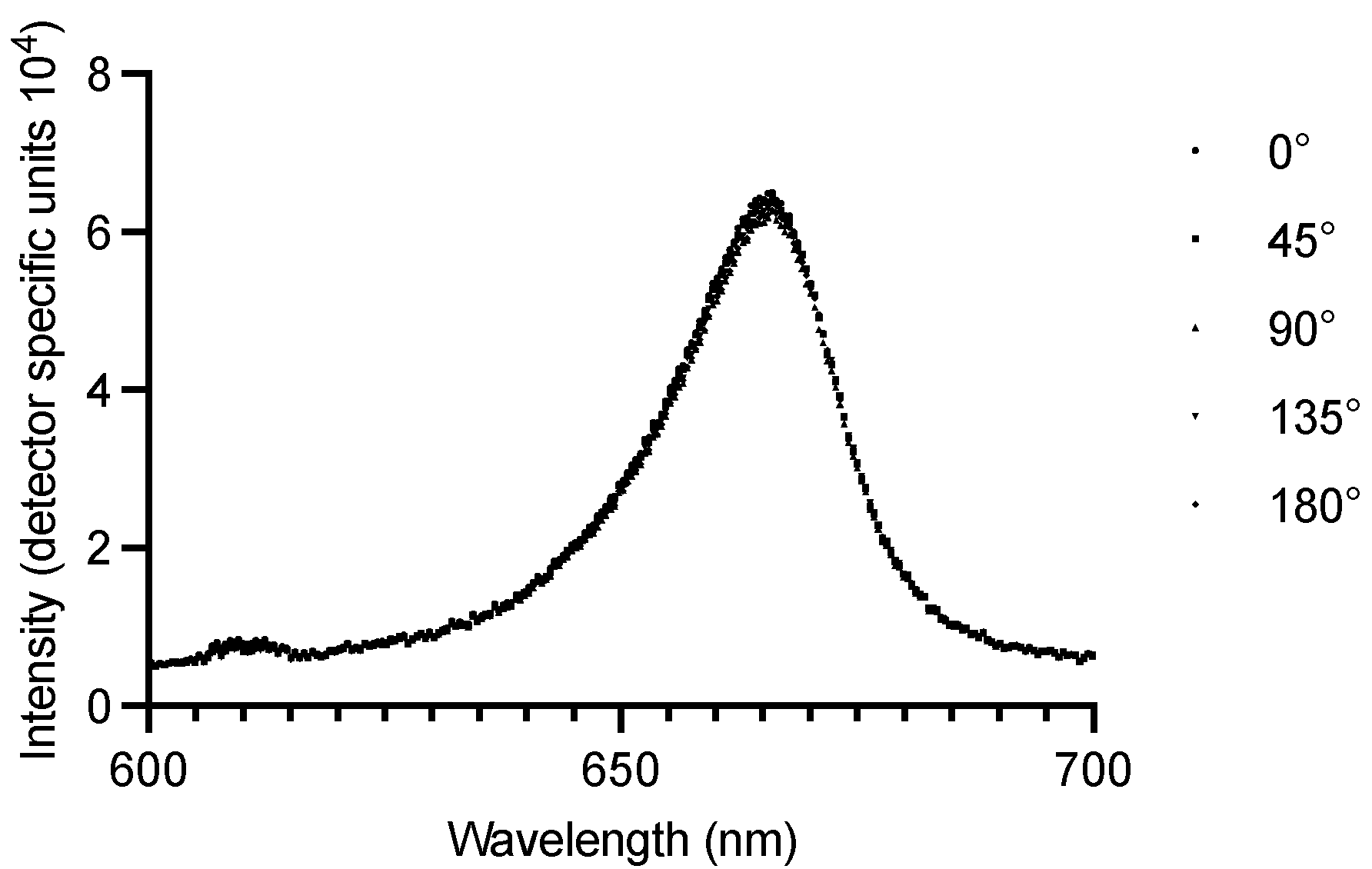
3.1. Zirconium Dental Implants Waveguiding Properties
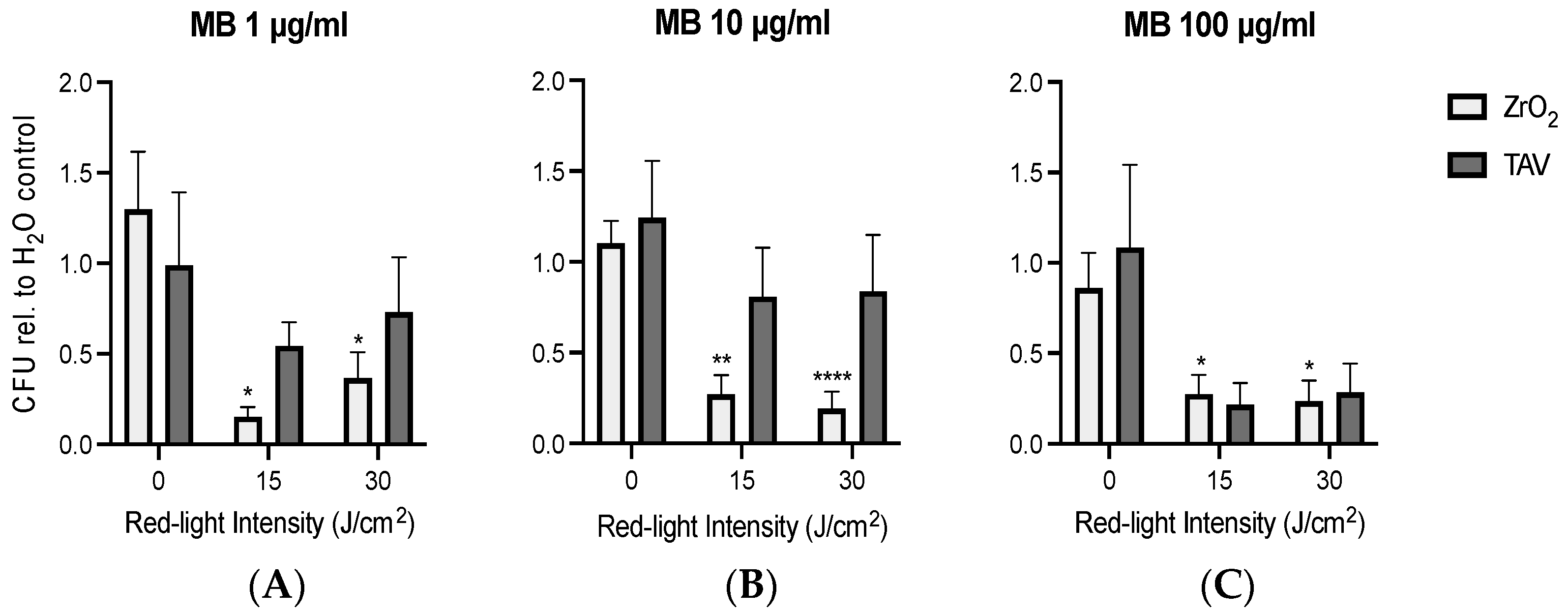
3.2. PDI Biofilm Destruction Comparison ZrO2 and TAV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| MB | Methylene Blue |
| PDI | Photodynamic Inactivation |
| PI | periimplantitis |
| ROS | reactive oxygen species |
| TAV | titanium alloy Ti-6AI-4V |
| ZrO2 | zirconium dioxide ceramics |
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Obreja, K.; Begic, A.; Ramanauskaite, A.; Schwarz, F. Periimplantitis Definition, Symptome und leitliniengerechte Empfehlungen. DFZ 2020, 64, 86–90. [Google Scholar] [CrossRef]
- Carvalho, É.B.S.; Romandini, M.; Sadilina, S.; Sant’Ana, A.C.P.; Sanz, M. Microbiota Associated with Peri-Implantitis—A Systematic Review with Meta-Analyses. Clin. Oral. Implant. Res. 2023, 34, 1176–1187. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of Bacteria Associated with Peri-Implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and Treatment of Peri-Implant Diseases—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N. A Comprehensive Review of Peri-Implantitis Risk Factors. Curr. Oral Health Rep. 2020, 7, 262–273. [Google Scholar] [CrossRef]
- Antonowicz, B.; Borys, J.; Zalewska, A.; Żendzian-Piotrowska, M.; Łukaszuk, K.; Woźniak, Ł.; Szuta, M.; Maciejczyk, M. Circulating Biomarkers of Nitrosative Stress, Protein Glycoxidation and Inflammation in Maxillofacial Surgery Patients Treated with Titanium Implants. Dent. Med. Probl. 2025. [Google Scholar] [CrossRef]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Risk Factors for Peri-Implantitis: An Umbrella Review of Meta-Analyses of Observational Studies and Assessment of Biases. J. Dent. 2024, 146, 105065. [Google Scholar] [CrossRef]
- Ardila, C.M.; Vivares-Builes, A.M. Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15609. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Mirra, D.; Roccuzzo, A. Surgical Treatment of Peri-Implantitis. Br. Dent. J. 2024, 236, 803–808. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-Implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef]
- Riben Grundström, C.; Lund, B.; Kämpe, J.; Belibasakis, G.N.; Hultin, M. Systemic Antibiotics in the Surgical Treatment of Peri-Implantitis: A Randomized Placebo-Controlled Trial. J. Clin. Periodontol. 2024, 51, 981–996. [Google Scholar] [CrossRef]
- Eufinger, H.; Kübler, A.; Schliephake, H. Mund-, Kiefer- und Gesichtschirurgie. Operationsleher und–Atlas, 5th ed.; Springer-Verlag GmbH: Heidelberg, Germany, 2021. [Google Scholar]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis: Case Definitions and Diagnostic Considerations. J. Periodontol. 2018, 89, S304–S312. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-Implant Diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef]
- Fernandes, G.V.D.O.; Martins, B.G.D.S.; Fraile, J.F. Revisiting Peri-Implant Diseases in Order to Rethink the Future of Compromised Dental Implants: Considerations, Perspectives, Treatment, and Prognosis. Dent. Med. Probl. 2024, 61, 637–640. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022, 1st ed.; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- WHO-EURO-2024-9510-49282-73655-Eng.Pdf,” Position Paper. Available online: https://iris.who.int/bitstream/handle/10665/376479/WHO-EURO-2024-9510-49282-73655-eng.pdf?sequence=1 (accessed on 26 January 2025).
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B.; Taba, M. Antimicrobial Photodynamic Therapy in the Non-Surgical Treatment of Aggressive Periodontitis: A Preliminary Randomized Controlled Clinical Study. J. Periodontol. 2007, 78, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In Vitro Resistance Selection Studies of RLP068/Cl, a New Zn(II) Phthalocyanine Suitable for Antimicrobial Photodynamic Therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Prinz, J.; Wink, M.; Neuhaus, S.; Grob, M.C.; Walt, H.; Bosshard, P.P.; Achermann, Y. Effective Biofilm Eradication on Orthopedic Implants with Methylene Blue Based Antimicrobial Photodynamic Therapy In Vitro. Antibiotics 2023, 12, 118. [Google Scholar] [CrossRef]
- Cwalinski, T.; Polom, W.; Marano, L.; Roviello, G.; D’Angelo, A.; Cwalina, N.; Matuszewski, M.; Roviello, F.; Jaskiewicz, J.; Polom, K. Methylene Blue—Current Knowledge, Fluorescent Properties, and Its Future Use. J. Clin. Med. 2020, 9, 3538. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef]
- Liebermann, A.; Freitas Rafael, C.; Colle Kauling, A.E.; Edelhoff, D.; Ueda, K.; Seiffert, A.; Maziero Volpato, C.A.; Güth, J.-F. Transmittance of Visible and Blue Light through Zirconia. Dent. Mater. J. 2018, 37, 812–817. [Google Scholar] [CrossRef]
- Maharishi, A.; McLaren, E.A.; White, S.N. Color- and Strength-Graded Zirconia: Strength, Light Transmission, and Composition. J. Prosthet. Dent. 2024, 131, e1–e1236. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bächle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm Formation and Composition on Different Implant Materials in Vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 101–109. [Google Scholar] [CrossRef]
- Azizi, B.; Budimir, A.; Bago, I.; Mehmeti, B.; Jakovljević, S.; Kelmendi, J.; Stanko, A.P.; Gabrić, D. Antimicrobial Efficacy of Photodynamic Therapy and Light-Activated Disinfection on Contaminated Zirconia Implants: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2018, 21, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Labban, N.; Shibani, N.A.; Al-Kattan, R.; Alfouzan, A.F.; Binrayes, A.; Assery, M.K. Clinical, Bacterial, and Inflammatory Outcomes of Indocyanine Green-Mediated Photodynamic Therapy for Treating Periimplantitis among Diabetic Patients: A Randomized Controlled Clinical Trial. Photodiagnosis Photodyn. Ther. 2021, 35, 102350. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowygh, Z.H. Efficacy of Periimplant Mechanical Curettage with and without Adjunct Antimicrobial Photodynamic Therapy in Smokeless-Tobacco Product Users. Photodiagnosis Photodyn. Ther. 2017, 18, 260–263. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Ta, S.; Cheng, Z.; Zhang, P.; Zeng, N.; Goodman, B.A.; Xu, S.; Deng, W. Optical Properties of Yttria-Stabilized Zirconia Single-Crystals Doped with Terbium Oxide. Crystals 2022, 12, 1081. [Google Scholar] [CrossRef]
- Rogers, J.D.; Radosevich, A.J.; Yi, J.; Backman, V. Modeling Light Scattering in Tissue as Continuous Random Media Using a Versatile Refractive Index Correlation Function. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 7000514. [Google Scholar] [CrossRef]
- Bashkatov, A.A.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. Available online: https://www.researchgate.net/publication/231124674_Bashkatov_AA_Genina_EA_Kochubey_VI_Tuchin_VVOptical_properties_of_human_skin_subcutaneous_and_mucous_tissues_in_the_wavelength_range_from_400_to_2000_nm_J_Phys_D_Appl_Phys_382543-2555 (accessed on 19 February 2025).
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef]
- Khan, A.S.; Syed, M.R. A Review of Bioceramics-Based Dental Restorative Materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 10.1128. [Google Scholar] [CrossRef]
- The Role of Bacterial Biofilms in Antimicrobial Resistance. ASM.org. Available online: https://asm.org:443/Articles/2023/March/The-Role-of-Bacterial-Biofilms-in-Antimicrobial-Re (accessed on 17 February 2025).
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Mackay, A.M. The Evolution of Clinical Guidelines for Antimicrobial Photodynamic Therapy of Skin. Photochem. Photobiol. Sci. 2022, 21, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.P.M.; da Silva, D.d.F.T.; Fernandes, K.P.S.; Ferreira, R.d.C.; Lino-dos-Santos-Franco, A.; Rodrigues, M.F.S.D.; Motta, L.J.; Cecatto, R.B. Antimicrobial Photodynamic Therapy with Methylene Blue and Its Derivatives in Animal Studies: Systematic Review. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12978. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Pavelka, M.S.; Baran, T.M. Methylene Blue Photodynamic Therapy of Bacterial Species Found in Human Abscesses: Planktonic, Biofilm, and 3D Silicone Models. Proc. SPIE Int. Soc. Opt. Eng. 2023, 12358, 1235805. [Google Scholar]
- Songsantiphap, C.; Vanichanan, J.; Chatsuwan, T.; Asawanonda, P.; Boontaveeyuwat, E. Methylene Blue–Mediated Antimicrobial Photodynamic Therapy Against Clinical Isolates of Extensively Drug Resistant Gram-Negative Bacteria Causing Nosocomial Infections in Thailand, An In Vitro Study. Front. Cell. Infect. Microbiol. 2022, 12, 929242. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chen, C.-J.; Ding, S.-J.; Chen, C.-C. Antimicrobial Efficacy of Methylene Blue-Mediated Photodynamic Therapy on Titanium Alloy Surfaces in Vitro. Photodiagnosis Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef]
- Campos Chaves Lamarque, G.; Cusicanqui Méndez, D.A.; Arruda Matos, A.; José Dionísio, T.; Andrade Moreira Machado, M.A.; Magalhães, A.C.; Cardoso Oliveira, R.; Cruvinel, T. Cytotoxic Effect and Apoptosis Pathways Activated by Methylene Blue-Mediated Photodynamic Therapy in Fibroblasts. Photodiagnosis Photodyn. Ther. 2020, 29, 101654. [Google Scholar] [CrossRef]
- Salahuddin, N.; Akelah, A.; Elnagar, M.; Abdelwahab, M.A. Antibacterial and Cytotoxicity of Methylene Blue Loaded-Cellulose Nanocarrier on Breast Cancer Cell Line. Carbohydr. Polym. Technol. Appl. 2021, 2, 100138. [Google Scholar] [CrossRef]
- Lian, C.; Piksa, M.; Yoshida, K.; Persheyev, S.; Pawlik, K.J.; Matczyszyn, K.; Samuel, I.D.W. Flexible Organic Light-Emitting Diodes for Antimicrobial Photodynamic Therapy. NPJ Flex. Electron. 2019, 3, 18. [Google Scholar] [CrossRef]
- Nammour, S.; Zeinoun, T.; Bogaerts, I.; Lamy, M.; Geerts, S.O.; Saba, S.B.; Lamard, L.; Peremans, A.; Limme, M. Evaluation of Dental Pulp Temperature Rise during Photo-Activated Decontamination (PAD) of Caries: An in Vitro Study. Lasers Med. Sci. 2010, 25, 651–654. [Google Scholar] [CrossRef]
- Risk Factors: Radiation—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation (accessed on 18 February 2025).
- Newsom, E.T.; Sadeghpour, A.; Entezari, A.; Vinzons, J.L.U.; Stanford, R.E.; Mirkhalaf, M.; Chon, D.; Dunstan, C.R.; Zreiqat, H. Design and Evaluation of 3D-Printed Sr-HT-Gahnite Bioceramic for FDA Regulatory Submission: A Good Laboratory Practice Sheep Study. Acta Biomater. 2023, 156, 214–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, K.; Kadler, G.; Kalyanov, A.; Schweizer, T.A.; Walt, H.; Essig, H. Zirconium Dental Implants as Potential Optical Waveguides in Photodynamic Inactivation of Bacterial Biofilms—A Pilot Study. Microorganisms 2025, 13, 850. https://doi.org/10.3390/microorganisms13040850
Lehmann K, Kadler G, Kalyanov A, Schweizer TA, Walt H, Essig H. Zirconium Dental Implants as Potential Optical Waveguides in Photodynamic Inactivation of Bacterial Biofilms—A Pilot Study. Microorganisms. 2025; 13(4):850. https://doi.org/10.3390/microorganisms13040850
Chicago/Turabian StyleLehmann, Kolja, Gabor Kadler, Alexander Kalyanov, Tiziano A. Schweizer, Heinrich Walt, and Harald Essig. 2025. "Zirconium Dental Implants as Potential Optical Waveguides in Photodynamic Inactivation of Bacterial Biofilms—A Pilot Study" Microorganisms 13, no. 4: 850. https://doi.org/10.3390/microorganisms13040850
APA StyleLehmann, K., Kadler, G., Kalyanov, A., Schweizer, T. A., Walt, H., & Essig, H. (2025). Zirconium Dental Implants as Potential Optical Waveguides in Photodynamic Inactivation of Bacterial Biofilms—A Pilot Study. Microorganisms, 13(4), 850. https://doi.org/10.3390/microorganisms13040850





