Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Conditions
2.2. Experimental Layout
2.3. Sampling for Microbiome Analysis
2.4. Harvesting of Plants for Biomass
2.5. Determination of PHC Concentrations in Soil by GC-FID Analysis
2.6. Analysis of Heavy Metals by Atomic Absorption Spectroscopy
2.7. Statistical Methods
2.8. Sequence Data Accession Numbers
3. Results
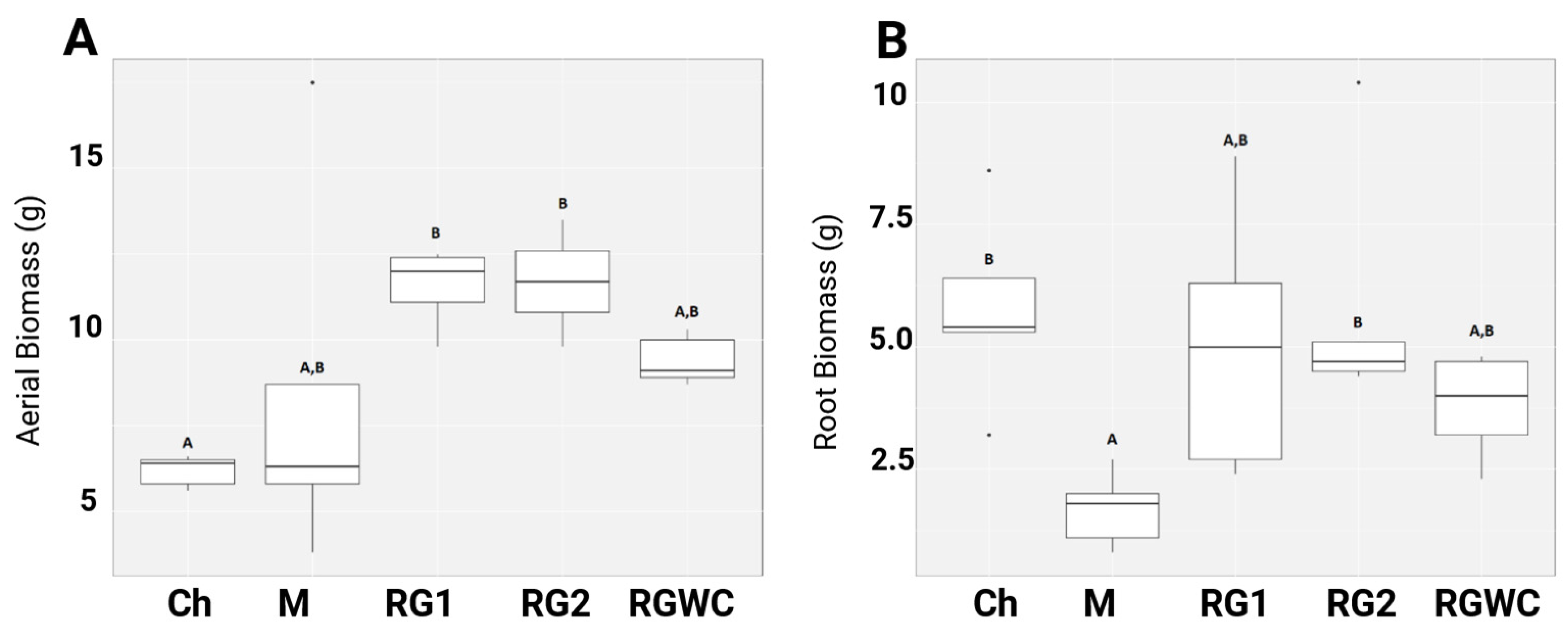
3.1. Aerial and Root Biomass After 14 Weeks of Growth
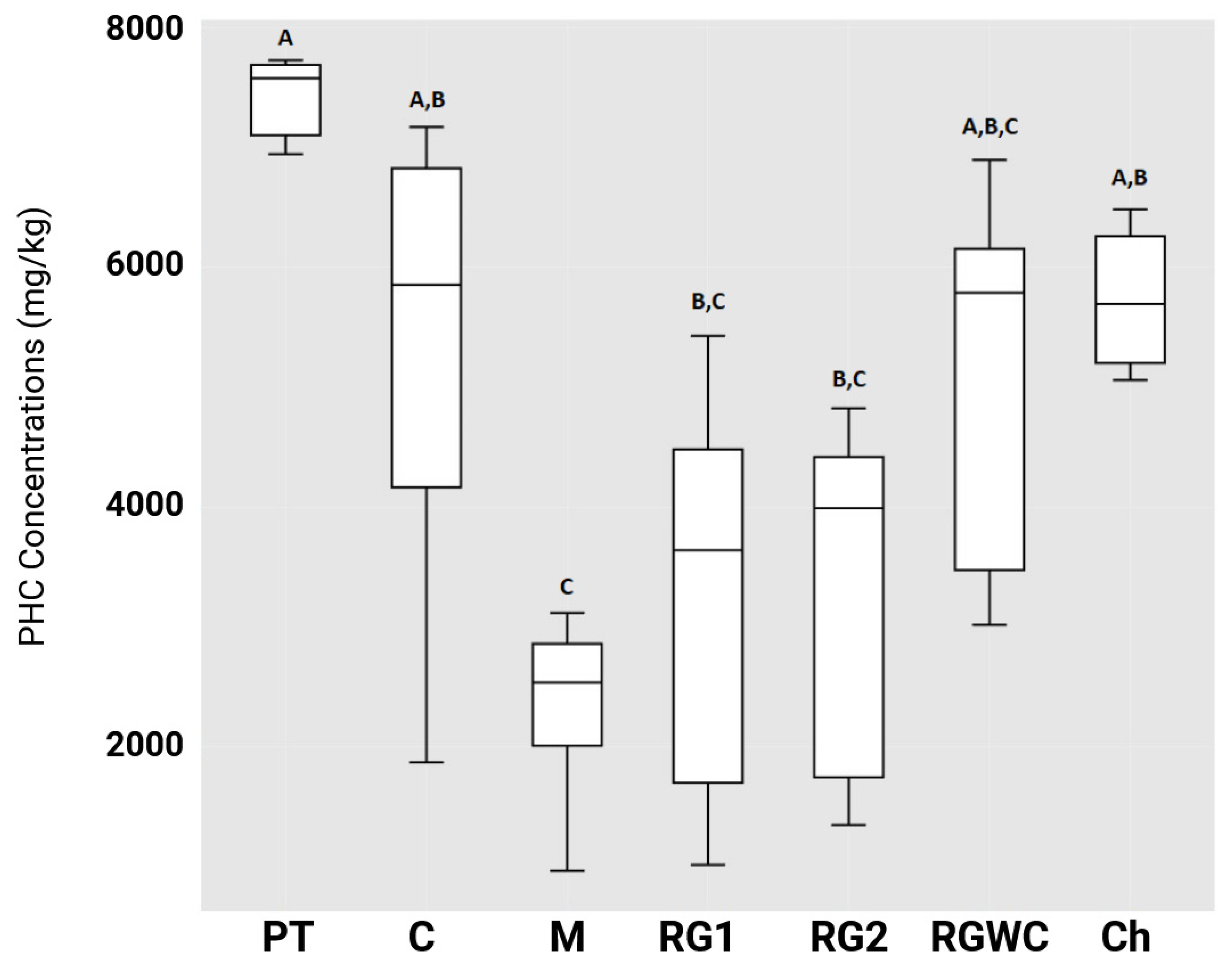
3.2. Reduction in Petroleum Hydrocarbon (PHC) Concentrations Under Microcosm Conditions
3.3. Heavy Metal Concentrations in Soil, Roots, and Shoots
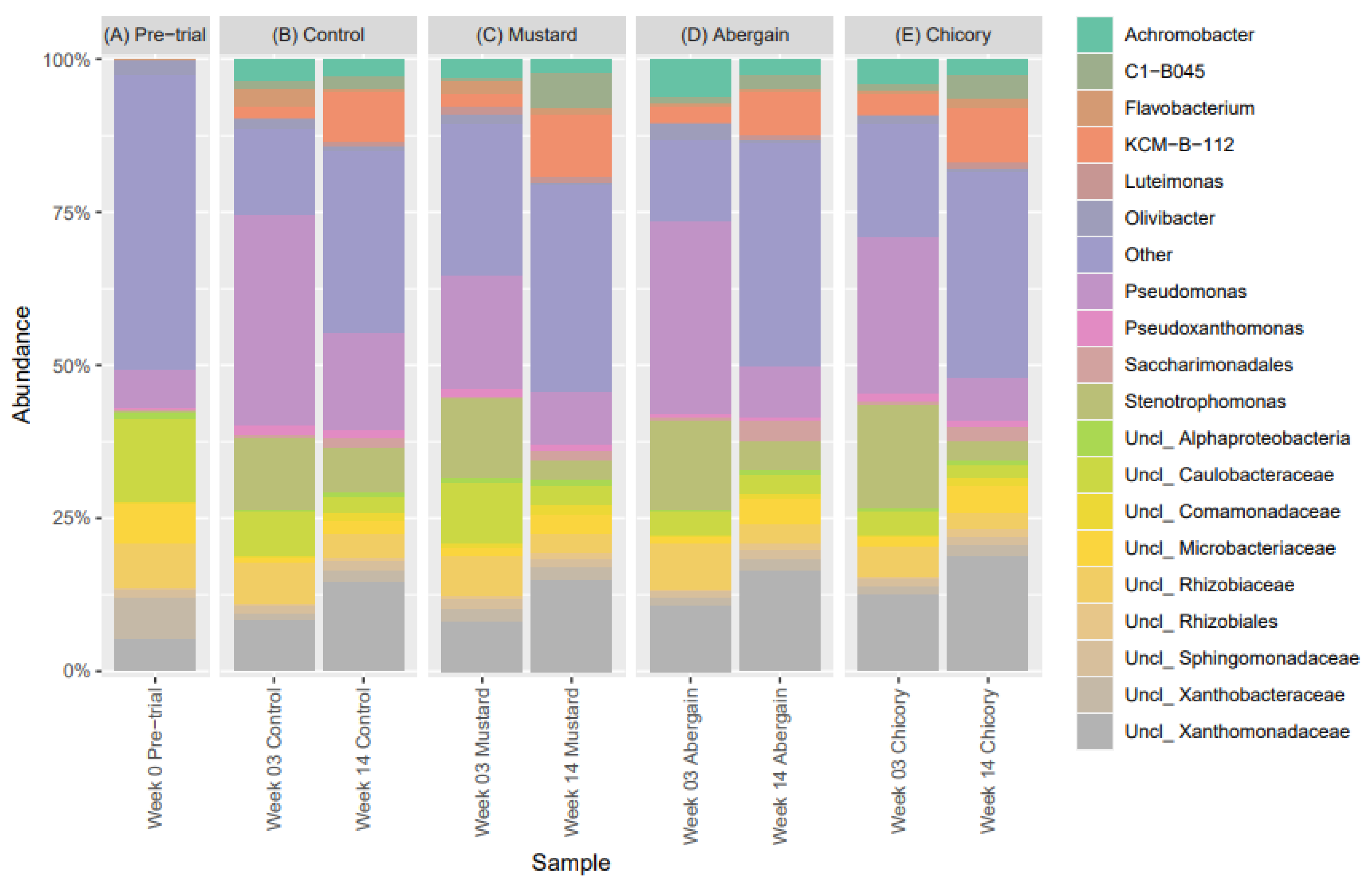
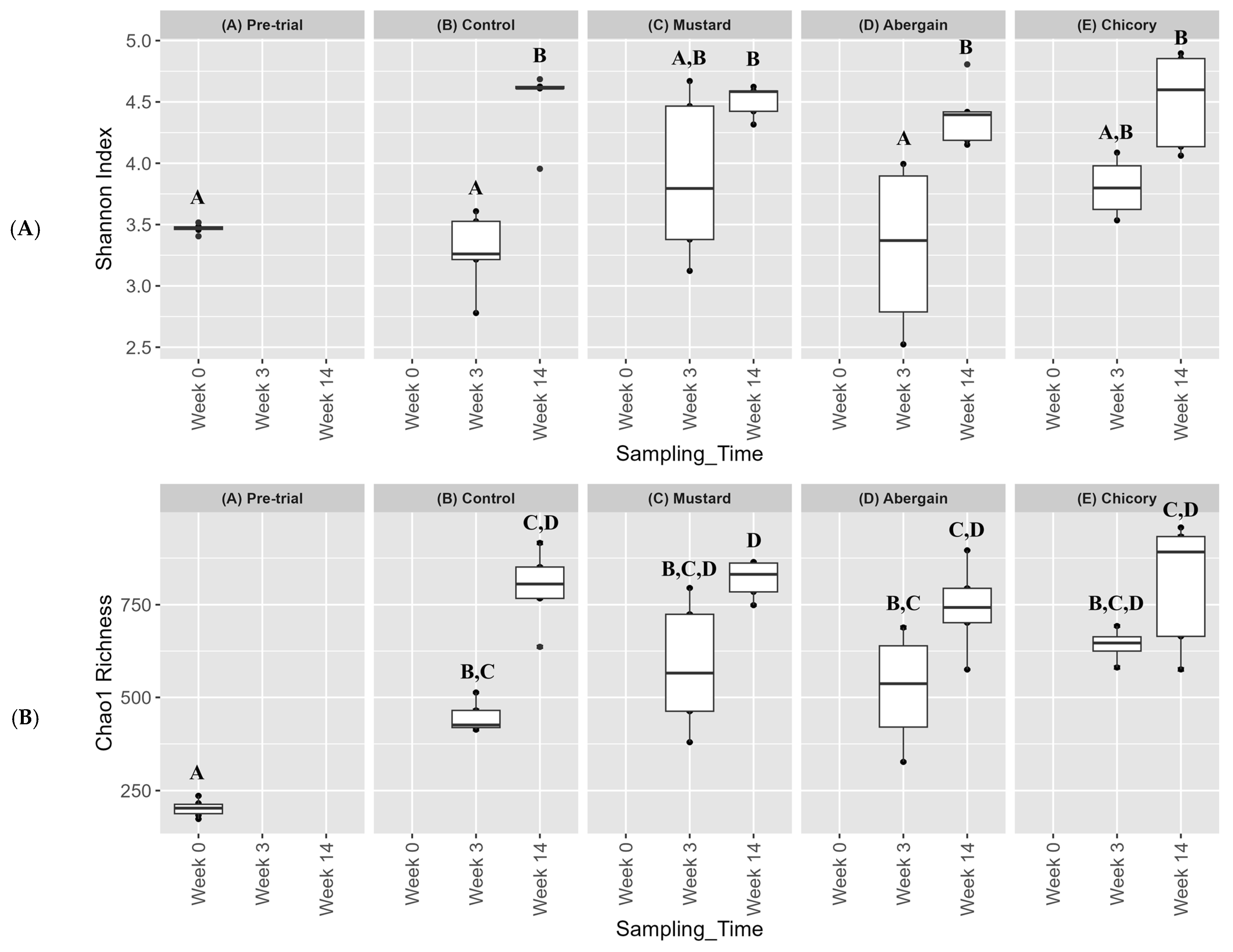
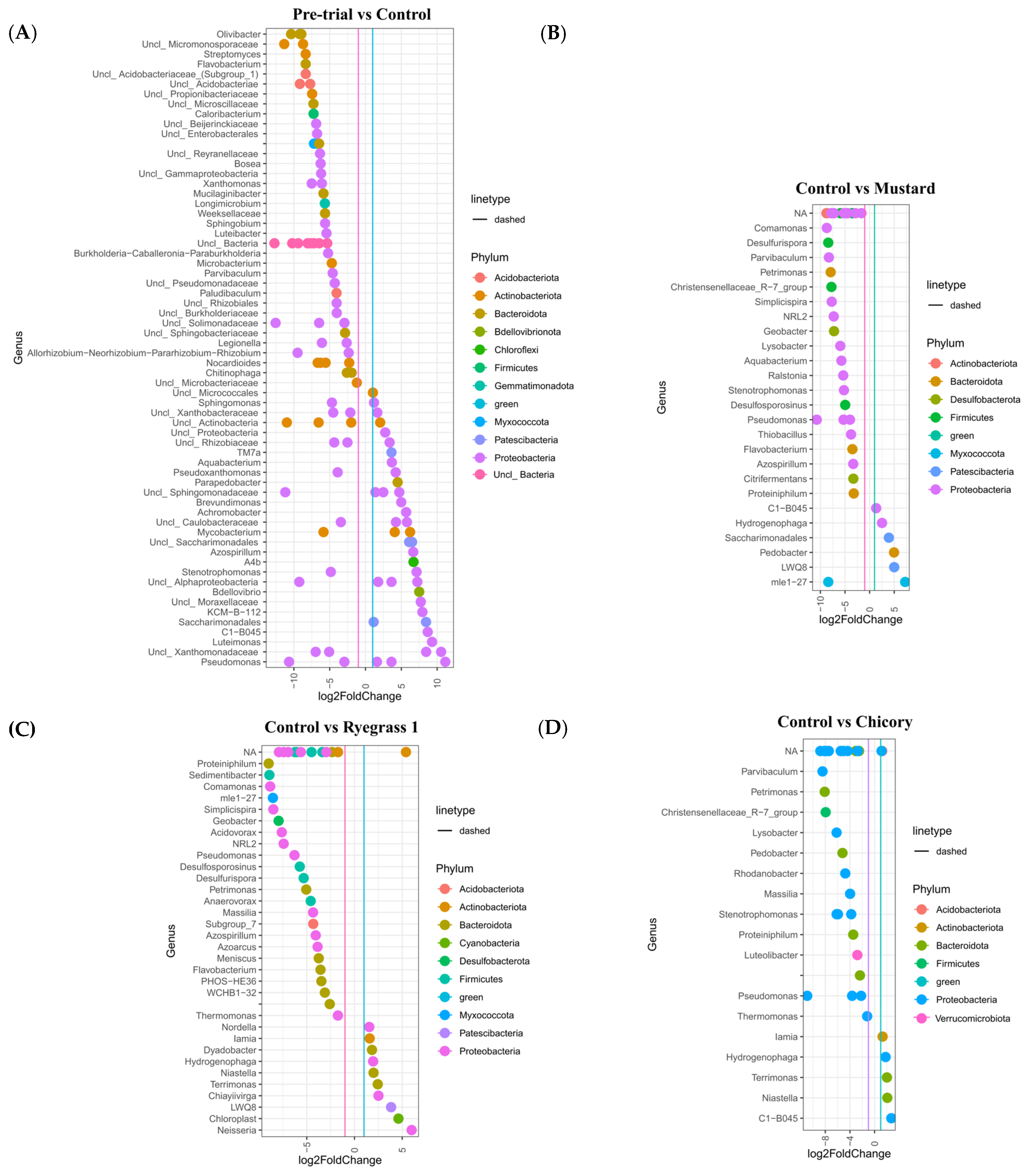
3.4. Bacterial Community Dynamics and Diversity During Phytoremediation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devatha, C.P.; Vishal, A.V.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics of soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Baoune, H.; Aparicio, J.D.; Acuña, A.; El Hadj-Khelil, A.O.; Sanchez, L.; Polti, M.A.; Alvarez, A. Effectiveness of the Zea mays-Streptomyces association for the phytoremediation of petroleum hydrocarbons impacted soils. Ecotoxicol. Environ. Saf. 2019, 184, 109591. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Ma, A.; Wei, X.; Chen, X.; Yin, J.; Hu, F.; Zhuang, X.; Song, M.; Zhuang, G. Interaction and spatio-taxonomic patterns of the soil microbiome around oil production wells impacted by petroleum hydrocarbons. Environ. Pollut. 2022, 307, 119531. [Google Scholar] [CrossRef]
- Das, N.; Bhuyan, B.; Pandey, P. Correlation of soil microbiome with crude oil contamination drives detection of hydrocarbon degrading genes which are independent of quantity and type of contaminants. Environ. Res. 2022, 215 Pt 1, 114185. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Shahsavari, E. Petroleum hydrocarbon contamination in terrestrial ecosystems—Fate and microbial responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef]
- Sun, S.; Liu, X.; Zhang, Y.; Chen, J.; Wang, H.; Li, P. Effects of petroleum hydrocarbons on microbial community structure and functional potential in contaminated soils. Environ. Pollut. 2022, 302, 119066. [Google Scholar] [CrossRef]
- Quintella, C.; Mata, A.M.T.; Lima, L. Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil-contaminated soils. J. Environ. Manag. 2019, 241, 156–166. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Kidd, P.S.; Monterroso, C. Influence of plant root exudates on the mobility of fuel volatile compounds in contaminated soils. Int. J. Phytoremediation 2014, 16, 824–839. [Google Scholar] [CrossRef]
- Martínez-Rabelo, F.; Gomez-Guzman, L.; García-Segura, D.R.; Villegas-García, E.; Rodriguez-Campos, J.; Velazquez, J.; Castellanos, B.; Barois, I.; Contreras-Ramos, S. Hydrocarbon Bioremediation in a Pilot-Scale: A Combination of Bioaugmentation, Phytoremediation, and Vermiremediation. Environ. Technol. Innov. 2023, 31, 103210. [Google Scholar] [CrossRef]
- Correa-García, S.; Pande, P.; Séguin, A.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of petroleum hydrocarbons: A model system for plant microbiome manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Mench, M. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.; Mao, H.; Wang, B.; Wang, P. Remediation of hydrocarbon–heavy metal co-contaminated soil by electrokinetics combined with biostimulation. Chem. Eng. J. 2018, 353, 4. [Google Scholar] [CrossRef]
- Rajkumari, J.; Das, S.; Borah, S.N.; Deka, M.; Sharma, R.K. Biodegradation of petroleum hydrocarbons by microbes: Mechanisms, pathways, and bioremediation technologies. Front. Microbiol. 2021, 12, 593023. [Google Scholar] [CrossRef]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant–microbiome interactions to improve phytoremediation: Engineering the meta-organism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef]
- He, M.; Li, Z.; Chen, C.; Mei, P. Impact of Soil Types and Root Exudates on Cadmium and Petroleum Hydrocarbon Phytoremediation by Sorghum sudanense, Festuca arundinacea, and Lolium perenne. Front. Ecol. Evol. 2022, 10, 1036765. [Google Scholar] [CrossRef]
- Płociniczak, T.; Kukla, M.; Wątroba, R.; Piotrowska-Seget, Z. The effect of soil bioaugmentation with strains of Pseudomonas on Cd, Zn and Cu uptake by Sinapis alba L. Chemosphere 2013, 91, 1332–1337. [Google Scholar] [CrossRef]
- Curiel-Alegre, S.; Velasco-Arroyo, B.; Rumbo, C.; Khan, A.H.A.; Tamayo-Ramos, J.A.; Rad, C.; Barros, R. Evaluation of biostimulation, bioaugmentation, and organic amendments application on the bioremediation of recalcitrant hydrocarbons of soil. SSRN Electron. J. 2022, 307, 135638. [Google Scholar] [CrossRef]
- Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, L181, 6–12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31986L0278 (accessed on 16 February 2025).
- Luo, H.; Yu, J.; Li, R.; Gu, J.-D.; Luo, L.; Zhang, Y.; He, Y.; Xiao, Y.; Deng, S.; Zhang, Y.; et al. Microbial biomass C:N:P as a better indicator than soil and ecoenzymatic C:N:P for microbial nutrient limitation and C dynamics in Zoige Plateau peatland soils. Int. Biodeterior. Biodegrad. 2022, 175, 105492. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Sadowska, M.; Biaduń, E.; Mazur, R.; Kowalska, J. Sinapis alba as a useful plant in bioremediation—Studies of defense mechanisms and accumulation of As, Tl and PGEs. Int. J. Phytoremediation 2022, 24, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Dionysiou, D.; Papadopoulou, M. Phytoremediation Potential of Cichorium intybus in Removing Heavy Metals and Its Ecological Benefits. Int. J. Phytoremediation 2021, 23, 368–380. [Google Scholar]
- García-Bayona, L.; Comstock, L.E. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cuesta, R.; Conlon, R.; Wang, M.; Blanco-Romero, E.; Durán, D.; Redondo-Nieto, M.; Rivilla, R. Field-scale biodegradation of total petroleum hydrocarbons and soil restoration by Ecopiles: Microbiological analysis of the process. Front. Microbiol. 2023, 14, 1158130. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wires Comput. Statistics 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Ruley, J.A.; Amoding, A.; Tumuhairwe, J.B.; Basamba, T.A.; Opolot, E.; Oryem-Origa, H. Enhancing the phytoremediation of hydrocarbon-contaminated soils in the Sudd Wetlands, South Sudan, using organic manure. Appl. Environ. Soil Sci. 2020, 2020, 4614286. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.S.; Hinton, J.C.D. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, R.; Niu, X.; Zhou, Q. Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil Tillage Res. 2010, 110, 87–93. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Feigl, G.; Duzs, Á.; Laczi, K.; Szilágyi, Á.; Rákhely, G.; Perei, K. Exploitation of extracellular organic matter from Micrococcus luteus to enhance ex situ bioremediation of soils polluted with used lubricants. J. Hazard. Mater. 2021, 417, 125996. [Google Scholar] [CrossRef]
- Mafiana, M.O.; Kang, X.H.; Leng, Y.; He, L.F.; Li, S.W. Petroleum contamination significantly changes soil microbial communities in three oilfield locations in Delta State, Nigeria. Environ. Sci. Pollut. Res. 2021, 28, 31447–31461. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.; Luan, X.; Bian, X.; Li, G.; Hao, T.; Liu, J.; Feng, K.; Song, Y. Degradation potential and pathways of methylcyclohexane by bacteria derived from Antarctic surface water. Chemosphere 2023, 329, 138647. [Google Scholar] [CrossRef]
- Uribe-Flores, M.M.; Cerqueda-García, D.; Hernández-Nuñez, E.; Cadena, S.; García-Cruz, N.U.; Trejo-Hernández, M.R.; García-Maldonado, J.Q. Bacterial succession and co-occurrence patterns of an enriched marine microbial community during light crude oil degradation in a batch reactor. J. Appl. Microbiol. 2019, 127, 495–507. [Google Scholar] [CrossRef]
- Peng, X.; Li, J.; Zhou, H.; Wu, Z.; Li, J.; Chen, S. Characteristics and source of inorganic and organic compounds in the sediments from two hydrothermal fields of the central Indian and Mid-Atlantic ridges. J. Asian Earth Sci. 2011, 41, 355–368. [Google Scholar] [CrossRef]
- Arrington, E.; Tarn, J.; Kittner, H.; Kivenson, V.; Liu, R.; Valentine, D. Methylated cycloalkanes fuel a novel genera in the Porticoccaceae family and inform substrate affinity for a unique copper membrane monooxygenase. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cui, Z.; Luan, X.; Li, S.; Li, Y.; Bian, X.; Li, G.; Wei, O.; Ran, X.; Bao, M.; Valentine, D. Occurrence and distribution of cyclic-alkane-consuming psychrophilic bacteria in the Yellow Sea and East China Sea. J. Hazard. Mater. 2022, 427, 128129. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Y.; Zhang, X.; Wang, Y.; Chen, Q.; Yang, F.; Wang, L. Biodegradation of polycyclic aromatic hydrocarbons by Hydrogenophaga sp. strain PAH-1 isolated from petroleum-contaminated soil. RSC Adv. 2017, 7, 56516–56524. [Google Scholar] [CrossRef]
- Abdul Majeed, Z.; Muhammad, Z.; Ullah, R.; Ullah, K.; Ali, H.; Inayat, N. Plant growth-promoting rhizobacteria as bioremediators of polluted agricultural soils: Challenges and prospects. In Hazardous and Trace Materials in Soil and Plants; Naeem, M., Aftab, T., Ansari, A.A., Gill, S.S., Macovei, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 265–275. ISBN 9780323916325. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, G.; Chen, X.; Long, H.; Wang, Y.; Chen, T.; Liu, G. The complete genome sequence of the cold adapted crude-oil degrader: Pedobacter steynii DX4. Stand. Genom. Sci. 2017, 12, 45. [Google Scholar] [CrossRef]
- Li, M.; Xu, M.; Su, A.; Zhang, Y.; Niu, L.; Xu, Y. Combined phenanthrene and copper pollution imposed a selective pressure on the rice root-associated microbiome. Front. Microbiol. 2022, 13, 888086. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, P.; He, Y.; Zeng, F.; Xu, J.; He, L. Enantioselective effects of cyflumetofen on microbial community and related nitrogen cycle gene function in acid-soil. Sci. Total Environ. 2021, 771, 144831. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Zhou, J.; Ju, F. Active predation, phylogenetic diversity, and global prevalence of myxobacteria in wastewater treatment plants. ISME J. 2023, 17, 671–681. [Google Scholar] [CrossRef]
- Hajzler, M.; Klimešová, J.; Středa, T. Biomass production of white mustard (Sinapis alba L.) varieties in relation to the root system size. J. Plant Nutr. Soil Sci. 2011, 2011, 105–108. [Google Scholar]
- Wang, Q.; Zheng, R.; Sun, X.; Jiang, Z.; Yang, F.; Lu, Q.; Cui, J. Effects of Comamonas testosteroni on PAHs degradation and bacterial community structure in Leymus chinensis rhizosphere soil. Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2020, 36, 2657–2673. (In Chinese) [Google Scholar] [CrossRef]
- Olowomofe, T.O.; Oluyege, J.O.; Aderiye, B.I.; Oluwole, O.A. Degradation of polyaromatic fractions of crude oil and detection of catabolic genes in hydrocarbon-degrading bacteria isolated from Agbabu bitumen sediments in Ondo State. AIMS Microbiol. 2019, 5, 308–323. [Google Scholar] [CrossRef]
- Song, M.; Luo, C.; Jiang, L.; Zhang, D.; Wang, Y.; Zhang, G. Identification of Benzo[a]pyrene*-metabolizing bacteria in forest soils by using DNA-based stable-isotope probing*. Appl. Environ. Microbiol. 2015, 81, 7368–7376. [Google Scholar] [CrossRef]
- Somee, M.R.; Amoozegar, M.A.; Dastgheib, S.M.M.; Shavandi, M.; Maman, L.G.; Bertilsson, S.; Mehrshad, M. Genome-Resolved Analyses Show an Extensive Diversification in Key Aerobic Hydrocarbon-Degrading Enzymes across Bacteria and Archaea. BMC Genom. 2022, 23, 690. [Google Scholar] [CrossRef] [PubMed]
- Kanaly, R.A.; Harayama, S.; Watanabe, K. Rhodanobacter sp. Strain BPC1 in a Benzo[a]pyrene-Mineralizing Bacterial Consortium. Appl. Environ. Microbiol. 2002, 68, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Gębicka, U.; Gałązka, I.; Włodarczyk, D.; Ławniczak, S. Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Braz. J. Microbiol. 2009, 40, 285–291. [Google Scholar] [CrossRef]
- Krishnaveni, V.; Mallavarapu, M. Identification of Electrode Respiring, Hydrocarbonoclastic Bacterial Strain Stenotrophomonas maltophilia MK2 Highlights the Untapped Potential for Environmental Bioremediation. Front. Microbiol. 2016, 7, 1965. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Hao, Z.; Ma, Y. Complete genome sequence of the aromatic-hydrocarbon-degrading bacterium Achromobacter xylosoxidans DN002. Arch. Microbiol. 2020, 202, 2849–2853. [Google Scholar] [CrossRef]
- Hong, Y.H.; Ye, C.C.; Zhou, Q.Z.; Wu, X.Y.; Yuan, J.P.; Peng, J.; Deng, H.; Wang, J.H. Genome Sequencing Reveals the Potential of Achromobacter sp. HZ01 for Bioremediation. Front. Microbiol. 2017, 8, 1507. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; Van Der Lelie, D.; Dow, J.M. The Versatility and Adaptation of Bacteria from the Genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef]
- Hoque, M.N.; Mannan, A.B.A.; Hossian, A.; Faisal, G.M.; Hossain, M.A.; Sultana, M. Arsenotrophic Achromobacter aegrifaciens Strains Isolated from Arsenic Contaminated Tubewell Water and Soil Sources Shared Similar Genomic Potentials. BMC Microbiol. 2024, 24, 518. [Google Scholar] [CrossRef]
- Sharma, A.; Sinharoy, S.; Bisht, N.C. The Mysterious Non-Arbuscular Mycorrhizal Status of Brassicaceae Species. Microorganisms 2023, 11, 123–134. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Guivernau, M.; Gallastegui, G.; Viñas, M.; de Hoog, G.S.; Elías, A. Fungal/Bacterial Interactions during the Biodegradation of TEX Hydrocarbons (Toluene, Ethylbenzene and p-Xylene) in Gas Biofilters Operated under Xerophilic Conditions. FEMS Microbiol. Ecol. 2012, 80, 722–734. [Google Scholar] [CrossRef] [PubMed]
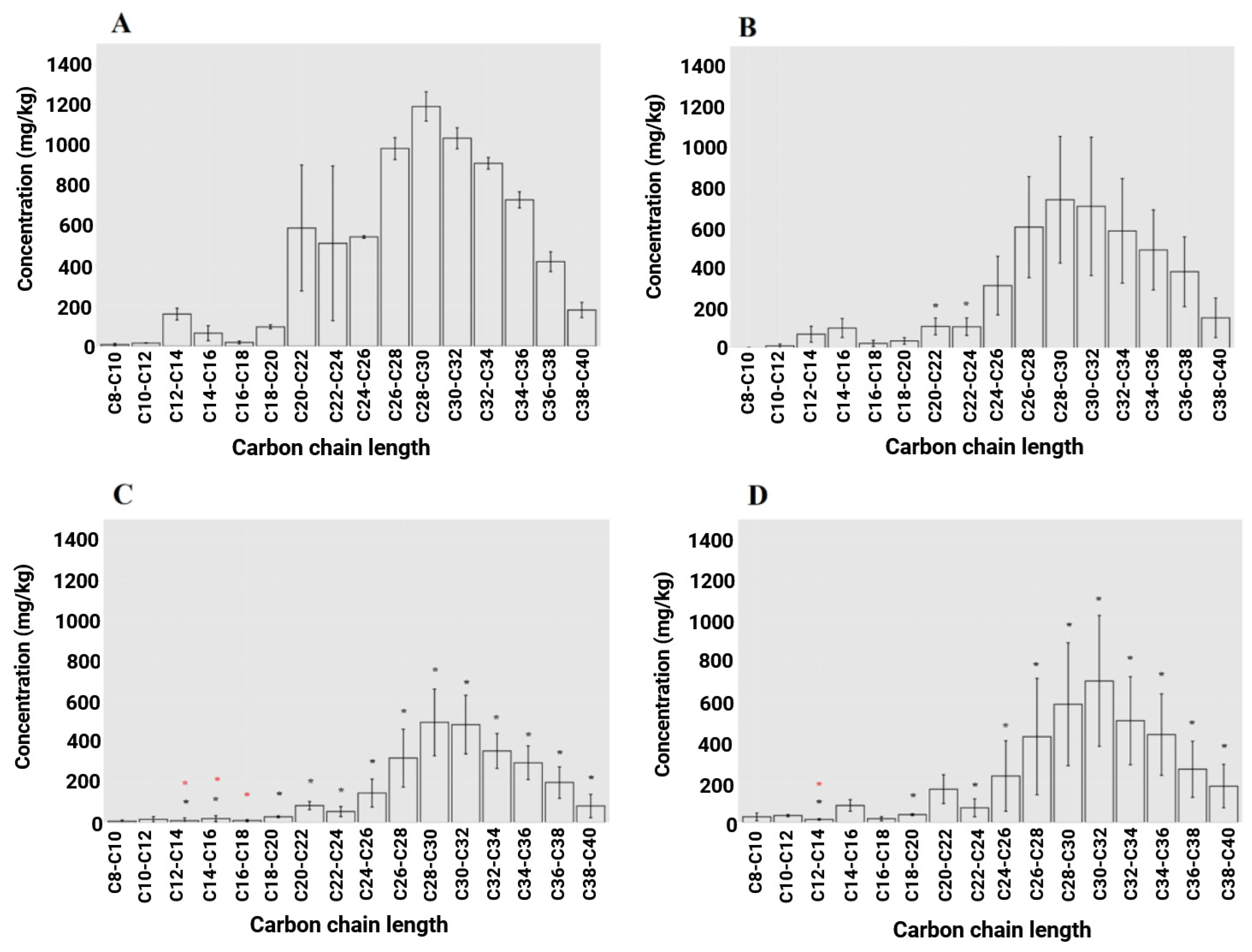

| C-Chain Length | Control | Mustard | Ryegrass (Abergain) | Ryegrass (Abergreen) | Ryegrass + White Clover | Chicory |
|---|---|---|---|---|---|---|
| c8–c10 | 64 ± 85 | 30 ± 102 | 0 ± 56 | 0 ± 90 | 0 ± 46 * | 0 ± 54 * |
| c10–c12 | 32 ± 81 | 56 ± 113 | 39 ± 54 | 28 ± 56 | 0 ± 49 | 0 ± 40 |
| c12–c14 | 57 ± 54 | 95 ± 158 * | 94 ± 74 * | 83 ± 88 | 85 ± 114 | 87 ± 56 |
| c14–c16 | 0 ± 47 | 75 ± 74 * | 17 ± 39 | 24 ± 39 | 18 ± 33 | 44 ± 25 |
| c16–c18 | 0 ± 55 | 52 ± 52 * | 19 ± 45 | 15 ± 57 | 0 ± 44 | 0 ± 32 |
| c18–c20 | 58 ± 40 | 72 ± 17 | 63 ± 12 | 57 ± 23 | 45 ± 27 * | 44 ± 34 |
| c20–c22 | 81 ± 37 | 86 ± 23 | 78 ± 24 | 72 ± 45 | 68 ± 32 * | 68 ± 17 * |
| c22–c24 | 85 ± 35 | 91 ± 45 | 89 ± 72 | 87 ± 55 | 81 ± 71 | 75 ± 33 |
| c24–c26 | 43 ± 45 | 74 ± 48 | 73 ± 95 | 63 ± 52 | 53 ± 74 | 46 ± 42 |
| c26–c28 | 39 ± 42 | 70 ± 46 | 62 ± 65 | 58 ± 42 | 39 ± 40 | 29 ± 15 |
| c28–c30 | 35 ± 45 | 66 ± 31 | 53 ± 51 | 51 ± 39 | 36 ± 26 | 25 ± 10 |
| c30–c32 | 27 ± 49 | 58 ± 26 | 43 ± 48 | 44 ± 42 | 11 ± 21 | 8 ± 7 |
| c32–c34 | 32 ± 44 | 63 ± 23 | 49 ± 43 | 52 ± 51 | 15 ± 19 | 1 ± 10 |
| c34–c36 | 32 ± 40 | 61 ± 28 | 49 ± 44 | 54 ± 51 | 18 ± 23 | 9 ± 14 |
| c36–c38 | 14 ± 45 | 57 ± 43 | 42 ± 51 | 45 ± 61 | 0 ± 29 | 0 ± 20 |
| c38–c40 | 4 ± 66 | 58 ± 72 | 28 ± 76 | 38 ± 97 | 0 ± 43 | 0 ± 47 |
| Treatment | Shoots Pb (mg/kg) | Roots Pb (mg/kg) | Total Plant Pb (mg/kg) | Soil Pb (mg/kg) | BCF | TF |
| M | 0.14 ± 0.11 | 0.42 ± 0.25 | 0.28 ± 0.18 | 4.4 ± 0.46 | 0.07 ± 0.04 | 0.35 ± 0.16 |
| RG1 | 0.26 ± 0.12 | 0.6 ± 0.23 | 0.43 ± 0.16 | 5.0 ± 0.24 | 0.08 ± 0.02 | 0.47 ± 0.21 |
| RG2 | 0.31 ± 0.02 | 0.94 ± 0.39 | 0.69 ± 0.21 | 4.5 ± 0.74 | 0.16 ± 0.04 | 0.41 ± 0.08 |
| RGWC | 0.41 ± 0.03 | 0.92 ± 0.36 | 0.66 ± 0.19 | 4.45 ± 0.30 | 0.15 ± 0.05 | 0.51 ± 0.17 |
| Ch | 0.33 ± 0.03 | 0.89 ± 0.50 | 0.61 ± 0.26 | 4.65 ± 1.33 | 0.13 ± 0.02 | 0.45 ± 0.16 |
| Treatment | Shoots Cu (mg/kg) | Roots Cu (mg/kg) | Total Plant Cu (mg/kg) | Soil Cu (mg/kg) | BCF | TF |
| M | 0.34 ± 0.09 | 0.36 ± 0.15 | 0.35 ± 0.07 | 0.11 ± 0.02 | 3.34 ± 1.05 | 1.27 ± 0.97 |
| RG1 | 0.38 ± 0.13 | 0.5 ± 0.08 | 0.44 ± 0.03 | 0.18 ± 0.01 | 2.47 ± 0.32 | 0.83 ± 0.48 |
| RG2 | 0.36 ± 0.16 | 0.71 ± 0.32 | 0.55 ± 0.18 | 0.14 ± 0.05 | 4.62 ± 1.81 | 0.46 ± 0.27 |
| RGWC | 0.3 ± 0.13 | 0.82 ± 0.42 | 0.56 ± 0.23 | 0.23 ± 0.07 | 2.64 ± 1.47 | 0.45 ± 0.28 |
| Ch | 0.23 ± 0.03 | 0.2 ± 0.02 | 0.21 ± 0.02 | 0.3 ± 0.08 | 0.80 ± 0.27 | 1.2 ± 0.24 |
| Treatment | Shoots Zn (mg/kg) | Roots Zn (mg/kg) | Total Plant Zn (mg/kg) | Soil Zn (mg/kg) | BCF | TF |
| M | 6.57 ± 1.25 | 3.96 ± 0.62 | 5.26 ± 0.91 | 8.94 ± 1.28 | 0.60 ± 0.15 | 1.66 ± 0.13 |
| RG1 | 3.92 ± 0.56 | 4.95 ± 0.43 | 4.44 ± 0.24 | 8.77 ± 0.28 | 0.51 ± 0.03 | 0.80 ± 0.18 |
| RG2 | 3.17 ± 0.31 | 5.29 ± 0.86 | 4.18 ± 0.45 | 7.9 ± 0.89 | 0.54 ± 0.07 | 0.53 ± 0.09 |
| RGWC | 2.97 ± 0.46 | 5.55 ± 0.90 | 4.26 ± 0.44 | 7.95 ± 0.89 | 0.54 ± 0.10 | 0.55 ± 0.13 |
| Ch | 3.29 ± 0.33 | 3.69 ± 0.63 | 3.49 ± 0.38 | 8.03 ± 0.84 | 0.43 ± 0.03 | 0.91 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conlon, R.; Dowling, D.N.; Germaine, K.J. Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil. Microorganisms 2025, 13, 848. https://doi.org/10.3390/microorganisms13040848
Conlon R, Dowling DN, Germaine KJ. Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil. Microorganisms. 2025; 13(4):848. https://doi.org/10.3390/microorganisms13040848
Chicago/Turabian StyleConlon, Robert, David N. Dowling, and Kieran J. Germaine. 2025. "Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil" Microorganisms 13, no. 4: 848. https://doi.org/10.3390/microorganisms13040848
APA StyleConlon, R., Dowling, D. N., & Germaine, K. J. (2025). Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil. Microorganisms, 13(4), 848. https://doi.org/10.3390/microorganisms13040848






