Abstract
Amebiasis is a globally prevalent infection that can lead to fatal outcomes if not adequately treated. Conventional treatment with imidazoles often fails due to side effects and resistance, emphasizing the need for alternative therapies. The probiotic Escherichia coli Nissle 1917 (EcN) has shown potential in combating intestinal pathogens. This study aimed to evaluate the amebicidal activity of EcN in vitro and its effect on the production of reactive oxygen species (ROS). Trophozoites of Entamoeba histolytica (2.5 × 10⁴ cells/mL) were cultured in 96-well plates and exposed to varying concentrations of EcN (102–109 cells/mL). Plates were incubated at 36 °C for 6, 12, and 18 h, after which trophozoite viability was assessed. Intracellular ROS production, including superoxide and hydrogen peroxide, was measured using fluorescent probes. The highest efficacy was observed after 18 h at a CFU concentration of 109 cells/mL. Increased ROS production at all probiotic concentrations suggested a role in EcN’s amebicidal mechanism. Morphological changes in trophozoites, such as rounding, vacuolization, and size reduction, were noted after EcN exposure, indicating growth inhibition. These findings suggest EcN induces structural and morphological changes in E. histolytica, inhibiting its growth in vitro. The findings suggest the potential efficacy of EcN; however, definitive confirmation requires data from human clinical trials.
1. Introduction
Entamoeba histolytica is an anaerobic eukaryotic protozoan and the etiological agent of amebiasis. The infection is globally distributed, with a higher prevalence in developing countries, particularly in regions with inadequate water treatment. There is a notable incidence in areas where contaminated water containing cysts, the infective form of the parasite, is used for vegetable cultivation [1,2].
Infection with E. histolytica can present as symptomatic or asymptomatic [3]. The primary site of infection is the intestine; however, the amoeba can disseminate to other organs, resulting in extraintestinal amebiasis, with the liver being the most common site. In developing countries, diarrhea remains the third leading cause of mortality in children under five years of age [4], and amebiasis is listed among the top 15 causes of mortality [5].
An estimated 50 million cases of invasive E. histolytica infection occur annually, resulting in approximately 100,000 deaths. The prevalence of infection exhibits global variation, ranging from 10% to 50% across different regions worldwide [6,7]. However, it is believed that this percentage may be underestimated, as asymptomatic infections can be caused by both E. histolytica and E. dispar, which are morphologically indistinguishable.
The treatment of choice for amebiasis is metronidazole [8]. However, in up to 50% of patients, this medication alone fails to eradicate intestinal colonization, leaving affected individuals at a substantial risk of relapse months later. This peculiarity underscores the necessity for research into alternative therapeutic options that, in addition to ensuring safety, demonstrate effective activity while minimizing adverse effects and resistance. In this context, probiotics emerge as promising alternatives for either curative or preventive treatment [9,10,11].
A well-known probiotic is Escherichia coli Nissle 1917 (EcN) [12,13,14]. EcN is a Gram-negative enterobacterium that can colonize the intestine within a few days and persist as a colonic microbiota for months after administration [15,16]. This interaction of EcN with the microbiota promotes direct antagonistic effects, such as the inhibition of the growth and death of pathogenic bacteria and yeasts. Indirect antagonistic effects involve the inhibition of the invasion of intestinal epithelial cells by invasive pathogens and include signaling with the intestinal mucosa [17]. EcN inhibited the growth of 21 bacterial isolates, including 40% for Pseudomonas, 50% for E. coli, Enterococcus, and Staphylococcus, and 100% for Klebsiella and Enterobacter [18]. EcN has also been shown to effectively inhibit the adhesion of the adherent-invasive E. coli isolated from Crohn’s disease patients, supporting its use as an adjunctive treatment for these patients [19]. There is limited research evaluating the effects of EcN in parasitic infections. The therapeutic potential of this probiotic in amebiasis has not yet been assessed. Therefore, the objective of our study was to expand research on probiotics for the treatment of amebiasis by assessing the amebicidal activity of EcN in vitro and its effect on the production of reactive oxygen species.
2. Materials and Methods
2.1. E. histolytica Strain
The axenic strain EGG of E. histolytica used in this study was isolated in our laboratory from a patient residing in Manaus, Amazonas, who presented with dysentery. The patient’s diagnosis was confirmed through serology, zymodeme analysis, and PCR, all of which were positive for E. histolytica [20].
The strain was maintained in axenic culture and grown in TYI-S-33 medium [21]. Inoculations were performed every 72–96 h, and the tubes were stored with an inclination of 30 degrees in a bacteriological oven under temperature of 37 °C.
2.2. Escherichia coli Nissle 1917
The probiotic EcN (Mutaflor; Ardeypharm, Herdecke, Germany) was acquired as a pharmaceutical product available in Canada. EcN was isolated and maintained in brain-heart infusion broth under aerobic conditions for 24 h at 37 °C. Subculturing was performed every 24 h to ensure its use during the exponential growth phase.
EcN was acclimated in TYI-S-33 medium prior to association with E. histolytica to ensure its viability during the assays. Colony-forming units (CFUs) were determined through serial dilution in TYI-S-33 medium to achieve concentrations ranging from 102 to 109 cells/mL. Culture viability, colony counts, and purity were assessed via Gram staining for quality control.
2.3. In Vitro Association
Initially, 40,000 E. histolytica trophozoites were distributed into a 96-well plate. The plate was then incubated at 37 °C for 1 h to ensure adherence of the trophozoites to the plate surface. After the incubation, the supernatant was discarded. To the wells containing E. histolytica, 270 µL of TYI-S-33 medium and 30 µL of E. coli Nissle (EcN) were added in serial dilutions, achieving concentrations ranging from 102 to 109 cells/mL. The plate was subsequently incubated with the probiotic concentrations at 37 °C for 6, 12, and 18 h. Following each incubation period, the viability of the amoebae was assessed by counting using a hemocytometer and trypan blue exclusion.
The wells containing only E. histolytica, without the addition of EcN, were considered as controls, with 100% viable cells. The number of viable trophozoites for each probiotic concentration was quantified and compared to the control to calculate the percentage of inhibition at different association times and concentrations.
The final inhibition percentage for each treatment was calculated using the following formula adapted from Edington [22]: I (%) = [(CEhC − CEhT)/CEhC] × 100, where I represents the inhibition percentage; CEhC denotes the trophozoite growth of Entamoeba histolytica in the control; and CEhT indicates the trophozoite growth of Entamoeba histolytica in the treatment.
2.4. Morphometric Analysis of the Trophozoites
For the morphometric analysis of trophozoite size, the area of each trophozoite was measured, considering that live trophozoites could assume an amoeboid shape. Images of 15 viable trophozoites and 15 dead trophozoites were captured using a JVC TK-1270/RGB microcamera (Tokyo, Japan) under a 40× objective for digitization. The area occupied by each trophozoite was measured using QuPath software version 0.5.1 (https://qupath.github.io, accessed on 19 February 2025).
2.5. Determination of Reactive Oxygen Species
To assess intracellular reactive oxygen species (ROS) production, specifically superoxide radicals and hydrogen peroxide, intracellular fluorescent probes were employed. Following the treatments, the cells were washed with PBS (pH 7.2) and subsequently incubated with fluorescent probes for superoxide (dihydroethidium, DHE, 5 µM; Invitrogen, Waltham, MA, USA) and hydrogen peroxide (dihydrodichlorofluorescein diacetate, H2-DCF-DA, 5 µM; Invitrogen, USA) for 30 min. The DAPI probe was used for nuclear staining (for 5 min). All procedures were conducted in the dark, and cells were washed with PBS after staining. Post-incubation, the cells were fixed with 4% paraformaldehyde and subsequently examined using fluorescence microscopy for further analysis.
2.6. Fluorescence
Initially, round cover slips of 13 mm diameter were placed into each well of a 24-well plate. Subsequently, 200 µL of a solution containing 40,000 trophozoites of E. histolytica were distributed into each well. To ensure the adherence of the amoebae to the glass cover slips, the plate was incubated at 37 °C for 1 h. Following incubation, the supernatant was discarded. In wells containing E. histolytica, 270 µL of TYI-S-33 medium and 30 µL of probiotic bacteria, previously diluted to achieve successive dilutions ranging from 104 to 109 CFU, were added. The plate was incubated again at 37 °C for 6, 12, and 18 h. No antibiotics were employed during the co-cultivation experiment. Finally, the cover slips were removed from the plate and mounted on slides for imaging under a fluorescence microscope, with emission/excitation wavelengths set at 525/470 nm and 629/572 nm for H2-DCF-DA and DHE probes, respectively.
After the incubation period, the plates were examined using an inverted microscope to assess cell growth, viability, and adherence. Images were captured with a 20× objective on a fluorescence microscope (Axiovision 3.1, Zeiss, Hallbergmoos, Germany). Immunoreactive cells were identified by their green cytoplasm and blue nuclei. Photographs of fields containing 5 to 10 cells were taken for each experimental treatment across three fluorescent channels—blue, green, and red.
2.7. Statistical Analysis
In the in vitro assay, a two-way ANOVA was conducted to compare the effects of different E. coli Nissle concentrations across different time points (6 h, 12 h, and 18 h). To identify the specific differences in viability at each time point, Tukey’s post-hoc test for multiple comparisons was employed (p < 0.05). Data were processed using the statistical software package GraphPad Prism, version 9.0.
3. Results
3.1. Effect of Probiotic EcN on E. histolytica Viability
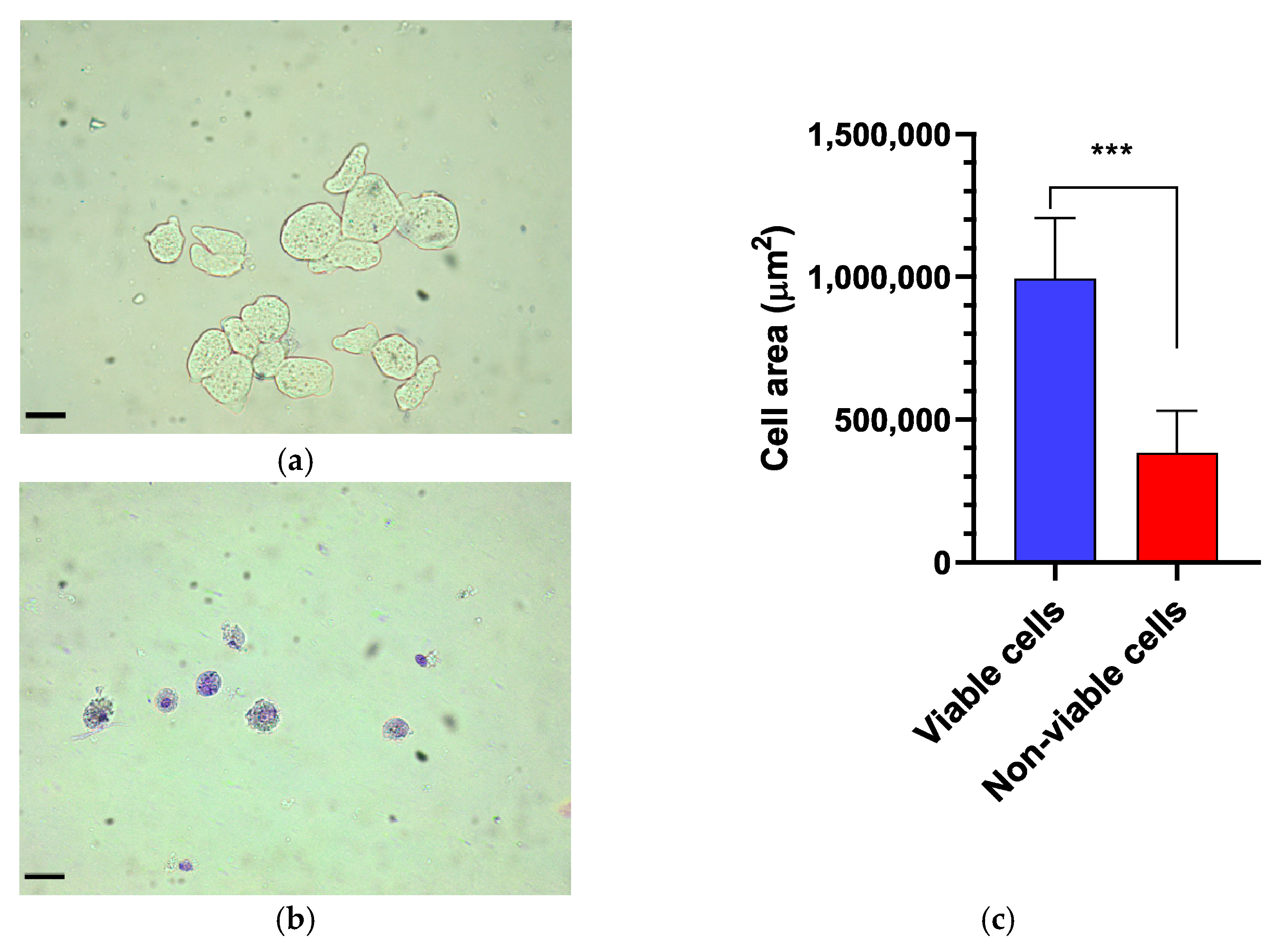
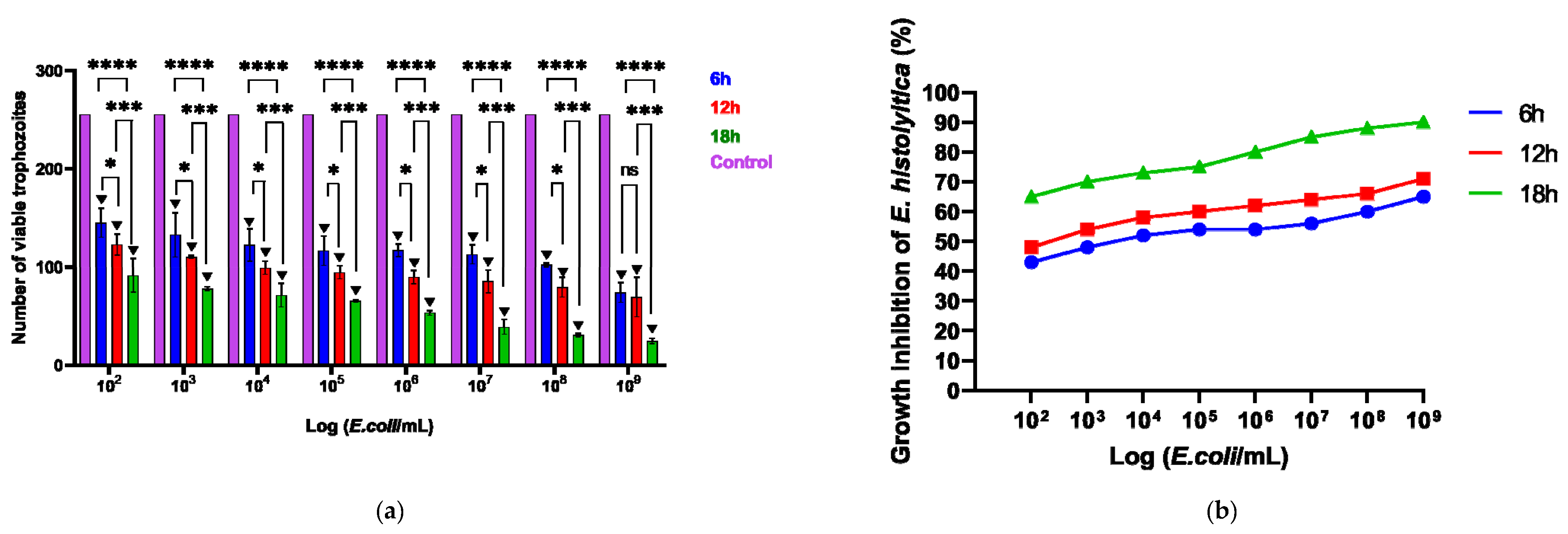
The viability of E. histolytica following association with EcN was assessed by counting viable trophozoites using a hemocytometer at intervals of 6, 12, and 18 h. Following exposure to the probiotic, the dead or distressed trophozoites, observed under an optical microscope, appeared stained with trypan blue and generally had a rounded shape (Figure 1a,b). Additionally, they were smaller in size compared to viable trophozoites, as confirmed by measuring the area occupied by each trophozoite (Figure 1c). The number of trophozoites was quantified and graphically represented in relation to the variation in EcN concentration (Figure 2a). Inhibition of E. histolytica growth was observed at all incubation times and across all probiotic concentrations. This inhibitory effect was most evident after 18 h of association at a CFU of 109 cells/mL (Figure 2b).
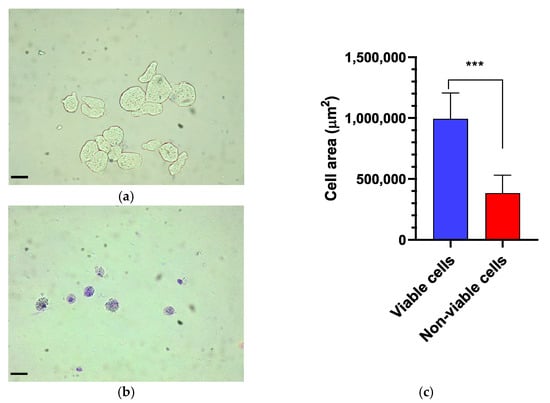
Figure 1.
Morphological assessment of Entamoeba histolytica trophozoites. (a) Control. Viable trophozoites exhibit a larger size and remain unstained by trypan blue. (b) In contrast, non-viable trophozoites display a rounded shape, increased cytoplasmic granularity, and intense staining with trypan blue. (c) Comparison of the trophozoite cell area between viable and non-viable cells, with a significant difference (p < 0.0007). Bar = 70 µm. *** p < 0.001.
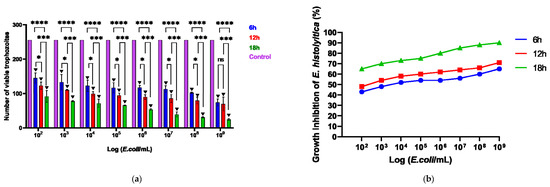
Figure 2.
(a) Number of viable cells following the association of the parasite E. histolytica with the probiotic E. coli Nissle, subjected to different concentrations (102–109) across various time intervals, in a 96-well plate. (b) Impact of probiotics on the inhibition of E. histolytica trophozoites over different time periods and at varying concentrations. * p < 0.05; *** p < 0.01; **** p < 0.001; ▼ p < 0.001 Significant difference when compared to the control group.
3.2. ROS Production
The production of superoxide and hydrogen peroxide was evaluated in the co-culture of EcN and E. histolytica. We focused on the analysis of ROS production at 6 h and 18 h. These were the two extremes of our experimental design and showed significant differences in the number of trophozoites between them.
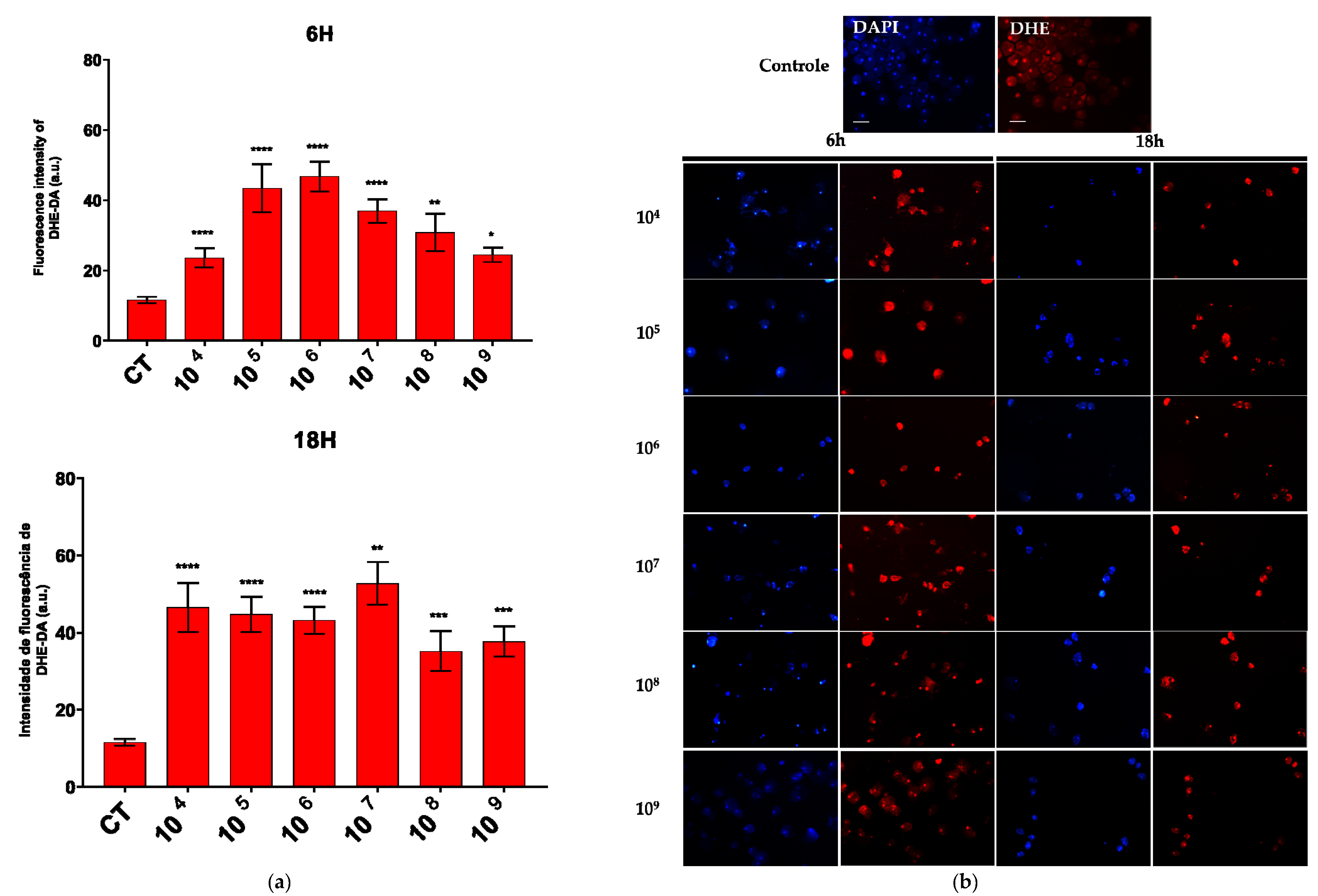
An increase in the production of both compounds was observed across all the associated time points and probiotic concentrations. Notably, a marked enhancement in superoxide production was detected at a concentration of 106 cells/mL after six hours of association and at 107 cells/mL after eighteen hours (Figure 3a).
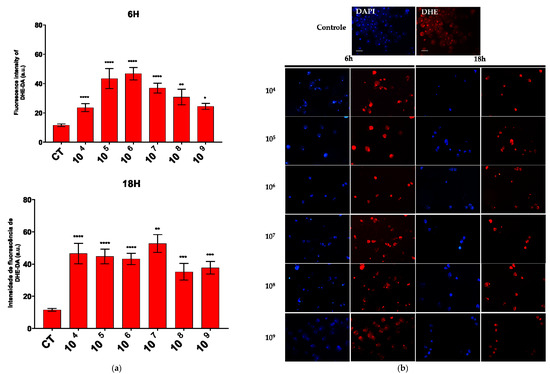
Figure 3.
ROS production evaluated by DHE and DCF fluorescence. (a) Quantification of DHE–DA fluorescence intensity in each group. (b) Fluorescence microscopic images of intracellular production of DHE–DA staining in E. histolytica after interaction with the probiotic E. coli Nissle at different concentrations over 6 h and 18 h. Bar = 50 µm. * p < 0.05; ** p < 0.002; *** p < 0.0005; **** p < 0.0001.
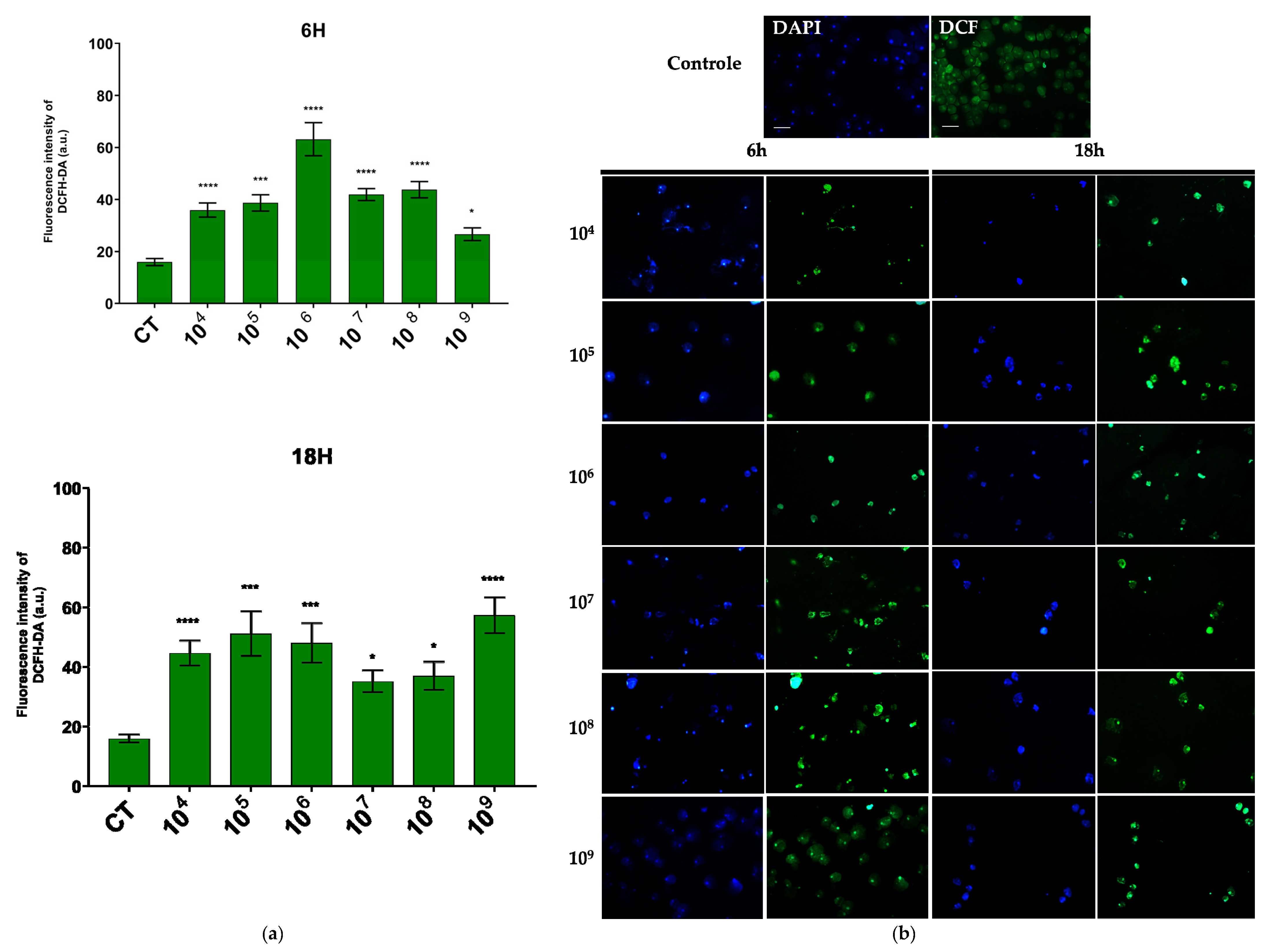
Regarding hydrogen peroxide, the concentration that showed the most pronounced reaction was also 106 cells/mL over a 6-h period, while in the 18-h period, the concentration of 109 cells/mL was most effective (Figure 4a).
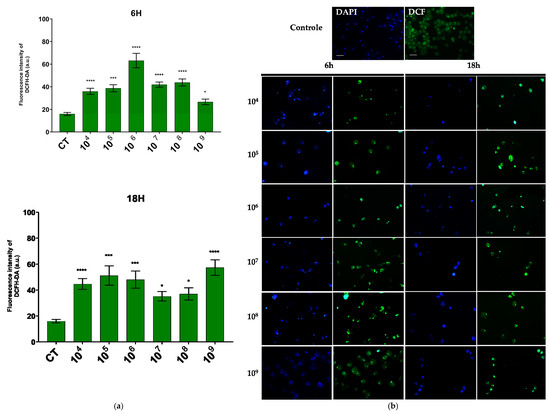
Figure 4.
ROS production evaluated by DCFH–DA fluorescence. (a) Quantification of DCFH–DA fluorescence intensity in each group. (b) Fluorescence microscopic images of intracellular production of DCFH–DA staining in E. histolytica after interaction with the probiotic E. coli Nissle at different concentrations over 6 h and 18 h. Bar = 50 µm. * p < 0.004; *** p < 0.0003; **** p < 0.0001.
In the fluorescence analysis, a clear morphological alteration of the trophozoites was observed following their interaction with the probiotic. The cells acquired a more rounded shape and exhibited vacuolation, accompanied by a reduction in size. Additionally, there was an apparent loss of the intracellular ameboid medium (Figure 3b and Figure 4b).
4. Discussion
The treatment of choice for amebiasis involves adverse effects that often lead to the discontinuation of therapy. Additionally, the suspicion of drug resistance signals the importance of alternative therapies in resolving the infection. Probiotics have emerged as potentially attractive options. However, the use of probiotics has been timidly evaluated in amebiasis. The combination of Saccharomyces boulardii and metronidazole for the treatment of amebiasis has been reported to reduce the duration of clinical symptoms and cyst excretion [23]. Furthermore, S. boulardii has demonstrated effectiveness in inhibiting the adherence of amoebae to the intestinal mucosal surface [24]. Lactobacillus helveticus has been identified as a potential probiotic for amebiasis [25,26], while Lactobacillus casei and Enterococcus faecium have demonstrated in vitro activity against E. invadens [27]. However, the precise mechanism of action of these probiotics remains incompletely understood. Our research group assessed the efficacy of the lactic acid bacterium Weissella paramesenteroides WpK in resolving the lesions induced by E. dispar in murine models. The bacterium facilitated the recovery of necrotic regions by enhancing intestinal mucosal protection through the upregulation of MUC-2 and epithelial regeneration [28].
EcN is a well-established probiotic with recognized benefits for intestinal homeostasis [29,30,31]. In this context, we investigated the potential of EcN to contribute to the control of E. histolytica infection. Our results demonstrated a reduction in the proliferation of E. histolytica trophozoites in the presence of EcN, suggesting an anti-amebic activity of the probiotic. This activity was time-dependent, with maximum efficacy observed at 18 h, resulting in over 80% inhibition of E. histolytica trophozoite growth. These findings support the therapeutic potential of EcN in the treatment of amebiasis.
In addition to the relationship between exposure time and the ability of EcN to inhibit the growth of E. histolytica, the quantity of probiotic cells is also crucial for the effectiveness of its activity. Inhibition of E. invadens growth was observed at a concentration of 108 CFU/mL, with E. faecium demonstrating approximately 71% inhibition of the parasite’s growth, and L. casei showing approximately 50% inhibition [27].
In this study, the most effective treatment was observed at a concentration of 109 CFU/mL of EcN. The efficacy of a probiotic in vivo depends on the specific strain, but generally, the effective dosage for inducing favorable changes in the intestinal microbiota ranges from 108 to 109 CFU/mL [32]. Although this study was conducted in vitro, the findings indicate that probiotic concentration plays a crucial role in the observed effects, aligning with dosages recommended in the in vivo studies. In vitro models are valuable tools for investigating initial mechanisms and providing insights for future in vivo research. Notably, all the concentrations of EcN tested resulted in a reduction in E. histolytica proliferation, reinforcing its potential therapeutic application in amoebiasis.
When associating EcN with E. histolytica, a clear reduction in the proliferation of the amoebas was observed. Anaerobic microorganisms possess inherently weak antioxidant defenses against oxidative stress. In E. histolytica, ROS act as significant cytotoxic effectors, causing protein oxidation which generally leads to the inhibition of protein synthesis [33]. Probiotics may serve as effective allies in controlling the proliferation of pathogenic microorganisms through the production of ROS.
In the association between EcN and E. histolytica, the production of hydrogen peroxide and superoxide was observed. The generation of free radicals is a common process during interactions between microorganisms. The probiotic effect of Lactobacillus helveticus has been attributed to its capacity to produce hydrogen peroxide [25,34].
E. histolytica trophozoites exhibited a more rounded and vacuolated morphology, along with a reduction in size following association with EcN. Additionally, a loss of intracellular amebic content was observed. Collectively, these findings indicate that EcN acts as a causative agent of the morphological and structural alterations observed in the amebic cells.
Among the potential mechanisms of action identified for the microbicidal activity of EcN are the enhancement of transepithelial resistance [35], a beneficial effect on the formation and stabilization of epithelial tight junctions [36], the positive regulation of zona occludens-1 mRNA expression [37], the induction of human β-defensin 2, an inducible antimicrobial peptide synthesized by the epithelium to counteract bacterial adhesion and invasion [38], and a protective role against pathogenic E. coli strains that colonize the intestines of patients with inflammatory bowel disease [19]. For the first time, the possibility of probiotic activity of EcN via reactive oxygen species is suggested. The production of hydrogen peroxide and superoxide by EcN may represent a mechanism through which the probiotic exerts its amebicidal activity.
5. Conclusions
The results of this study provide good evidence that the probiotic EcN inhibits the growth of E. histolytica trophozoites. This inhibitory effect was accompanied by significant morphological changes in the trophozoites, including rounding, vacuolization, and reduction in size. A notable decrease in trophozoite numbers was observed in cultures exposed to EcN, with the most pronounced effect occurring at a concentration of 109 cells/mL and after an 18-h incubation period, suggesting a dose- and time-dependent relationship. Additionally, for the first time, this study identifies the production of hydrogen peroxide and superoxide as a novel mechanism of action for EcN, which likely contributes to its inhibitory effects. Together, these findings shed new light on the therapeutic potential of EcN in the treatment of amoebiasis and encourage further research to explore the underlying mechanisms involved.
Author Contributions
Conceptualization: M.A.G. and F.M.S.O. Data Curation: V.M.-O., F.M.S.O., L.S.A.C., F.S.M. and M.A.G. Formal Analysis: V.M.-O., F.M.S.O., F.S.M., L.S.A.C. and M.A.G. Investigation: V.M.-O., O.L.M.M., J.R.F., J.T., F.M.S.O. and L.S.A.C. Methodology: V.M.-O., O.L.M.M., J.R.F., J.T., R.E.S. and A.C.C.-S. Writing—Original Draft Preparation: V.M.-O. and M.A.G. Review and Editing: F.M.S.O., L.S.A.C., F.S.M. and M.A.G. Funding Acquisition: M.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), grant number: APQ-02829-18 and by FAPEMIG and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq—Brasil grant number BPQ-06575-24. VMO was funded by CNPq/Brazil.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barlaam, A.; Sannella, A.R.; Ferrari, N.; Temesgen, T.T.; Rinaldi, L.; Normanno, G.; Cacciò, S.M.; Robertson, L.J.; Giangaspero, A. Ready-to-eat salads and berry fruits purchased in Italy contaminated by Cryptosporidium spp., Giardia duodenalis, and Entamoeba histolytica. Int. J. Food Microbiol. 2022, 370, 109634. [Google Scholar] [CrossRef]
- Saleh, F.E.R.; Gad, M.A.; Ashour, A.A.; Soliman, M.I.; El-Senousy, W.M.; Al-Herrawy, A.Z. Molecular detection of Entamoeba histolytica in fresh vegetables and irrigation. Egypt. J. Aquat. Biol. Fish. 2019, 22, 551–561. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Singh, U. Diversity and Plasticity of Virulent Characteristics of Entamoeba histolytica. Trop. Med. Infect. Dis. 2023, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Shirley, D.-A.T.; Farr, L.; Watanabe, K.; Moonah, S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef]
- Nasrallah, J.; Akhoundi, M.; Haouchine, D.; Marteau, A.; Mantelet, S.; Wind, P.; Benamouzing, R.; Bouchaud, R.; Dhote, R.; Izri, A. Updates on the worldwide burden of amoebiasis: A case series and literature review. J. Infect. Public Health 2022, 15, 1134–1141. [Google Scholar] [CrossRef]
- Ximénez, C.; Morán, P.; Rojas, L.; Valadez, A.; Gómez, A. Reassessment of the epidemiology of amebiasis: State of the art. Infect. Genet. Evol. 2009, 9, 1023–1032. [Google Scholar] [CrossRef]
- Kumanan, T.; Sujanitha, V.; Sri Ranganathan, S. Metronidazole for Amoebiasis: A tale of more than half a century. Jaffna Med. J. 2021, 33, 6–13. [Google Scholar] [CrossRef]
- Van de Wijgert, J.H.H.M.; Verwijs, M.C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 287–299. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmed, S.; Tabassum, N.; Bhattacharya, R.; Bose, D. Role of probiotics in prevention and treatment of enteric infections: A comprehensive review. 3 Biotech 2021, 11, 242. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.C.; Regal, P.; Miranda, J.M. Probiotics as a possible strategy for the prevention and treatment of allergies. A narrative review. Foods 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, T.; Tang, H.; Feng, P.; Ali, G.; Liu, P.; Li, X. Genetically encoded probiotic EcN 1917 alleviates alcohol-induced acute liver injury and restore gut microbiota homeostasis. J. Funct. Foods 2021, 85, 104661. [Google Scholar] [CrossRef]
- Michael, H.; Miyazaki, A.; Langel, S.N.; Amimo, J.O.; Kick, M.K.; Chepngeno, J.; Paim, F.C.; Fischer, D.D.; Rajashekara, G.; Saif, L.J.; et al. Escherichia coli Nissle 1917 Enhances Efficacy of Oral Attenuated Human Rotavirus Vaccine in a Gnotobiotic Piglet Model. Vaccines 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hao, L.; Liu, X.; Borrás-Hidalgo, O.; Zhang, Y. Enhanced anti-proliferative efficacy of epothilone B loaded with Escherichia coli Nissle 1917 bacterial ghosts on the HeLa cells by mitochondrial pathway of apoptosis. Drug Dev. Ind. Pharm. 2018, 44, 1328–1335. [Google Scholar] [CrossRef]
- Blum, G.; Marre, R.; Hacker, J. Properties of Escherichia coli Strains of Serotype 06. Infection 1994, 23, 234–236. [Google Scholar] [CrossRef]
- Kruis, W. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U. Escherichia coli strain Nissle 1917—From bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016, 363, fnw212. [Google Scholar] [CrossRef]
- Storm, D.W.; Koff, S.A.; Horvath, D.J.; Li, B.; Justice, S.S. In Vitro Analysis of the Bactericidal Activity of Escherichia Coli Nissle 1917 Against Pediatric Uropathogens. J. Urol. 2011, 186, 1678–1683. [Google Scholar] [CrossRef]
- Boudeau, J.; Glasser, A.L.; Julien, S.; Colombel, J.F.; Darfeuille-Michaud, A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent–invasive E. coli strains isolated from patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 18, 45–56. [Google Scholar] [CrossRef]
- Gomes, M.A.; Melo, M.N.; Macedo, A.M.; Pena, G.P.M.; Caliari, M.V.; Silva, E.F. Characterization of Entamoeba histolytica and Entamoeba dispar by Biological, Biochemical, and Molecular Parameters. Arch. Med. Res. 2000, 31, S249–S250. [Google Scholar] [CrossRef]
- Clark, C.G.; Diamond, L.S. Methods for Cultivation of Luminal Parasitic Protists of Clinical Importance. Clin. Microbiol. Rev. 2002, 15, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Edgington, L.V. Fungitoxic Spectrum of Benzimidazole Compounds. Phytopathology 1971, 61, 42–44. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Dehbashi, N.; Yazdanparast, K.; Shafaghi, A. Efficacy of saccharomyces boulardii with antibiotics in acute amoebiasis. World J. Gastroenterol. 2003, 9, 1832–1833. [Google Scholar] [CrossRef] [PubMed]
- Rigothier, M.C.; Maccario, J.; Gayral, P. Inhibitory activity of saccharomyces yeasts on the adhesion ofEntamoeba histolytica trophozoites to human erythrocytes in vitro. Parasitol. Res. 1994, 80, 10–15. [Google Scholar] [CrossRef]
- Sarid, L.; Zanditenas, E.; Ye, J.; Trebicz-Geffen, M.; Ankri, S. Insights into the Mechanisms of Lactobacillus acidophilus Activity against Entamoeba histolytica by Using Thiol Redox Proteomics. Antioxidants 2022, 11, 814. [Google Scholar] [CrossRef]
- Varet, H.; Shaulov, Y.; Sismeiro, O.; Trebicz-Geffen, M.; Legendre, R.; Coppée, J.-Y.; Ankri, S.; Guillen, N. Enteric bacteria boost defences against oxidative stress in Entamoeba histolytica. Sci. Rep. 2018, 8, 9042. [Google Scholar] [CrossRef]
- Sarjapuram, N.; Mekala, N.; Singh, M.; Tatu, U. The Potential of Lactobacillus casei and Entercoccus faecium Combination as a Preventive Probiotic Against Entamoeba. Probiotics Antimicrob. Proteins 2017, 9, 142–149. [Google Scholar] [CrossRef]
- Prado, G.K.S.; Torrinha, K.C.; Cruz, R.E.; Gonçalves, A.B.B.; Silva, C.A.V.; Oliveira, F.M.S.; Nunes, A.C.; Gomes, M.A.; Caliari, M.V. Weissella paramesenteroides WpK4 ameliorate the experimental amoebic colitis by increasing the expression of MUC-2 and the intestinal epithelial regeneration. J. Appl. Microbiol. 2020, 129, 1706–1719. [Google Scholar] [CrossRef]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef]
- Martens, K.; Pugin, B.; De Boeck, I.; Spacova, I.; Steelant, B.; Seys, S.F.; Lebeer, S.; Hellings, P.W. Probiotics for the airways: Potential to improve epithelial and immune homeostasis. Allergy 2018, 73, 1954–1963. [Google Scholar] [CrossRef]
- Shini, S.; Bryden, W.L. Probiotics and gut health: Linking gut homeostasis and poultry productivity. Anim. Prod. Sci. 2021, 62, 1090–1112. [Google Scholar] [CrossRef]
- Bai, J.C.; Ciacci, C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J. Clin. Gastroenterol. 2017, 51, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Shahi, P.; Trebicz-Geffen, M.; Nagaraja, S.; Alterzon-Baumel, S.; Hertz, R.; Methling, K.; Lalk, M.; Ankri, S. Proteomic Identification of Oxidized Proteins in Entamoeba histolytica by Resin-Assisted Capture: Insights into the Role of Arginase in Resistance to Oxidative Stress. PLoS Neglected Trop. Dis. 2016, 10, e0004340. [Google Scholar] [CrossRef]
- Collins, E.B.; Aramaki, K. Production of Hydrogen Peroxide by Lactobacillus acidophilus. J. Dairy Sci. 1980, 63, 353–357. [Google Scholar] [CrossRef]
- Hering, N.A.; Richter, J.F.; Fromm, A.; Wieser, A.; Hartmann, S.; Günzel, D.; Bucker, R.; Fromm, M.; Schulzke, J.D.; Troeger, H. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014, 7, 369–378. [Google Scholar] [CrossRef]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKC? redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic Escherichia coli Nissle 1917 Inhibits Leaky Gut by Enhancing Mucosal Integrity. PLoS ONE 2007, 2, e1308. [Google Scholar] [CrossRef]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of Human β-Defensin 2 by the Probiotic Escherichia coli Nissle 1917 Is Mediated through Flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).