Gut Microbiome—Brain Crosstalk in the Early Life of Chicken Reveals the Circadian Regulation of Key Metabolic and Immune Signaling Processes
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Ethics Statement
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. RNA Isolation and Quantification
2.5. RNA Library Preparation and Transcriptome Profile Generation
2.6. Transcriptome Data Analysis
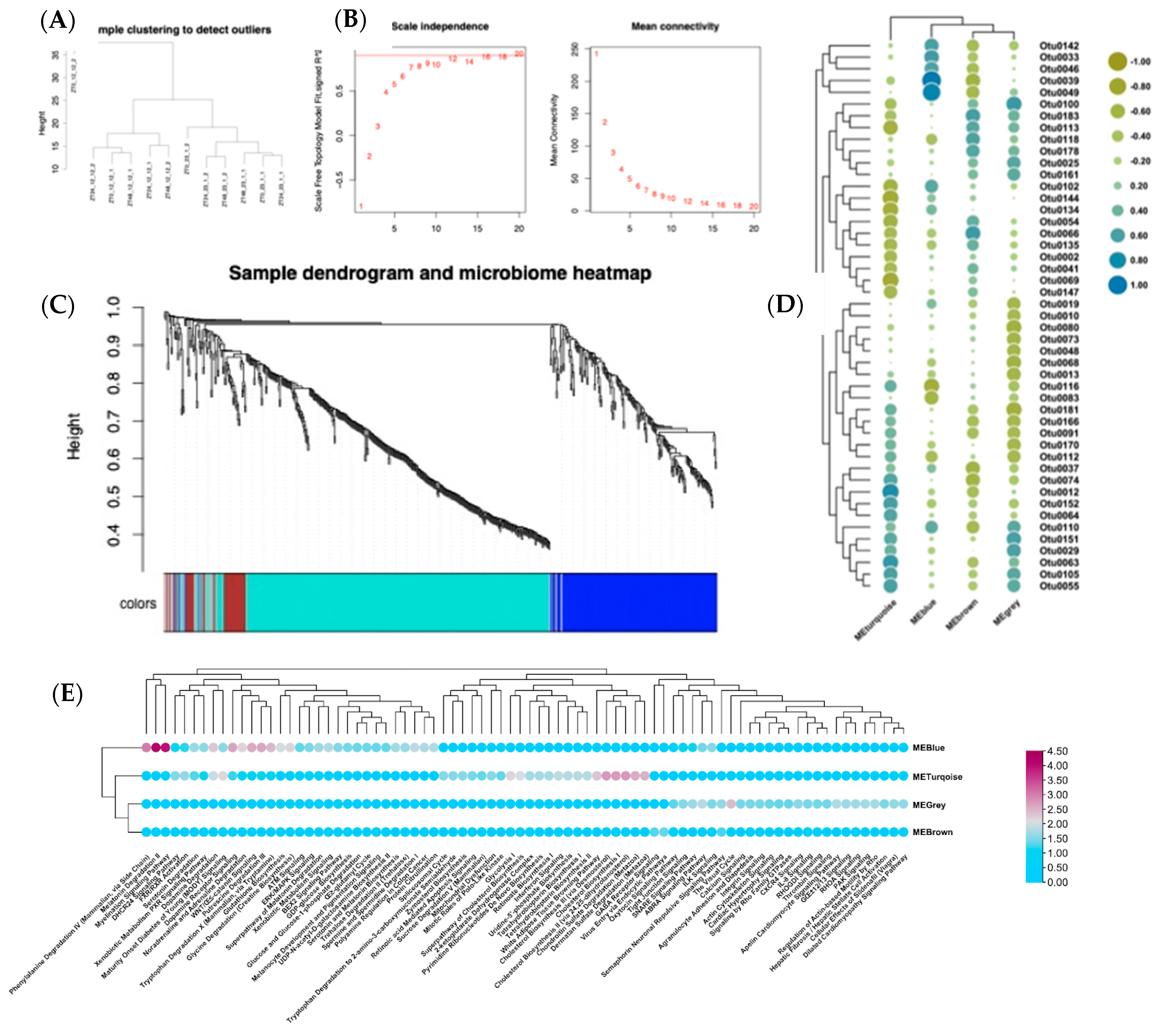
2.7. Microbiota Analysis
2.8. Microbiota Abundance—Gene Co-Expression Quantification
3. Results and Discussion
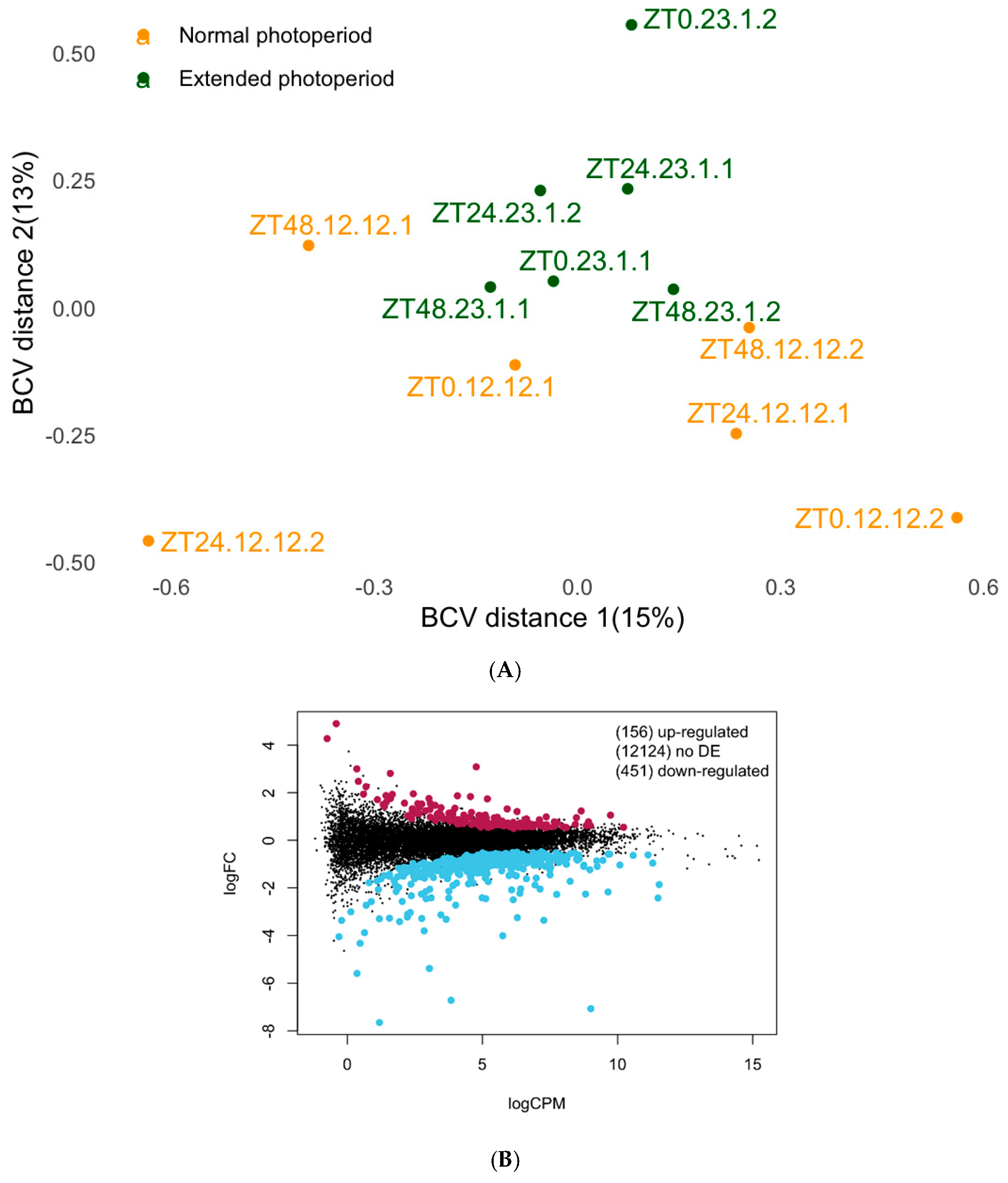
3.1. The Differential Expression Analysis of Brain Tissue
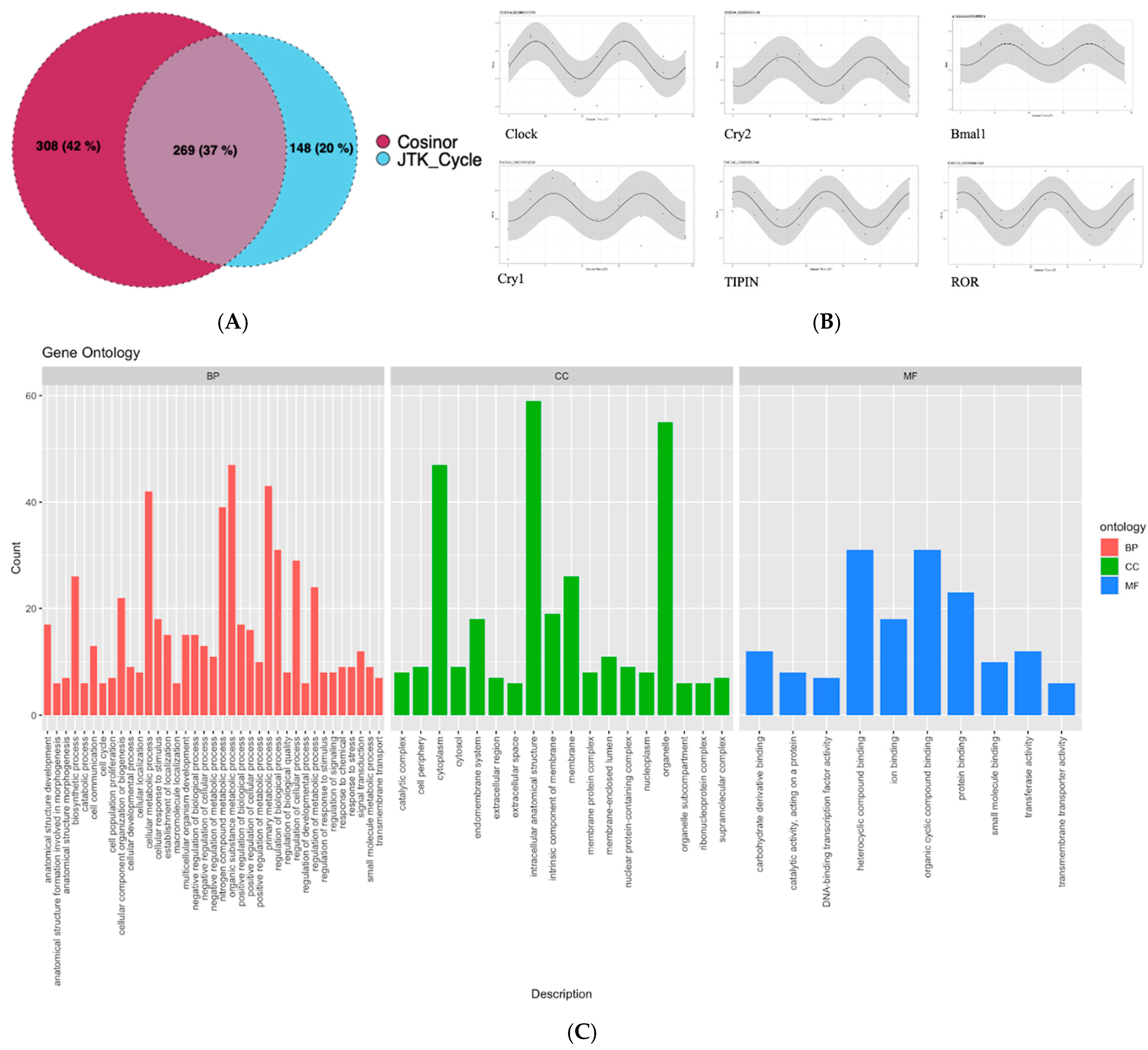
3.2. Genes Following Circadian Rhythm
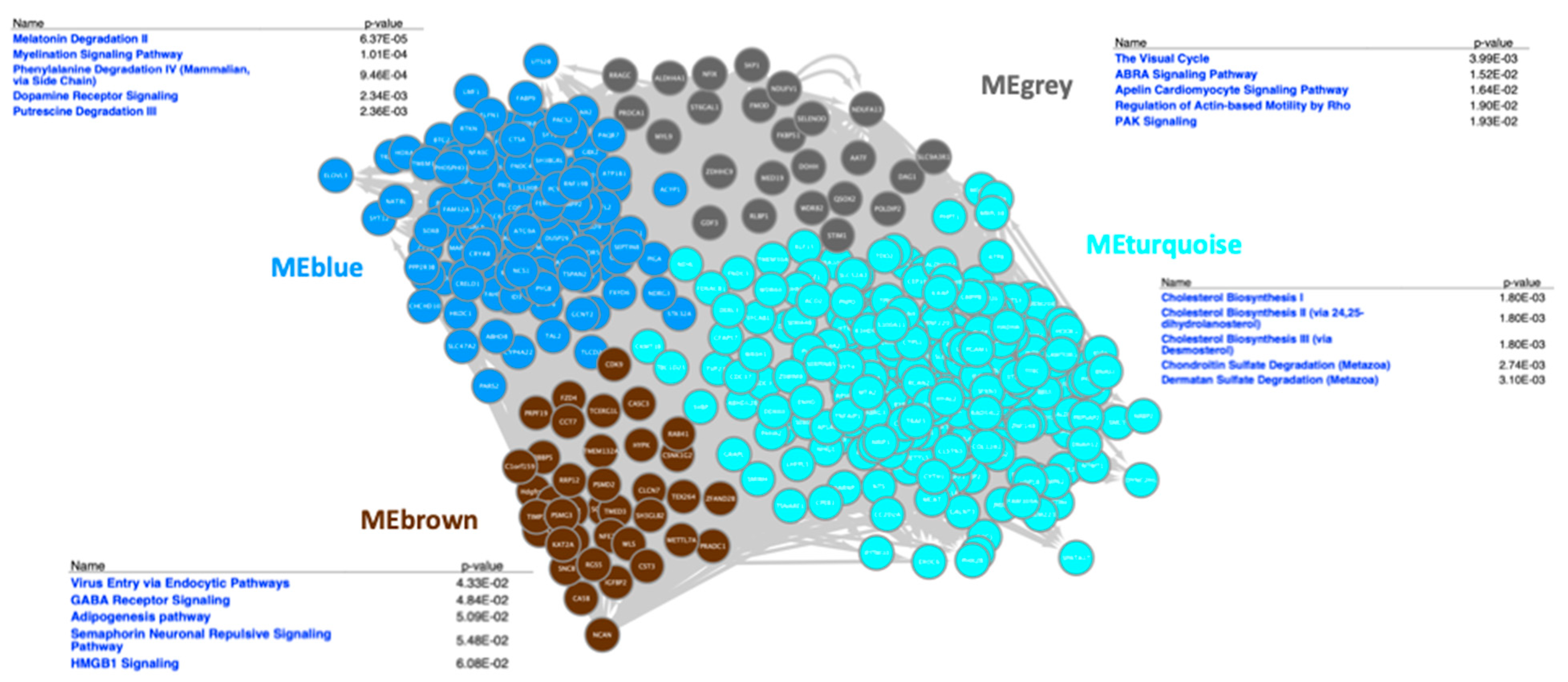
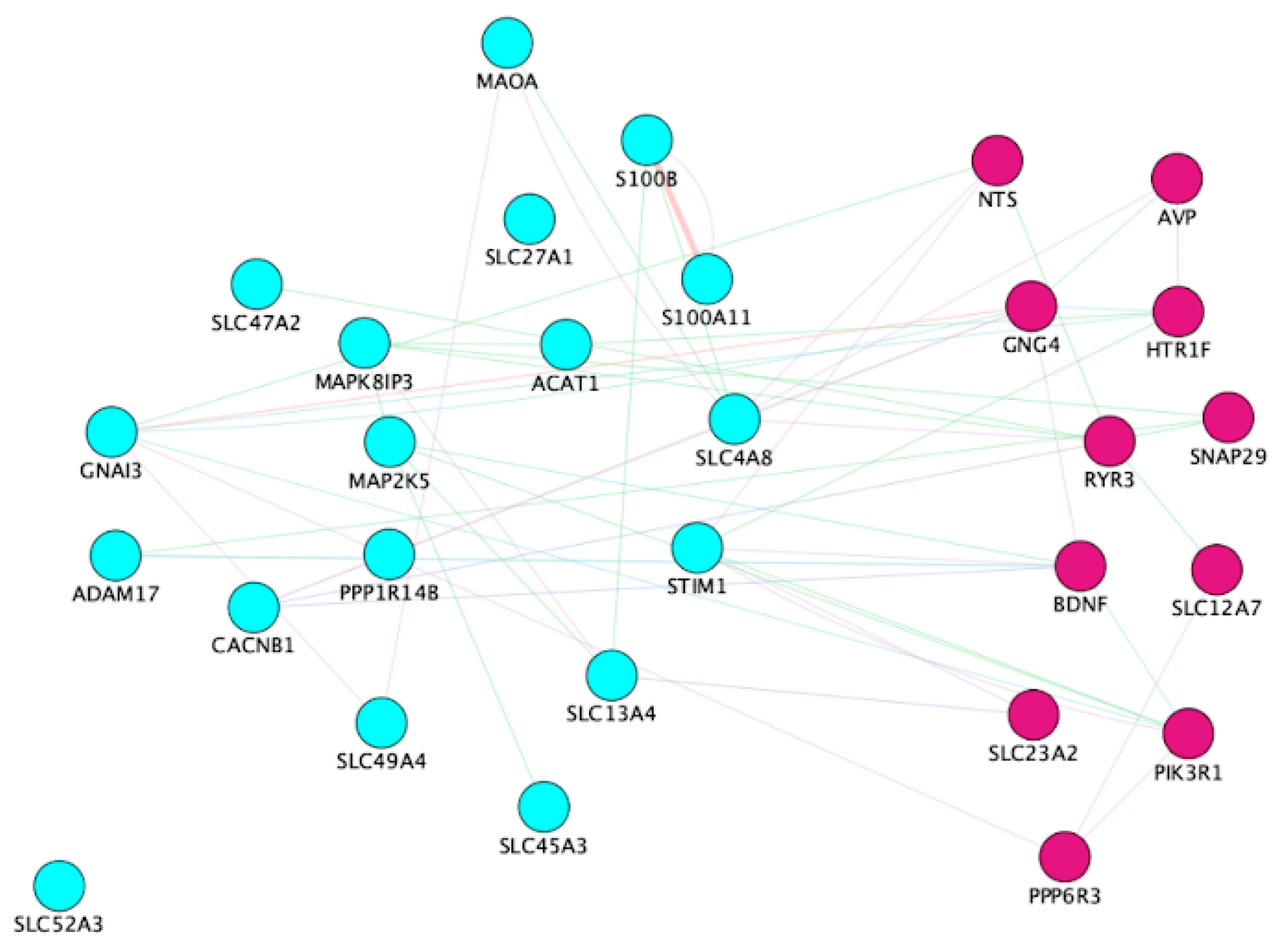
3.3. Correlation of Gene Expression and Gut Microbiota
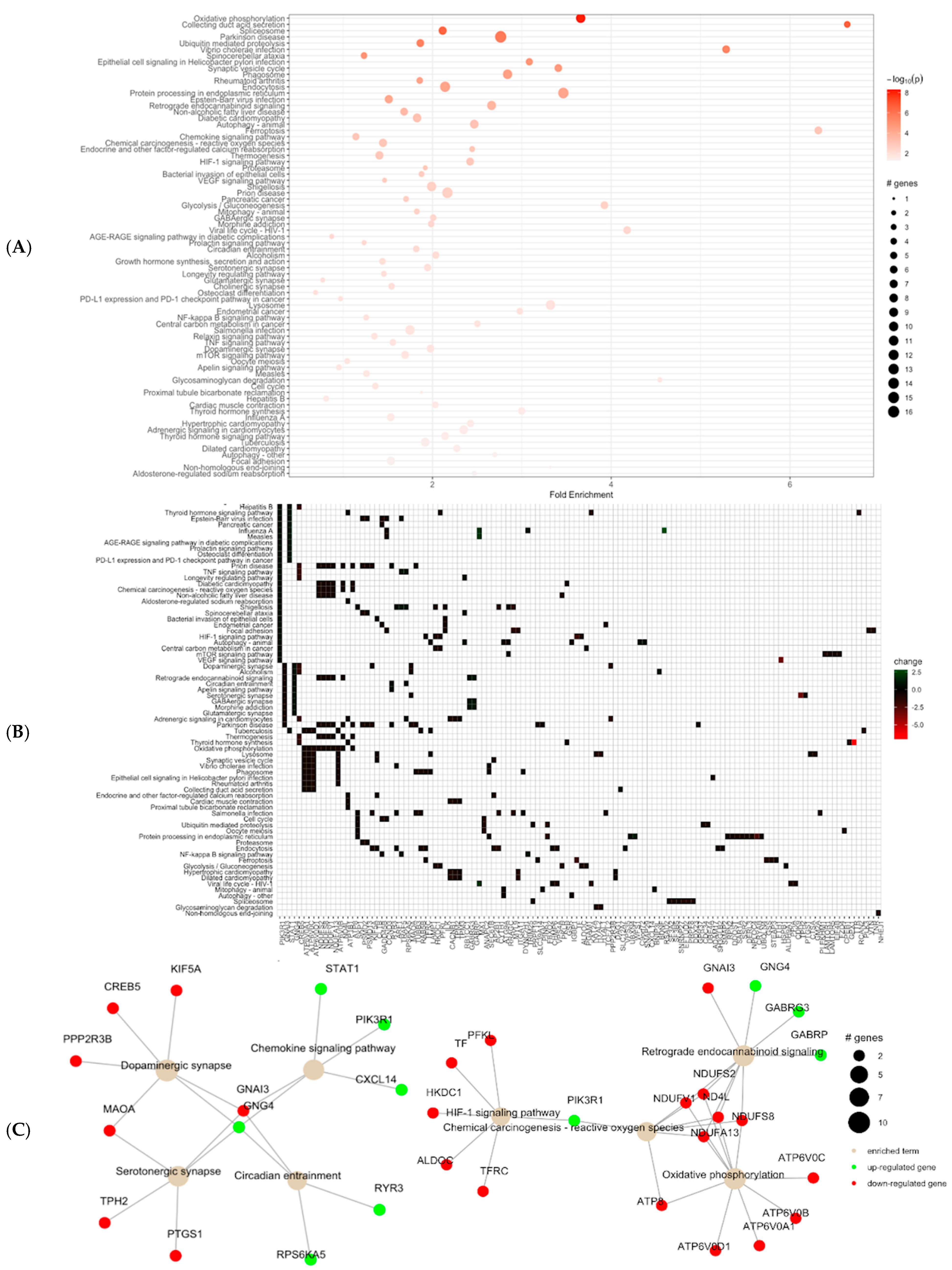
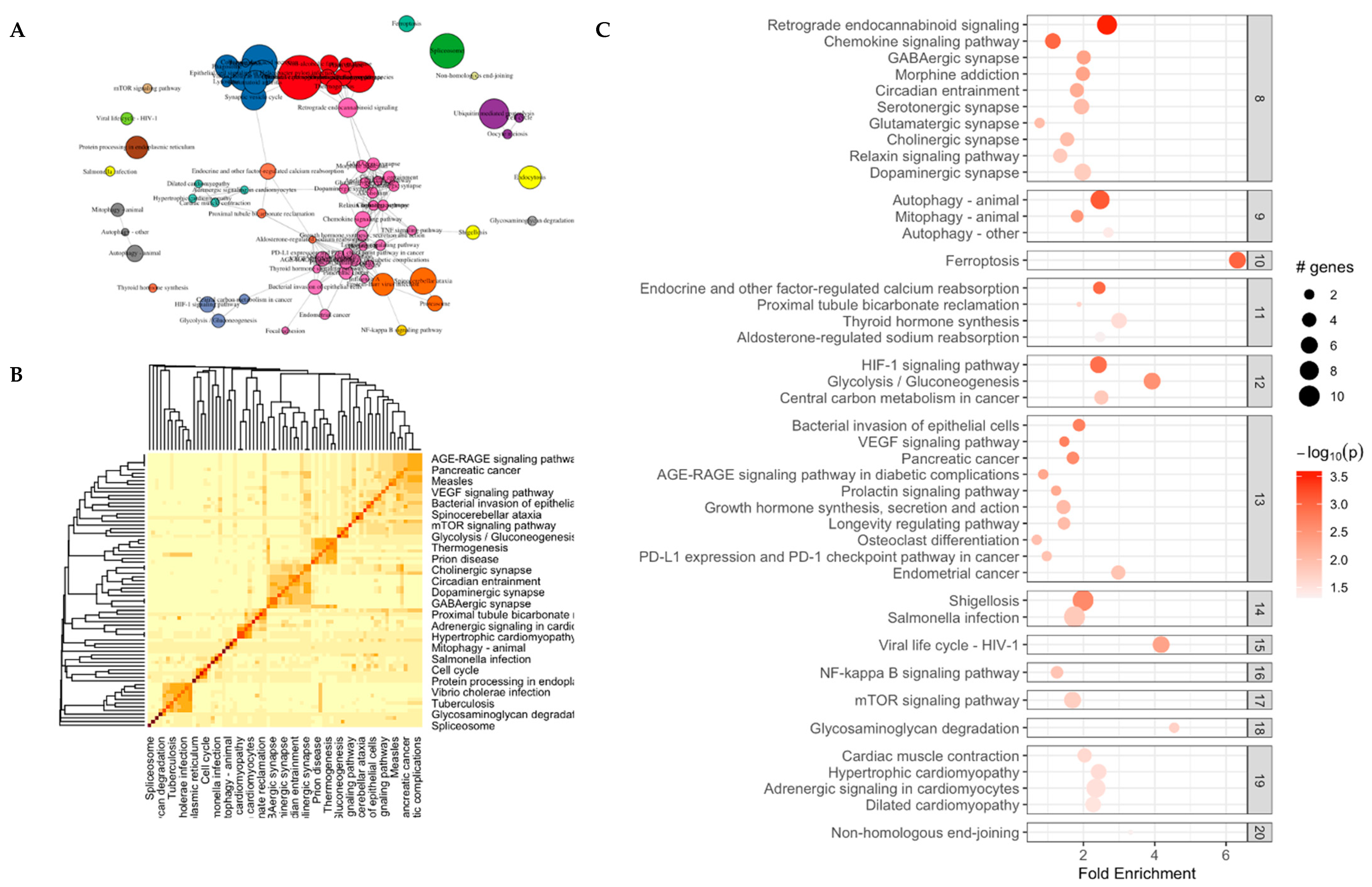
3.4. KEGG Pathway Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, S.; Ma, K. Circadian clock regulation of skeletal muscle growth and repair. F1000Research 2016, 5, 1549. [Google Scholar] [CrossRef]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian disruption and metabolic disease: Findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Ceglia, N.; Zeller, M.; Eckel-Mahan, K.; Sassone-Corsi, P.; Baldi, P. The pervasiveness and plasticity of circadian oscillations: The coupled circadian-oscillators framework. Bioinformatics 2015, 31, 3181–3188. [Google Scholar] [CrossRef]
- Tong, Q.; McGonnell, I.M.; Demmers, T.G.M.; Roulston, N.; Bergoug, H.; Romanini, C.E.; Verhelst, R.; Guinebretière, M.; Eterradossi, N.; Berckmans, D.; et al. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal 2018, 12, 765–773. [Google Scholar] [CrossRef]
- Bloch, G.; Barnes, B.M.; Gerkema, M.P.; Helm, B. Animal activity around the clock with no overt circadian rhythms: Patterns, mechanisms and adaptive value. Proc. Biol. Sci. 2013, 280, 20130019. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-C. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed. J. 2018, 41, 5–8. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- He, Q.; Wu, B.; Price, J.L.; Zhao, Z. Circadian rhythm neuropeptides in drosophila: Signals for normal circadian function and circadian neurodegenerative disease. Int. J. Mol. Sci. 2017, 18, 886. [Google Scholar] [CrossRef]
- Rosselot, A.E.; Hong, C.I.; Moore, S.R. Rhythm and bugs: Circadian clocks, gut microbiota, and enteric infections. Curr. Opin. Gastroenterol. 2016, 32, 7–11. [Google Scholar] [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.-D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Murakami, M.; Tognini, P. The circadian clock as an essential molecular link between host physiology and microorganisms. Front. Cell. Infect. Microbiol. 2019, 9, 469. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Ibrahim, M.M.A. The Functional Profiles of Chicken Eggs Incubated Under Monochromatic Lighting. Doctoral Dissertation, Texas A&M University, College Station, TX, USA, 2021. [Google Scholar]
- Ibrahim, M.M.A.; Nelson, J.R.; Archer, G.S.; Athrey, G. Effects of monochromatic lighting during incubation and vaccination on the splenic transcriptome profiles of chicken. Front. Genet. 2021, 12, 628041. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Time in motion: The molecular clock meets the microbiome. Cell 2014, 159, 469–470. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies—SRI. Available online: https://www.sri.com/publication/circadian-disruption-and-mental-health-the-chronotherapeutic-potential-of-microbiome-based-and-dietary-strategies/ (accessed on 16 April 2024).
- Li, X.; Zheng, Z.; Pan, J.; Jiang, D.; Tian, Y.; Fang, L.; Huang, Y. Impacts of colored light-emitting diode illumination on the growth performance and fecal microbiota in goose. Poult. Sci. 2020, 99, 1805–1812. [Google Scholar] [CrossRef]
- Pang, X.; Chen, L.; Xu, G. New awareness of the interplay between the gut microbiota and circadian rhythms. Pol. J. Microbiol. 2023, 72, 355–363. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Li, S.; Wu, Z.; Xie, Y.; Qian, Q.; Yang, H.; Li, X. Apple polyphenol extract modulates bile acid metabolism and gut microbiota by regulating the circadian rhythms in daytime-restricted high fat diet feeding C57BL/6 male mice. Food Funct. 2022, 13, 2805–2822. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Gombert, M.; Martínez-Raga, J.; Cenit, M.C. Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies. Int. J. Mol. Sci. 2023, 24, 7579. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Han, J.; Fasina, Y.; Harrison, S.H. Connecting gut microbiomes and short chain fatty acids with the serotonergic system and behavior in Gallus gallus and other avian species. Front. Physiol. 2022, 13, 1035538. [Google Scholar] [CrossRef]
- Slevin, M.C.; Houtz, J.L.; Bradshaw, D.J.; Anderson, R.C. Evidence supporting the microbiota-gut-brain axis in a songbird. Biol. Lett. 2020, 16, 20200430. [Google Scholar] [CrossRef]
- Lyte, J.M.; Keane, J.; Eckenberger, J.; Anthony, N.; Shrestha, S.; Marasini, D.; Daniels, K.M.; Caputi, V.; Donoghue, A.M.; Lyte, M. Japanese quail (Coturnix japonica) as a novel model to study the relationship between the avian microbiome and microbial endocrinology-based host-microbe interactions. Microbiome 2021, 9, 38. [Google Scholar] [CrossRef]
- Hieke, A.-S.C.; Hubert, S.M.; Athrey, G. Circadian disruption and divergent microbiota acquisition under extended photoperiod regimens in chicken. PeerJ 2019, 7, e6592. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- USADELLAB.org—Trimmomatic: A Flexible Read Trimming Tool for Illumina NGS Data. Available online: http://www.usadellab.org/cms/?page=trimmomatic (accessed on 25 August 2023).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S. HTSeq: Analysing high-throughput sequencing data with Python. 2010.
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef]
- Carlucci, M.; Kriščiūnas, A.; Li, H.; Gibas, P.; Koncevičius, K.; Petronis, A.; Oh, G. DiscoRhythm: An easy-to-use web application and R package for discovering rhythmicity. Bioinformatics 2019, 36, 1952–1954. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Ulgen, E.; Ozisik, O.; Sezerman, O.U. pathfindR: An R Package for Comprehensive Identification of Enriched Pathways in Omics Data Through Active Subnetworks. Front. Genet. 2019, 10, 858. [Google Scholar] [CrossRef]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.-Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Diao, M.; Dyksma, S.; Koeksoy, E.; Ngugi, D.K.; Anantharaman, K.; Loy, A.; Pester, M. Global diversity and inferred ecophysiology of microorganisms with the potential for dissimilatory sulfate/sulfite reduction. FEMS Microbiol. Rev. 2023, 47, fuad058. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Nakagawa, S.; Nguyen Pham, K.T.; Shao, X.; Doi, M. Time-Restricted G-Protein Signaling Pathways via GPR176, Gz, and RGS16 Set the Pace of the Master Circadian Clock in the Suprachiasmatic Nucleus. Int. J. Mol. Sci. 2020, 21, 5055. [Google Scholar] [CrossRef] [PubMed]
- Hampp, G.; Ripperger, J.A.; Houben, T.; Schmutz, I.; Blex, C.; Perreau-Lenz, S.; Brunk, I.; Spanagel, R.; Ahnert-Hilger, G.; Meijer, J.H.; et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr. Biol. 2008, 18, 678–683. [Google Scholar] [CrossRef]
- González, S.; Moreno-Delgado, D.; Moreno, E.; Pérez-Capote, K.; Franco, R.; Mallol, J.; Cortés, A.; Casadó, V.; Lluís, C.; Ortiz, J.; et al. Circadian-related heteromerization of adrenergic and dopamine D4 receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012, 10, e1001347. [Google Scholar] [CrossRef]
- Sellix, M.T.; Freeman, M.E. Circadian rhythms of neuroendocrine dopaminergic neuronal activity in ovariectomized rats. Neuroendocrinology 2003, 77, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Manella, G.; Asher, G. The circadian nature of mitochondrial biology. Front. Endocrinol. 2016, 7, 162. [Google Scholar] [CrossRef]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Liu, J.; Kang, R.; Tang, D. The circadian clock protects against ferroptosis-induced sterile inflammation. Biochem. Biophys. Res. Commun. 2020, 525, 620–625. [Google Scholar] [CrossRef]
- Mizushima, N.; Klionsky, D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007, 27, 19–40. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Yang, Y.; Wang, Q. Association between succinate receptor SUCNR1 expression and immune infiltrates in ovarian cancer. Front. Mol. Biosci. 2020, 7, 150. [Google Scholar] [CrossRef]
- Kono, M.; Komatsuda, H.; Yamaki, H.; Kumai, T.; Hayashi, R.; Wakisaka, R.; Nagato, T.; Ohkuri, T.; Kosaka, A.; Ohara, K.; et al. Immunomodulation via FGFR inhibition augments FGFR1 targeting T-cell based antitumor immunotherapy for head and neck squamous cell carcinoma. Oncoimmunology 2022, 11, 2021619. [Google Scholar] [CrossRef]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. 2016, 13, 1. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Role of 5-HT7 receptors in the immune system in health and disease. Mol. Med. 2019, 26, 2. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Ceperuelo-Mallafré, V.; Vendrell, J. Rethinking succinate: An unexpected hormone-like metabolite in energy homeostasis. Trends Endocrinol. Metab. 2021, 32, 680–692. [Google Scholar] [CrossRef]
- Villanueva-Carmona, T.; Cedó, L.; Madeira, A.; Ceperuelo-Mallafré, V.; Rodríguez-Peña, M.-M.; Núñez-Roa, C.; Maymó-Masip, E.; Repollés-de-Dalmau, M.; Badia, J.; Keiran, N.; et al. SUCNR1 signaling in adipocytes controls energy metabolism by modulating circadian clock and leptin expression. Cell Metab. 2023, 35, 601–619.e10. [Google Scholar] [CrossRef]
- Li, X.; Mao, M.; Zhang, Y.; Yu, K.; Zhu, W. Succinate modulates intestinal barrier function and inflammation response in pigs. Biomolecules 2019, 9, 486. [Google Scholar] [CrossRef]
- Sun, X.; Deng, J.; Liu, T.; Borjigin, J. Circadian 5-HT production regulated by adrenergic signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 4686–4691. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Yu, W.; Gong, J.-S.; Ko, M.; Garver, W.S.; Yanagisawa, K.; Michikawa, M. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J. Biol. Chem. 2005, 280, 11731–11739. [Google Scholar] [CrossRef]
- Duarte, A.; Castillo, A.F.; Podestá, E.J.; Poderoso, C. Mitochondrial fusion and ERK activity regulate steroidogenic acute regulatory protein localization in mitochondria. PLoS ONE 2014, 9, e100387. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Liu, X.; Zheng, N.; Gu, Y.; Song, Y.; Wang, X. Regulatory roles of external cholesterol in human airway epithelial mitochondrial function through STARD3 signalling. Clin. Transl. Med. 2022, 12, e902. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; A, A.K.; Sanawar, R.; Jaleel, A.; Santhosh Kumar, T.R.; Kartha, C.C. Chronic pressure overload results in deficiency of mitochondrial membrane transporter ABCB7 which contributes to iron overload, mitochondrial dysfunction, metabolic shift and worsens cardiac function. Sci. Rep. 2019, 9, 13170. [Google Scholar] [CrossRef]
- Radka, C.D.; Frank, M.W.; Rock, C.O.; Yao, J. Fatty acid activation and utilization by Alistipes finegoldii, a representative Bacteroidetes resident of the human gut microbiome. Mol. Microbiol. 2020, 113, 807–825. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, K.; Ma, M.; Ma, J.; Tian, J.; Jin, Y. Lactobacillus mediates the expression of NPC1L1, CYP7A1, and ABCG5 genes to regulate cholesterol. Food Sci. Nutr. 2021, 9, 6882–6891. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Lu, T.-Y.; Tseng, Y.-Y.; Pan, T.-M. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2006, 71, 238–245. [Google Scholar] [CrossRef]
- Salaj, R.; Stofilová, J.; Soltesová, A.; Hertelyová, Z.; Hijová, E.; Bertková, I.; Strojný, L.; Kružliak, P.; Bomba, A. The effects of two Lactobacillus plantarum strains on rat lipid metabolism receiving a high fat diet. Sci. World J. 2013, 2013, 135142. [Google Scholar] [CrossRef]
- Yang, D.; Lyu, W.; Hu, Z.; Gao, J.; Zheng, Z.; Wang, W.; Firrman, J.; Ren, D. Probiotic Effects of Lactobacillus fermentum ZJUIDS06 and Lactobacillus plantarum ZY08 on Hypercholesteremic Golden Hamsters. Front. Nutr. 2021, 8, 705763. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Chen, X.; Chen, Y.; Bao, Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J. Dairy Sci. 2012, 95, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hu, J.; Erasmus, M.A.; Zhang, H.; Johnson, T.A.; Cheng, H. Cecal microbiota transplantation: Unique influence of cecal microbiota from divergently selected inbred donor lines on cecal microbial profile, serotonergic activity, and aggressive behavior of recipient chickens. J. Anim. Sci. Biotechnol. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Ali, Z.A.; Kojima, Y.; Kundu, R.K.; Sheikh, A.Y.; Agrawal, R.; Zheng, L.; Leeper, N.J.; Pearl, N.E.; Patterson, A.J.; et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Investig. 2008, 118, 3343–3354. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium Signaling Network in Plants. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Mellström, D.; Vandenput, L.; Mallmin, H.; Holmberg, A.H.; Lorentzon, M.; Odén, A.; Johansson, H.; Orwoll, E.S.; Labrie, F.; Karlsson, M.K.; et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J. Bone Miner. Res. 2008, 23, 1552–1560. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Y.; Zhang, F.; Yao, F.; Sun, C. Calcium signaling pathways: Key pathways in the regulation of obesity. Int. J. Mol. Sci. 2019, 20, 2768. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.-I.; Honma, S. Roles of neuropeptides, VIP and AVP, in the mammalian central circadian clock. Front. Neurosci. 2021, 15, 650154. [Google Scholar] [CrossRef]
- Nahm, S.-S.; Farnell, Y.Z.; Griffith, W.; Earnest, D.J. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J. Neurosci. 2005, 25, 9304–9308. [Google Scholar] [CrossRef] [PubMed]
- Burkeen, J.F.; Womac, A.D.; Earnest, D.J.; Zoran, M.J. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J. Neurosci. 2011, 31, 8432–8440. [Google Scholar] [CrossRef] [PubMed]
- Enoki, R.; Ono, D.; Kuroda, S.; Honma, S.; Honma, K.-I. Dual origins of the intracellular circadian calcium rhythm in the suprachiasmatic nucleus. Sci. Rep. 2017, 7, 41733. [Google Scholar] [CrossRef] [PubMed]
- Fon, E.A.; Edwards, R.H. Molecular mechanisms of neurotransmitter release. Muscle Nerve 2001, 24, 581–601. [Google Scholar] [CrossRef]
- Jackman, S.L.; Turecek, J.; Belinsky, J.E.; Regehr, W.G. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 2016, 529, 88–91. [Google Scholar] [CrossRef]
- Kim, J.W.; Yong, A.J.H.; Dawson, T.M.; Dawson, V.L.; Jan, Y.-N.; Ingolia, N.T. Molecular recording of calcium signaling via calcium-dependent protein proximity labeling. BioRxiv 2022. [Google Scholar] [CrossRef]
- Sanborn, V.; Azcarate-Peril, M.A.; Updegraff, J.; Manderino, L.; Gunstad, J. Randomized Clinical Trial Examining the Impact of Lactobacillus rhamnosus GG Probiotic Supplementation on Cognitive Functioning in Middle-aged and Older Adults. Neuropsychiatr. Dis. Treat. 2020, 16, 2765–2777. [Google Scholar] [CrossRef]

| QC | NP_ZT0 | NP_ZT24 | NP_ZT48 | EP_ZT0 | EP_ZT24 | EP_ZT48 |
|---|---|---|---|---|---|---|
| Total M Seqs | 21.48 | 16.43 | 15.31 | 16.25 | 14.66 | 17.84 |
| Length | 73 | 73 | 73 | 73 | 73 | 73 |
| M Aligned | 18.81 | 14.8 | 13.24 | 14.32 | 12.78 | 14.55 |
| % Aligned | 87% | 90% | 86% | 88% | 87% | 84% |
| M Assigned | 10.54 | 8.29 | 6.93 | 6.96 | 6.34 | 6.99 |
| % Assigned | 47% | 50% | 44% | 43% | 42% | 41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.; Cilkiz, M.; Ibrahim, M.M.A.; Athrey, G. Gut Microbiome—Brain Crosstalk in the Early Life of Chicken Reveals the Circadian Regulation of Key Metabolic and Immune Signaling Processes. Microorganisms 2025, 13, 789. https://doi.org/10.3390/microorganisms13040789
Gupta M, Cilkiz M, Ibrahim MMA, Athrey G. Gut Microbiome—Brain Crosstalk in the Early Life of Chicken Reveals the Circadian Regulation of Key Metabolic and Immune Signaling Processes. Microorganisms. 2025; 13(4):789. https://doi.org/10.3390/microorganisms13040789
Chicago/Turabian StyleGupta, Mridula, Mustafa Cilkiz, Mohamed M. A. Ibrahim, and Giridhar Athrey. 2025. "Gut Microbiome—Brain Crosstalk in the Early Life of Chicken Reveals the Circadian Regulation of Key Metabolic and Immune Signaling Processes" Microorganisms 13, no. 4: 789. https://doi.org/10.3390/microorganisms13040789
APA StyleGupta, M., Cilkiz, M., Ibrahim, M. M. A., & Athrey, G. (2025). Gut Microbiome—Brain Crosstalk in the Early Life of Chicken Reveals the Circadian Regulation of Key Metabolic and Immune Signaling Processes. Microorganisms, 13(4), 789. https://doi.org/10.3390/microorganisms13040789








