Uncovering Microbial Diversity and Community Structure of Black Spots Residing in Tomb Mural Painting
Abstract
1. Introduction
2. Materials and Methods
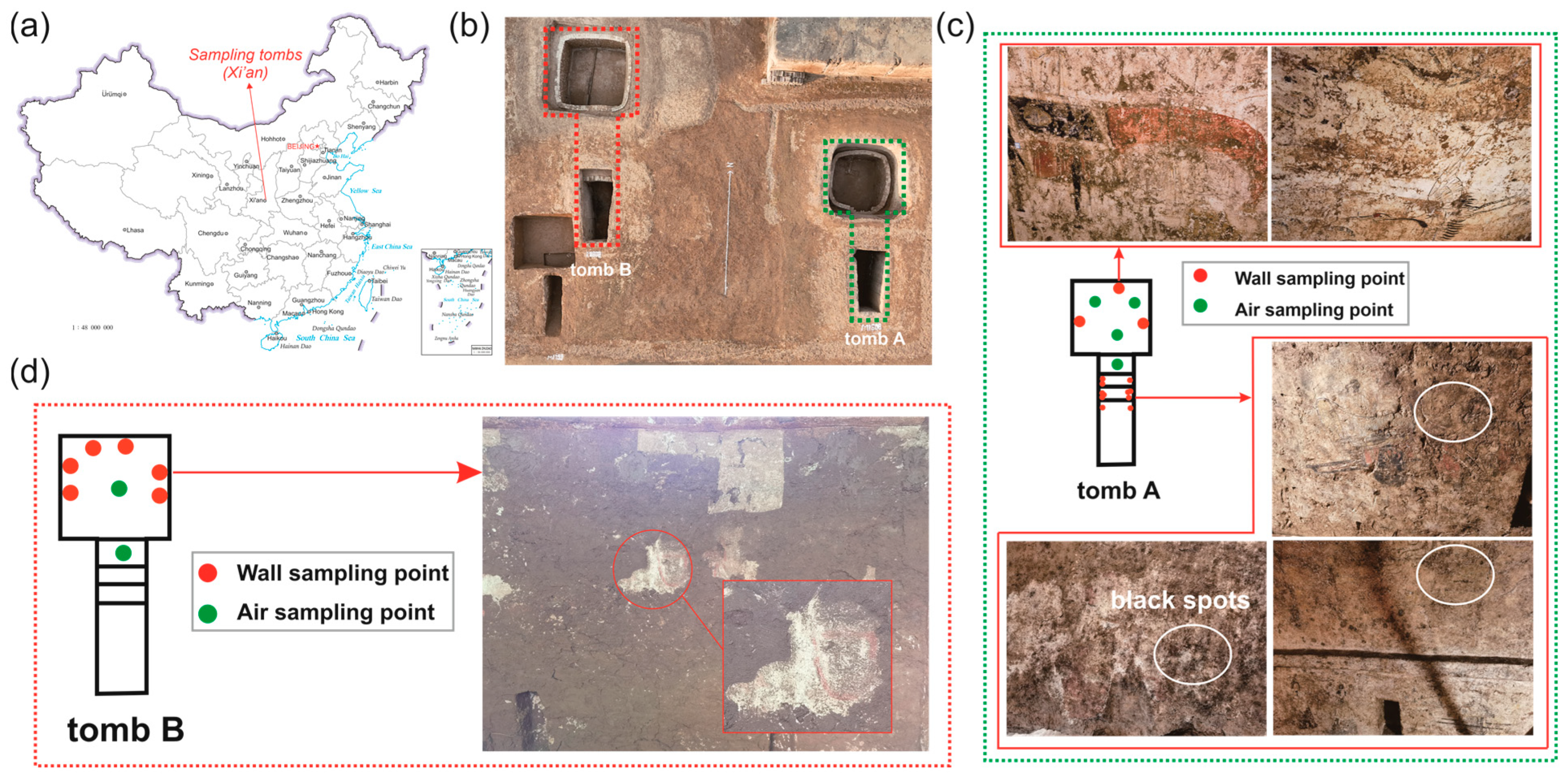
2.1. Site Description and Sampling
2.2. DNA Extraction and Sequencing
2.3. Bioinformatic and Statistical Analyses
2.4. Data of Microclimate Parameters
3. Results
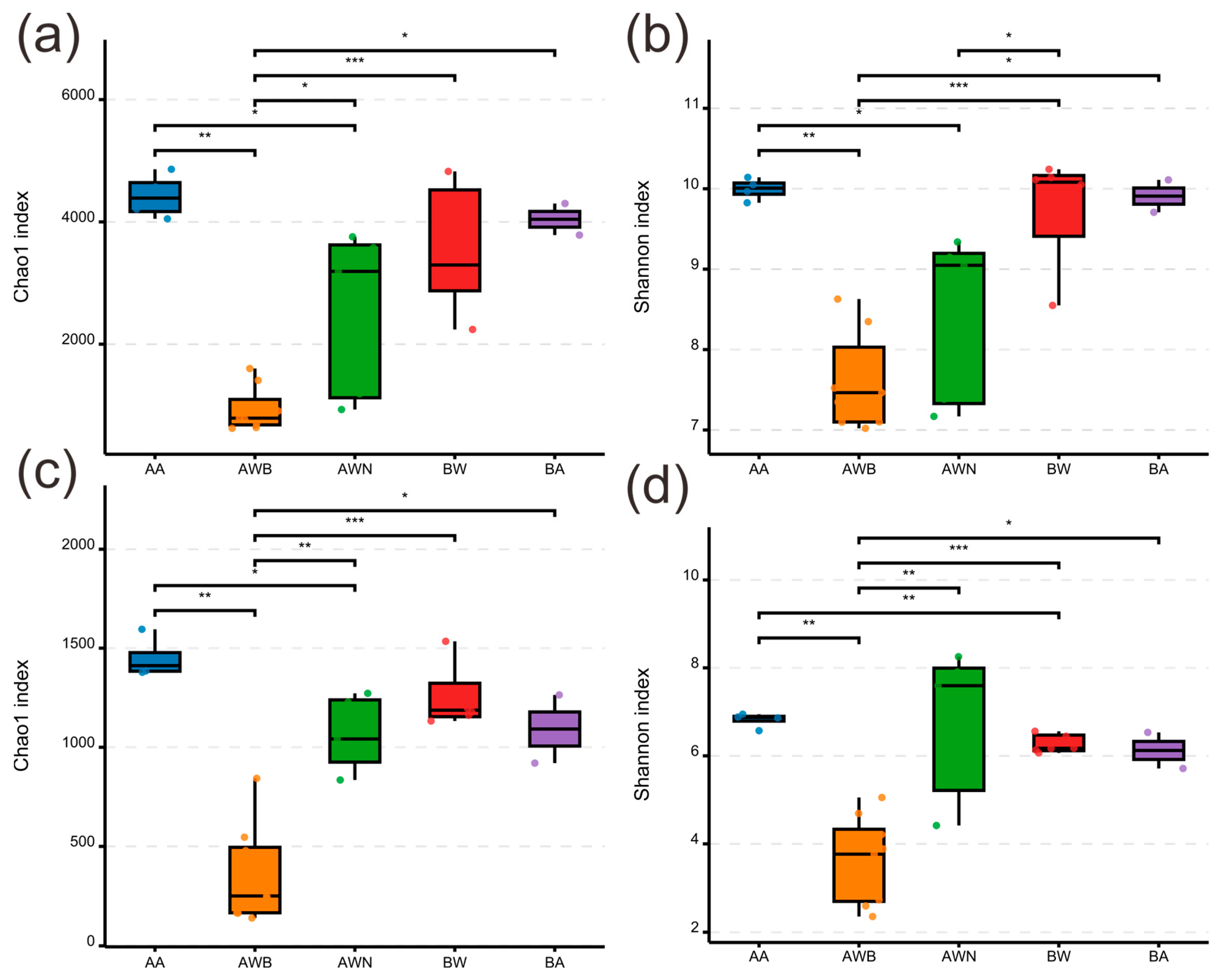
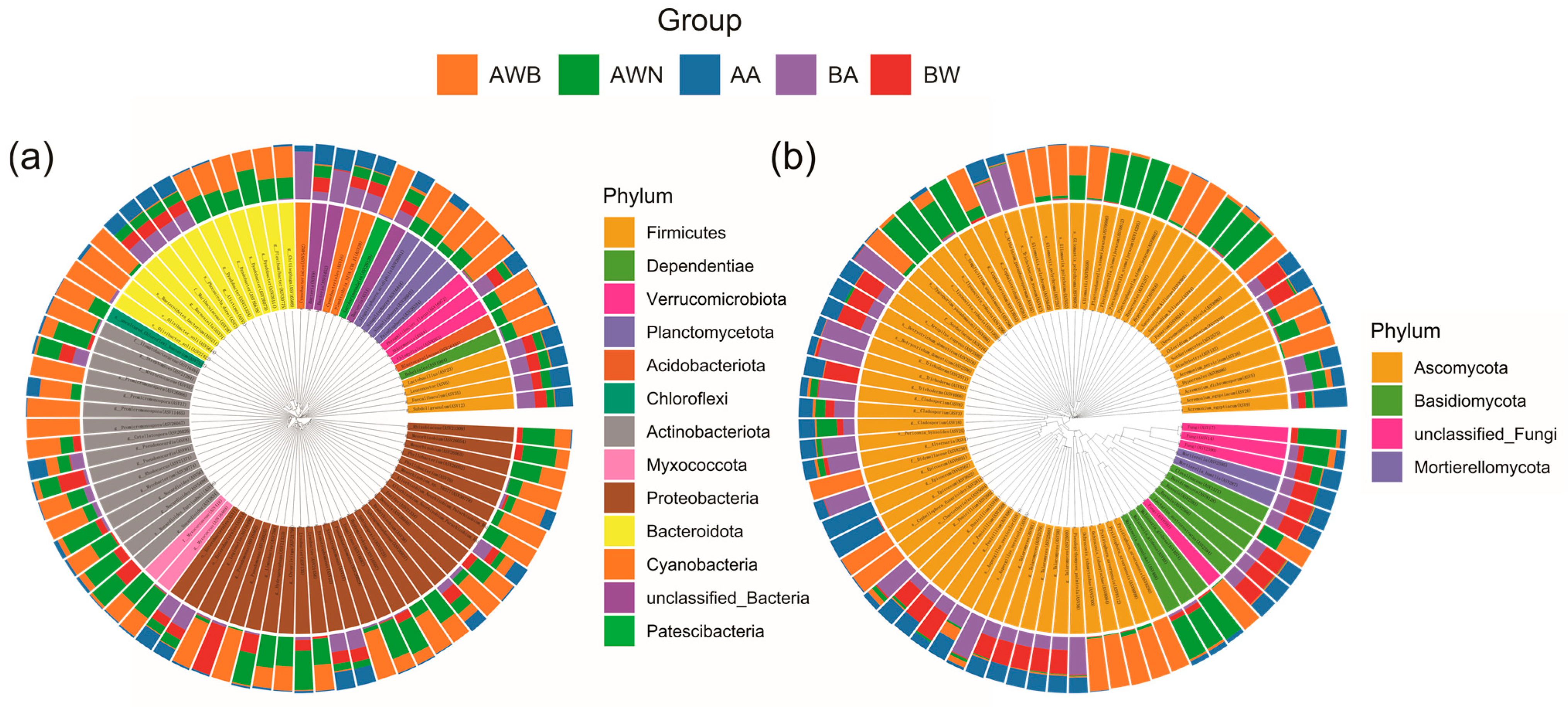
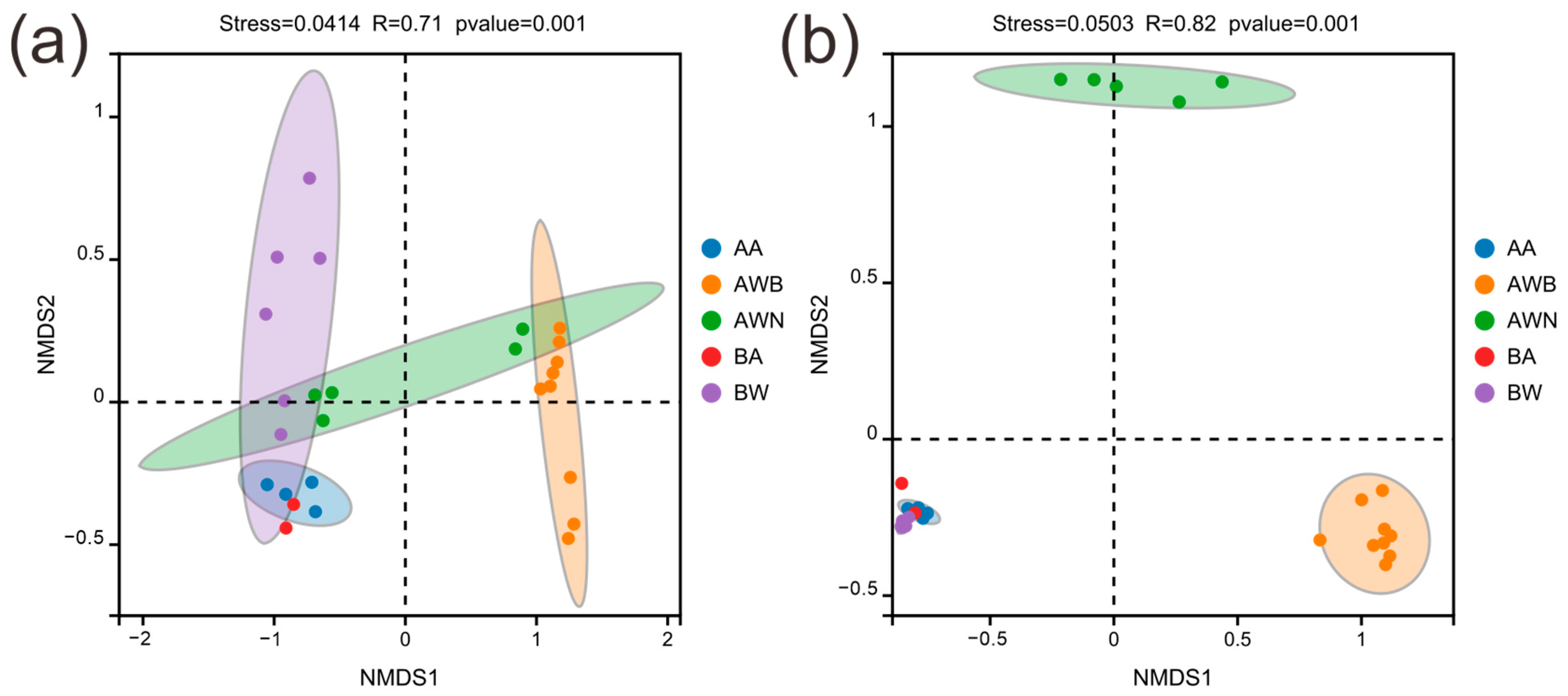
3.1. Microbial Diversity Patterns
3.2. Microbial Assembly Processes
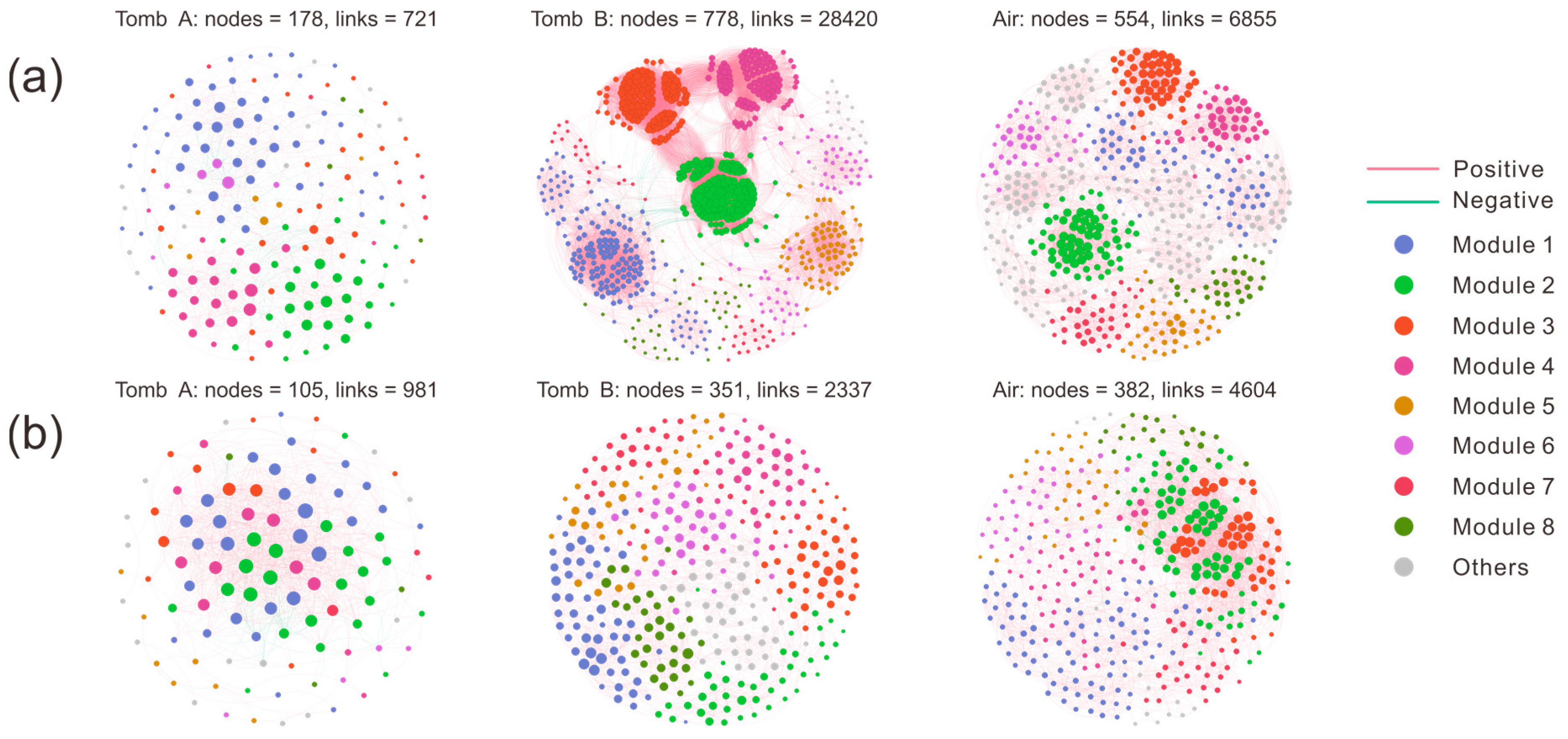
3.3. Analysis of Microbial Co-Occurrence Networks
3.4. Potential Functional Pathways in Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, L.; Creuzé-des-Châtelliers, C.; Trabac, T.; Dubost, A.; Moënne-Loccoz, Y.; Pommier, T. Rock substrate rather than black stain alterations drives microbial community structure in the passage of Lascaux Cave. Microbiome 2018, 6, 216. [Google Scholar] [CrossRef]
- He, D.; Wu, F.; Ma, W.; Zhang, Y.; Gu, J.-D.; Duan, Y.; Xu, R.; Feng, H.; Wang, W.; Li, S.-W. Insights into the bacterial and fungal communities and microbiome that causes a microbe outbreak on ancient wall paintings in the Maijishan Grottoes. Int. Biodeterior. Biodegrad. 2021, 163, 105250. [Google Scholar] [CrossRef]
- Mugnai, G.; Borruso, L.; Wu, Y.-L.; Gallinaro, M.; Cappitelli, F.; Zerboni, A.; Villa, F. Ecological strategies of bacterial communities in prehistoric stone wall paintings across weathering gradients: A case study from the Borana zone in southern Ethiopia. Sci. Total Environ. 2024, 907, 168026. [Google Scholar] [CrossRef] [PubMed]
- Farda, B.; Djebaili, R.; Vaccarelli, I.; Del Gallo, M. Actinomycetes from Caves: An Overview of Their Diversity, Biotechnological Properties, and Insights for Their Use in Soil Environments. Microorganisms 2022, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pimentel, J.L.; Dominguez-Moñino, I.; Jurado, V.; Laiz, L.; Caldeira, A.T.; Saiz-Jimenez, C. The Rare Actinobacterium Crossiella sp. is a Potential Source of New Bioactive Compounds with Activity against Bacteria and Fungi. Microorganisms 2022, 10, 1575. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Jin, T.; Li, Y.; Wu, B.; Yu, D.; Yu, Z.; Su, B.; Chen, R.; Feng, Y.; et al. Multikingdom interactions govern the microbiome in subterranean cultural heritage sites. Proc. Natl. Acad. Sci. USA 2022, 119, e2121141119. [Google Scholar] [CrossRef] [PubMed]
- Elhagrassy, A.F. Isolation and characterization of actinomycetes from Mural paintings of Snu-Sert-Ankh tomb, their antimicrobial activity, and their biodeterioration. Microbiol. Res. 2018, 216, 47–55. [Google Scholar] [CrossRef]
- Xing, W.; Qi, B.; Chen, R.; Ding, W.; Zhang, F. Metagenomic analysis reveals taxonomic and functional diversity of microbial communities on the deteriorated wall paintings of Qinling Tomb in the Southern Tang Dynasty, China. BMC Microbiol. 2023, 23, 140. [Google Scholar] [CrossRef]
- Cennamo, P.; Montuori, N.; Trojsi, G.; Fatigati, G.; Moretti, A. Biofilms in churches built in grottoes. Sci. Total Environ. 2016, 543, 727–738. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Komar, M.; Gutarowska, B. Towards understanding the link between the deterioration of building materials and the nature of aerophytic green algae. Sci. Total Environ. 2022, 802, 149856. [Google Scholar] [CrossRef] [PubMed]
- Paiva de Carvalho, H.; Sequeira, S.O.; Pinho, D.; Trovão, J.; da Costa, R.M.F.; Egas, C.; Macedo, M.F.; Portugal, A. Combining an innovative non-invasive sampling method and high-throughput sequencing to characterize fungal communities on a canvas painting. Int. Biodeterior. Biodegrad. 2019, 145, 104816. [Google Scholar] [CrossRef]
- Vieto, S.; Escudero-Leyva, E.; Avendaño, R.; Rechnitzer, N.; Barrantes-Madrigal, M.D.; Conejo-Barboza, G.; Herrera-Sancho, O.A.; Chaverri, P.; Chavarría, M. Biodeterioration and cellulolytic activity by fungi isolated from a nineteenth-century painting at the National Theatre of Costa Rica. Fungal Biol. 2022, 126, 101–112. [Google Scholar] [CrossRef]
- Leplat, J.; Francois, A.; Bousta, F. White fungal covering on the wall paintings of the Saint-Savin-sur-Gartempe Abbey church crypt: A case study. Int. Biodeterior. Biodegrad. 2017, 122, 29–37. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, H.; Wu, M.; Li, Y.; Cao, H.; Rong, R. Seasonal dynamics of airborne culturable fungi and its year-round diversity monitoring in Dahuting Han Dynasty Tomb of China. Sci. Total Environ. 2022, 838, 155990. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.; Xu, Q.; Yu, H. A new technique for the removal of red fungal stains on traditional Chinese painting on silk. Int. Biodeterior. Biodegrad. 2023, 181, 105622. [Google Scholar] [CrossRef]
- Fomina, M.; Cuadros, J.; Pinzari, F.; Hryshchenko, N.; Najorka, J.; Gavrilenko, M.; Hong, J.W.; Gadd, G.M. Fungal transformation of mineral substrata of biodeteriorated medieval murals in Saint Sophia’s cathedral, Kyiv, Ukraine. Int. Biodeterior. Biodegrad. 2022, 175, 105486. [Google Scholar] [CrossRef]
- Capodicasa, S.; Fedi, S.; Porcelli, A.M.; Zannoni, D. The microbial community dwelling on a biodeteriorated 16th century painting. Int. Biodeterior. Biodegrad. 2010, 64, 727–733. [Google Scholar] [CrossRef]
- Portillo, M.C.; Gonzalez, J.M. Sulfate-reducing bacteria are common members of bacterial communities in Altamira Cave (Spain). Sci. Total Environ. 2009, 407, 1114–1122. [Google Scholar] [CrossRef]
- Okpalanozie, O.E.; Adebusoye, S.A.; Troiano, F.; Polo, A.; Cappitelli, F.; Ilori, M.O. Evaluating the microbiological risk to a contemporary Nigerian painting: Molecular and biodegradative studies. Int. Biodeterior. Biodegrad. 2016, 114, 184–192. [Google Scholar] [CrossRef]
- Pavić, A.; Ilić-Tomić, T.; Pačevski, A.; Nedeljković, T.; Vasiljević, B.; Morić, I. Diversity and biodeteriorative potential of bacterial isolates from deteriorated modern combined-technique canvas painting. Int. Biodeterior. Biodegrad. 2015, 97, 40–50. [Google Scholar] [CrossRef]
- Sansupa, C.; Suphaphimol, N.; Nonthijun, P.; Ronsuek, T.; Yimklan, S.; Semakul, N.; Khrueraya, T.; Suwannarach, N.; Purahong, W.; Disayathanoowat, T. Life on the wall: The diversity and activity of microbes on 13th-century AD. Lan Na mural painting. Front. Microbiol. 2023, 14, 1220901. [Google Scholar] [CrossRef]
- Piano, E.; Bona, F.; Falasco, E.; La Morgia, V.; Badino, G.; Isaia, M. Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps). Sci. Total Environ. 2015, 536, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Viles, H.A.; Cutler, N.A. Global environmental change and the biology of heritage structures. Glob. Change Biol. 2012, 18, 2406–2418. [Google Scholar] [CrossRef]
- Sanchez-Moral, S.; Luque, L.; Cuezva, S.; Soler, V.; Benavente, D.; Laiz, L.; Gonzalez, J.M.; Saiz-Jimenez, C. Deterioration of building materials in Roman catacombs: The influence of visitors. Sci. Total Environ. 2005, 349, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Dang, R. Review of lighting deterioration, lighting quality, and lighting energy saving for paintings in museums. Build. Environ. 2022, 208, 108608. [Google Scholar] [CrossRef]
- Melo, M.J.; Araújo, R.; Castro, R.; Casanova, C. Colour degradation in medieval manuscripts. Microchem. J. 2016, 124, 837–844. [Google Scholar] [CrossRef]
- Pellegrini, D.; Duce, C.; Bonaduce, I.; Biagi, S.; Ghezzi, L.; Colombini, M.P.; Tinè, M.R.; Bramanti, E. Fourier transform infrared spectroscopic study of rabbit glue/inorganic pigments mixtures in fresh and aged reference paint reconstructions. Microchem. J. 2016, 124, 31–35. [Google Scholar] [CrossRef]
- Baquedano Estevez, C.; Moreno-Merino, L.; de la Losa Roman, A.; Duran Valsero, J.J. The lampenflora in show caves and its treatment: An emerging ecological problem. Int. J. Speleol. 2019, 48, 249–277. [Google Scholar] [CrossRef]
- Liu, W.; Bao, Y.; Zhang, J.; Ma, Y.; Cui, X.; Li, Y.; Feng, Y. Unearthing lights-induced epilithic bacteria community assembly and associated function potentials in historical relics. Int. Biodeterior. Biodegrad. 2024, 186, 105701. [Google Scholar] [CrossRef]
- Bontemps, Z.; Moënne-Loccoz, Y.; Hugoni, M. Stochastic and deterministic assembly processes of microbial communities in relation to natural attenuation of black stains in Lascaux Cave. mSystems 2024, 9, e01233-23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, X.; Xie, H.; Wu, R.; Zhang, Z.; Guo, Q.; Hokoi, S. The impact of cave opening and closure on murals hygrothermal behavior in Cave 98 of Mogao Caves, China. Build. Environ. 2024, 256, 111502. [Google Scholar] [CrossRef]
- Basile, A.; Riggio, F.P.; Tescari, M.; Chebbi, A.; Sodo, A.; Bartoli, F.; Imperi, F.; Caneva, G.; Visca, P. Metagenome-resolved functional traits of Rubrobacter species implicated in rosy discoloration of ancient frescoes in two Georgian Cathedrals. Sci. Total Environ. 2025, 958, 178135. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Cañaveras, J.C.; Benavente, D.; Duarte, E.; Saiz-Jimenez, C.; Sanchez-Moral, S. New insights into the structure, microbial diversity and ecology of yellow biofilms in a Paleolithic rock art cave (Pindal Cave, Asturias, Spain). Sci. Total Environ. 2023, 897, 165218. [Google Scholar] [CrossRef]
- Bontemps, Z.; Alonso, L.; Pommier, T.; Hugoni, M.; Moënne-Loccoz, Y. Microbial ecology of tourist Paleolithic caves. Sci. Total Environ. 2022, 816, 151492. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, W.; Xie, C.; Zhu, Y.; Tang, W.; Zhou, X.; Xiao, H. Airborne bacterial communities in the poultry farm and their relevance with environmental factors and antibiotic resistance genes. Sci. Total Environ. 2022, 846, 157420. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Guimerà, R.; Nunes Amaral, L.A. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Mircea, C.; Vulpoi, A.; Rusu, I.; Radu, C.; Pârvu, M.; Kelemen, B. Exploring post-excavation degradation potential of fungal communities associated with archaeological human remains. Archaeometry 2019, 61, 750–763. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, C.; Hu, Y.; Zhang, B.; Wang, J. Dynamics of microbial community composition during degradation of silks in burial environment. Sci. Total Environ. 2023, 883, 163694. [Google Scholar] [CrossRef]
- Xiong, J.; Qi, X.; Wu, D.; Zhang, Y.; Yang, C.; Ma, Y.; Yang, J.; Wang, H.; Han, J.; Li, A. Microbial pollution assessment in semi-exposed relics: A case study of the K9901 pit of the mausoleum of emperor Qin Shihuang. Build. Environ. 2024, 262, 111744. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, X.; Hu, P.; Gao, L.; Gu, J.-D. Identifying the major metabolic potentials of microbial-driven carbon, nitrogen and sulfur cycling on stone cultural heritage worldwide. Sci. Total Environ. 2024, 954, 176757. [Google Scholar] [CrossRef]
- Dupont, J.; Jacquet, C.; Dennetière, B.; Lacoste, S.; Bousta, F.; Orial, G.; Cruaud, C.; Couloux, A.; Roquebert, M.-F. Invasion of the French Paleolithic painted cave of Lascaux by members of the Fusarium solani species complex. Mycologia 2007, 99, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Jurado, V.; Sanchez-Moral, S.; Saiz-Jimenez, C. Entomogenous fungi and the conservation of the cultural heritage: A review. Int. Biodeterior. Biodegrad. 2008, 62, 325–330. [Google Scholar] [CrossRef]
- Kiyuna, T.; An, K.-D.; Kigawa, R.; Sano, C.; Miura, S.; Sugiyama, J. Molecular assessment of fungi in ‘‘black spots’’ that deface murals in the Takamatsuzuka and Kitora Tumuli in Japan: Acremonium sect. Gliomastix including Acremonium tumulicola sp. nov. and Acremonium felinum comb. nov. Mycoscience 2011, 52, 1–17. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol. 2012, 116, 574–589. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Real-time PCR detection of Ochroconis lascauxensis involved in the formation of black stains in the Lascaux Cave, France. Sci. Total Environ. 2013, 443, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Hirao, A.; Yamasaki, R.; Hara, K.; Ishihara, Y. Seasonal variation in airborne microbial communities of the Akiyoshido Cave: Lampenflora dispersed by phototrophic bioaerosols. Int. Biodeterior. Biodegrad. 2024, 195, 105905. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Jurado, V.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Ontañon, R.; Arias, P.; Cañaveras, J.C.; Sanchez-Moral, S.; et al. Microclimate, airborne particles, and microbiological monitoring protocol for conservation of rock-art caves: The case of the world-heritage site La Garma cave (Spain). J. Environ. Manag. 2024, 351, 119762. [Google Scholar] [CrossRef]
- Branysova, T.; Petru, N.; Lopez Marin, M.A.; Solcova, M.; Demnerova, K.; Stiborova, H. Uncovering the microbial diversity of Czech Republic archives: A study of metabolically active airborne microbes. Heliyon 2024, 10, e27930. [Google Scholar] [CrossRef] [PubMed]
- Bastholm, C.J.; Madsen, A.M.; Andersen, B.; Frisvad, J.C.; Richter, J. The mysterious mould outbreak—A comprehensive fungal colonisation in a climate-controlled museum repository challenges the environmental guidelines for heritage collections. J. Cult. Herit. 2022, 55, 78–87. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Rochefort, A.; Simonin, M.; Marais, C.; Guillerm-Erckelboudt, A.-Y.; Barret, M.; Sarniguet, A. Transmission of Seed and Soil Microbiota to Seedling. mSystems 2021, 6, e00446-21. [Google Scholar] [CrossRef]
- Lepinay, C.; Mihajlovski, A.; Touron, S.; Seyer, D.; Bousta, F.; Di Martino, P. Bacterial diversity associated with saline efflorescences damaging the walls of a French decorated prehistoric cave registered as a World Cultural Heritage Site. Int. Biodeterior. Biodegrad. 2018, 130, 55–64. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Muhammad, A.; Shen, X.; Sun, C.; Li, Q.; Hu, Y.; Shao, Y. From surviving to thriving, the assembly processes of microbial communities in stone biodeterioration: A case study of the West Lake UNESCO World Heritage area in China. Sci. Total Environ. 2022, 805, 150395. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Jiang, Y.; Cheng, M.; Dini-Andreote, F.; Sui, Y.; Xu, Q.; Geisen, S.; Sun, B. Organism body size structures the soil microbial and nematode community assembly at a continental and global scale. Nat. Commun. 2020, 11, 6406. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.M.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Xiong, J.; Li, A.; Liu, C.; Dong, J.; Yang, B.; Cao, J.; Ren, T. Probing the historic thermal and humid environment in a 2000-year-old ancient underground tomb and enlightenment for cultural heritage protection and preventive conservation. Energy Build. 2021, 251, 111388. [Google Scholar] [CrossRef]
- Li, Q.; Wu, C.; He, J.; Zhang, B. Unraveling the microbiotas and key genetic contexts identified on stone heritage using illumina and nanopore sequencing platforms. Int. Biodeterior. Biodegrad. 2023, 185, 105688. [Google Scholar] [CrossRef]
- Ceci, A.; Pinzari, F.; Russo, F.; Persiani, A.M.; Gadd, G.M. Roles of saprotrophic fungi in biodegradation or transformation of organic and inorganic pollutants in co-contaminated sites. Appl. Microbiol. Biotechnol. 2019, 103, 53–68. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Chen, C.; Jin, X.; Chen, Z.; Xiong, C.; Li, P.; Zhao, J.; Huang, W. Characterization and comparative mitogenomic analysis of six newly sequenced mitochondrial genomes from ectomycorrhizal fungi (Russula) and phylogenetic analysis of the Agaricomycetes. Int. J. Biol. Macromol. 2018, 119, 792–802. [Google Scholar] [CrossRef]
- Ianiri, G.; Averette, A.F.; Kingsbury, J.M.; Heitman, J.; Idnurm, A. Gene Function Analysis in the Ubiquitous Human Commensal and Pathogen Malassezia Genus. mBio 2016, 7, e01853-16. [Google Scholar] [CrossRef]
- Armer, V.J.; Kroll, E.; Darino, M.; Smith, D.P.; Urban, M.; Hammond-Kosack, K.E. Navigating the Fusarium species complex: Host-range plasticity and genome variations. Fungal Biol. 2024, 128, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Warinner, C.; Speller, C.; Collins, M.J.; Lewis, C.M. Ancient human microbiomes. J. Hum. Evol. 2015, 79, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Abia, A.L.K.; Alisoltani, A.; Ubomba-Jaswa, E.; Dippenaar, M.A. Microbial life beyond the grave: 16S rRNA gene-based metagenomic analysis of bacteria diversity and their functional profiles in cemetery environments. Sci. Total Environ. 2019, 655, 831–841. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; He, Z.; Wang, Z.; Chen, A.; Wu, C. Uncovering Microbial Diversity and Community Structure of Black Spots Residing in Tomb Mural Painting. Microorganisms 2025, 13, 755. https://doi.org/10.3390/microorganisms13040755
Li Q, He Z, Wang Z, Chen A, Wu C. Uncovering Microbial Diversity and Community Structure of Black Spots Residing in Tomb Mural Painting. Microorganisms. 2025; 13(4):755. https://doi.org/10.3390/microorganisms13040755
Chicago/Turabian StyleLi, Qiang, Zhang He, Zeng Wang, Aidong Chen, and Chao Wu. 2025. "Uncovering Microbial Diversity and Community Structure of Black Spots Residing in Tomb Mural Painting" Microorganisms 13, no. 4: 755. https://doi.org/10.3390/microorganisms13040755
APA StyleLi, Q., He, Z., Wang, Z., Chen, A., & Wu, C. (2025). Uncovering Microbial Diversity and Community Structure of Black Spots Residing in Tomb Mural Painting. Microorganisms, 13(4), 755. https://doi.org/10.3390/microorganisms13040755




