Neisseria gonorrhoeae—Susceptibility Trends and Basic Molecular Mapping of Isolates Collected in Israel in 2016–2022
Abstract
1. Introduction
- 1.
- Globally Prevalent Resistant Sequence Types: ST1901 is frequently associated with decreased susceptibility or resistance to extended-spectrum cephalosporins, particularly ceftriaxone [13,14]. ST1901 has been identified in multiple countries across different continents, indicating its global spread [15].
- 2.
- Emerging Resistant Sequence Types: ST13871 is associated with high-level azithromycin resistance, and ST-13871 has been reported in multiple European countries and the United States [16,17]. ST14422 has been identified as a prevalent sequence type in some regions, and ST14422 has been associated with resistance to multiple antibiotics, including tetracycline and ciprofloxacin [13,17]. ST11210 has been linked to decreased susceptibility to extended-spectrum cephalosporins and resistance to fluoroquinolones in some studies [13,16].
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Bacterial Growth and Testing
2.3. Antimicrobial Susceptibility Testing (AST)
- 1.
- Decreased susceptibility to cephalosporins or resistance to azithromycin, along with resistance to at least two other antimicrobials, was defined as MDR-NG;
- 2.
- Decreased susceptibility to a cephalosporin plus resistance to azithromycin as well as resistance to at least two other antimicrobials was defined as XDR-NG [24].
2.4. Molecular Characterization Using NG-MAST Genotyping
3. Results
3.1. The Demographic Characteristics of the Study Group
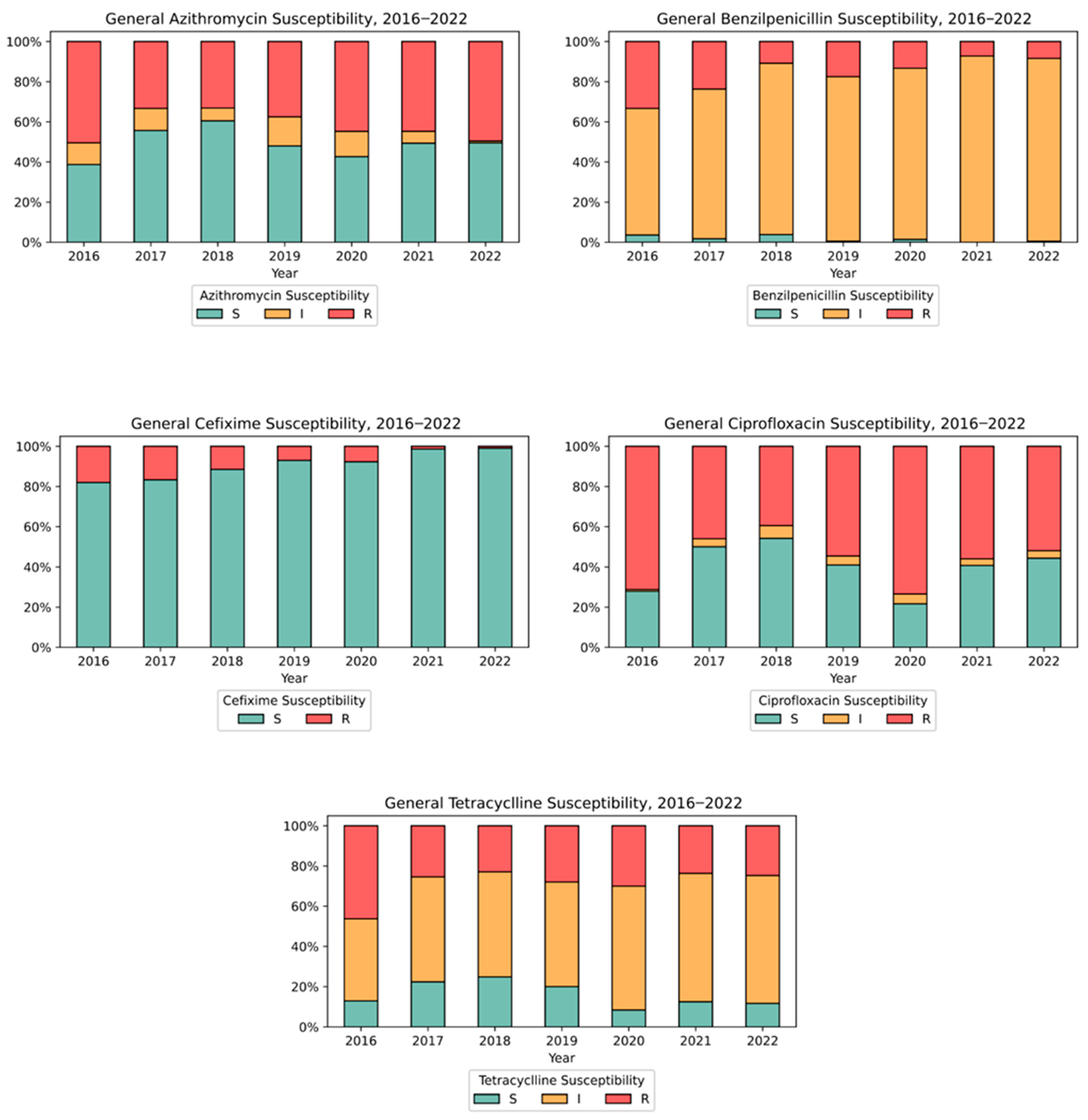
3.2. Antimicrobial Susceptibility Testing (AST)
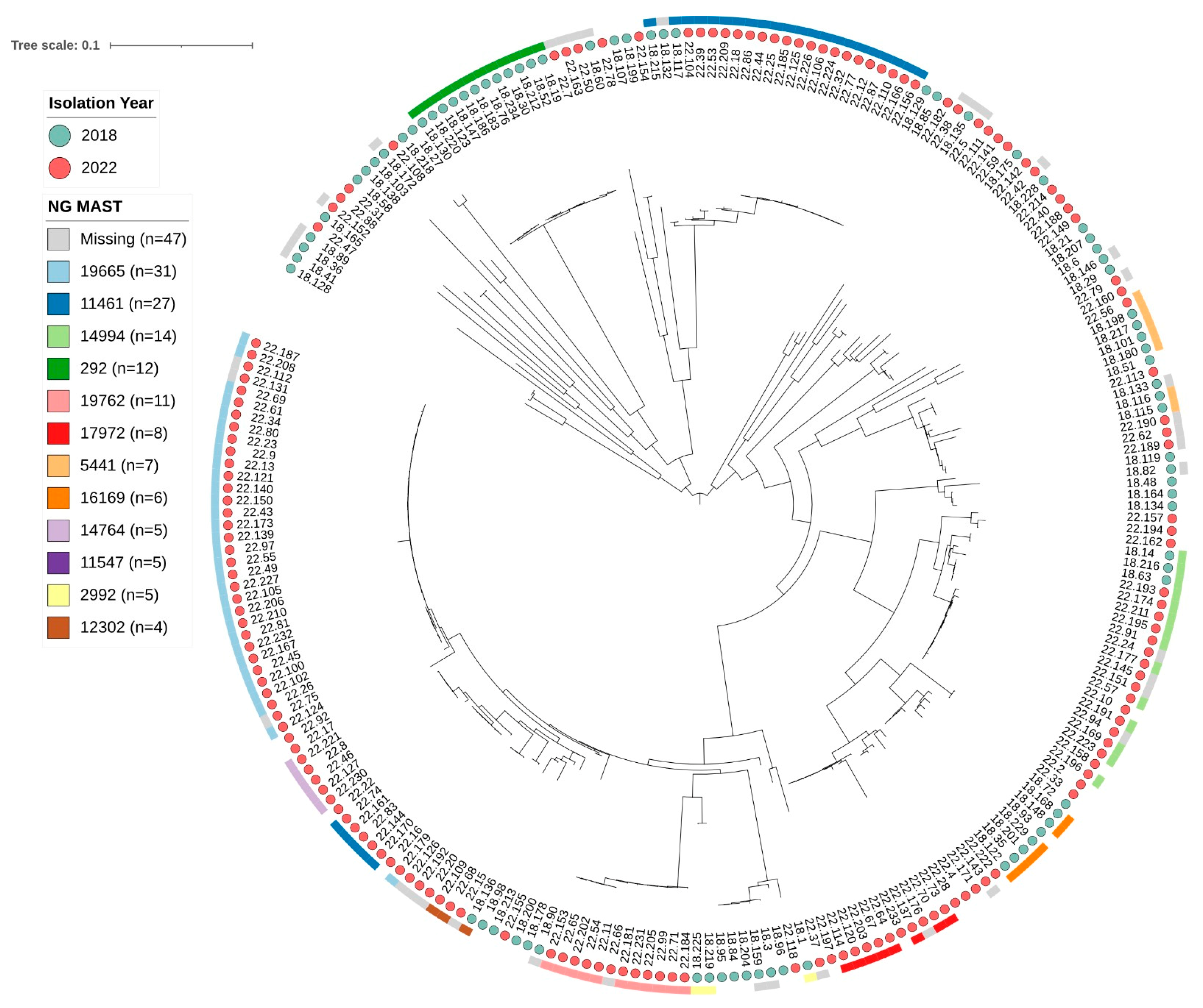
3.3. Molecular Characterization by NG-MAST Genotyping
4. Discussion
4.1. Demographic Patterns and Surveillance
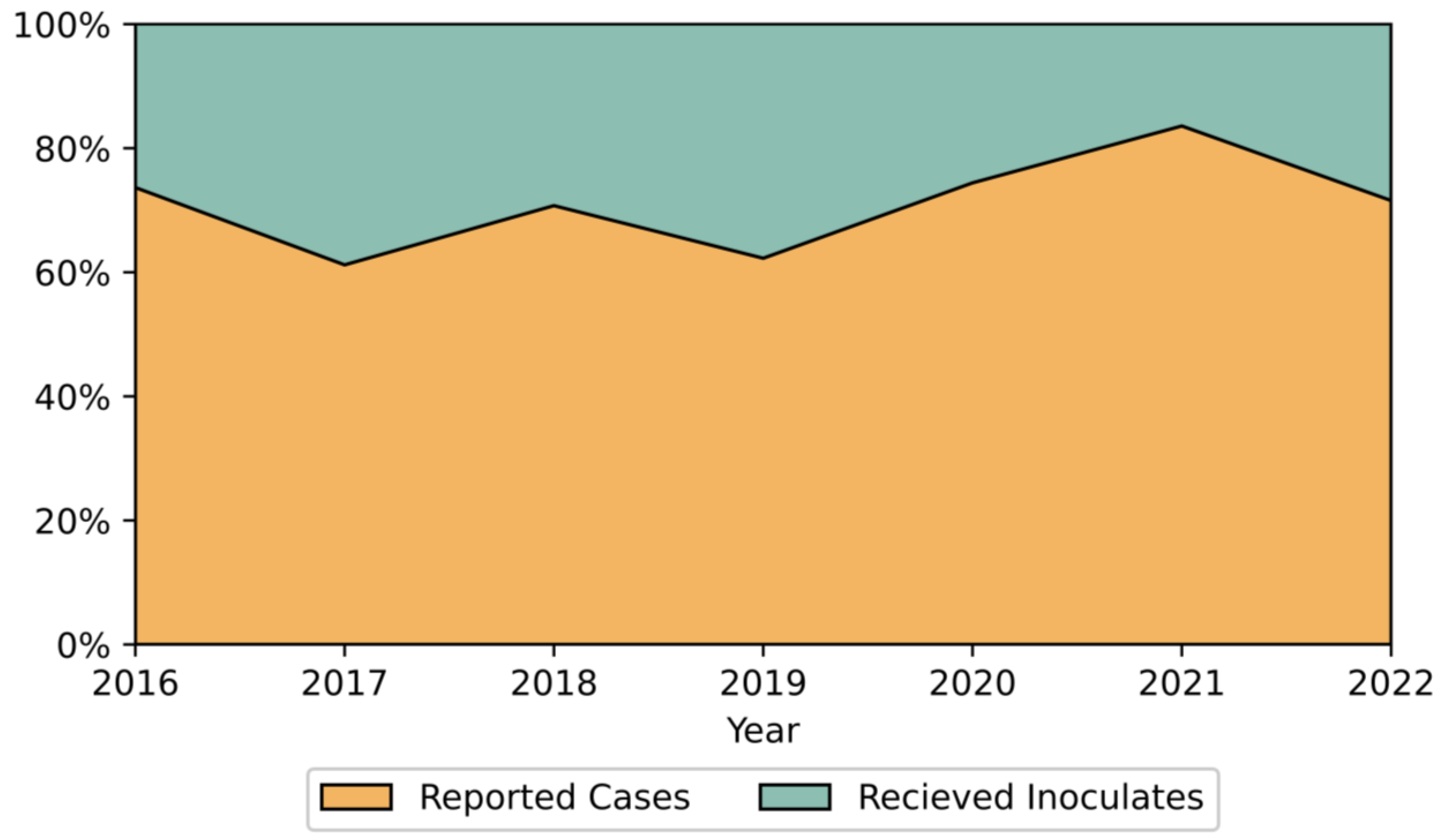
4.2. Surveillance Systems and Reported Trends
- 1.
- International travel-mediated pathogen importation;
- 2.
- Evolving sexual behaviors;
- 3.
- Reduced condom use, potentially linked to increased PrEP adoption;
- 4.
- Enhanced anonymous testing availability;
- 5.
- Establishment of dedicated sexual health clinics within HMOs.
4.3. Non-Endemic Status and AMR Implications
4.4. Antimicrobial Resistance Patterns
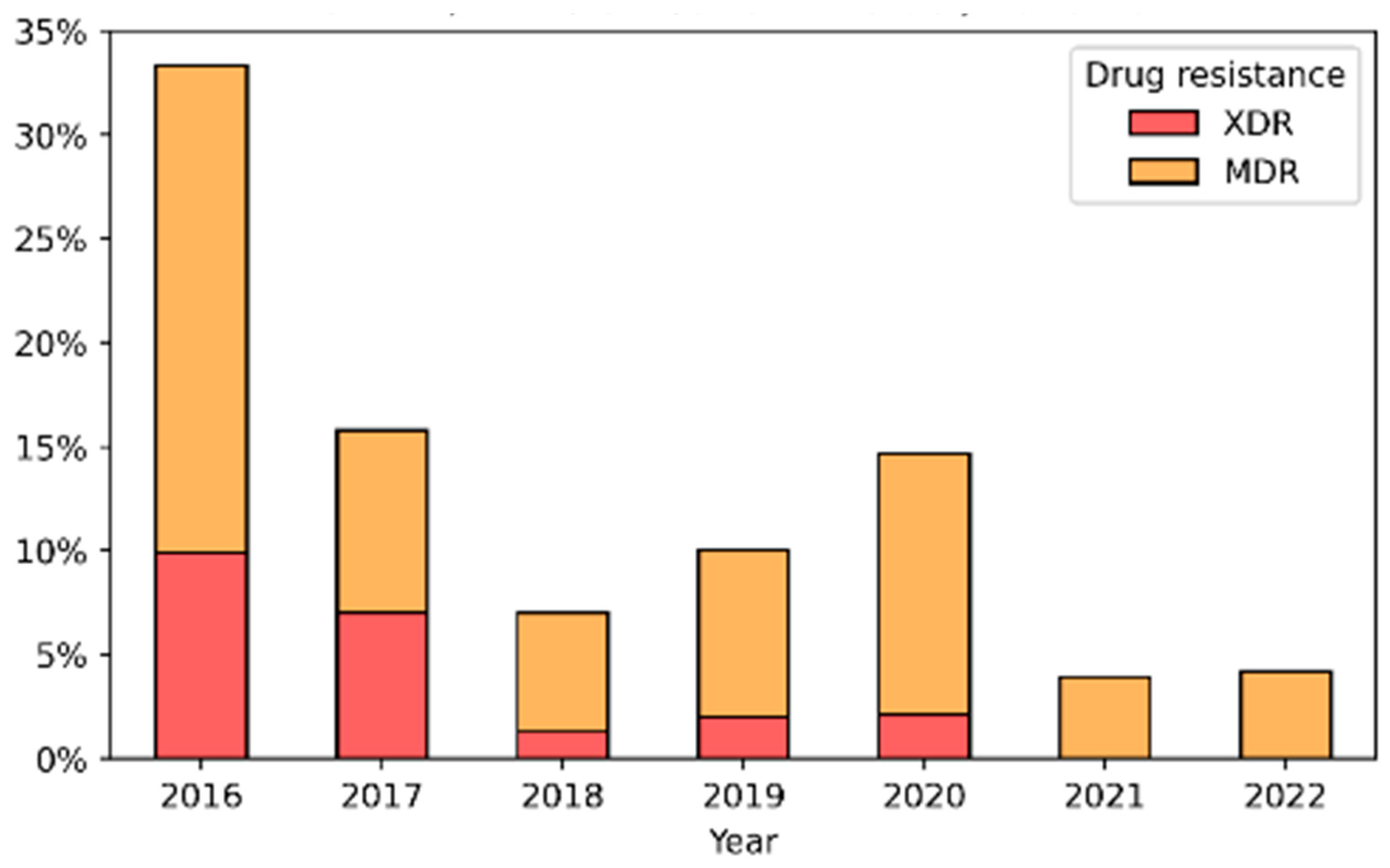
4.5. Multi-Drug Resistance Trends
4.6. Genetic Diversity and Sequence Types
4.7. Future Directions and Recommendations
5. Conclusions
6. Study Limitations and Strengths
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Gonococcal Antimicrobial Susceptibility Surveillance in Europe—Results Summary 2017; ECDC: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Euro-GASP-2017.pdf (accessed on 26 February 2025).
- Barry, P.; Klausner, J. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert. Opin. Pharmacother. 2009, 10, 555–577. [Google Scholar] [CrossRef]
- Ison, C.A.; Hussey, J.; Sankar, K.N.; Evans, J.; Alexander, S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 2011, 16, 19833. [Google Scholar] [CrossRef] [PubMed]
- Aniskevich, A.; Shimanskaya, I.; Boiko, I. Antimicrobial resistance in Neisseria gonorrhoeae isolates and gonorrhoea treatment in the Republic of Belarus, Eastern Europe, 2009–2019. BMC Infect. Dis. 2021, 21, 520. [Google Scholar] [CrossRef]
- Tapsall, J. Multidrug-resistant Neisseria gonorrhoeae. CMAJ 2009, 180, 268–269. [Google Scholar] [CrossRef]
- Antimicrobial resistance in Neisseria gonorrhoeae in New Zealand 2022 surveillance Report. In Report; The Institute of Environmental Science and Research Ltd.: Wellington, New Zealand, 2023; Available online: https://www.esr.cri.nz/media/jt2f3hzt/esr-antimicrobial-resistance-in-neisseria-gonorrhoeae-surveillance-report-2022.pdf (accessed on 26 February 2025).
- Hooshiar, M.H.; Sholeh, M.; Beig, M.; Azizian, K.; Kouhsari, E. Global trends of antimicrobial resistance rates in Neisseria gonorrhoeae: A systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1284665. [Google Scholar] [CrossRef]
- Schaeffer, J.; Lippert, K.; Pleininger, S.; Stöger, A.; Hasenberger, P.; Stadlbauer, S.; Heger, F.; Eigentler, A.; Geusau, A.; Indra, A.; et al. Association of Phylogenomic Relatedness among Neisseria gonorrhoeae Strains with Antimicrobial Resistance. Emerg. Infect. Dis. 2016, 28, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Nicholas, R.A. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012, 7, 1401–1422. [Google Scholar] [CrossRef]
- Kakooza, F.; Kiggundu, R.; Mboowa, G.; Kateete, P.D.; Nsangi, O.T.; Kabahita, J.M.; Ssentalo Bagaya, B.; Golparian, D.; Unemo, M. Antimicrobial susceptibility surveillance and antimicrobial resistance in Neisseria gonorrhoeae in Africa from 2001 to 2020: A mini-review. Front. Microbiol. 2023, 14, 2023. [Google Scholar] [CrossRef]
- Martin, I.M.; Ison, C.A.; Aanensen, D.M.; Fenton, K.A.; Spratt, B.G. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 2004, 189, 1497–1505. [Google Scholar] [CrossRef]
- Hadad, R.; Golparian, D.; Velicko, I.; Ohlsson, A.K.; Lindroth, Y.; Ericson, E.L.; Fredlund, H.; Engstrand, L.; Unemo, M. First National Genomic Epidemiological Study of Neisseria gonorrhoeae Strains Spreading Across Sweden in 2016. Front. Microbiol. 2022, 12, 820998. [Google Scholar] [CrossRef]
- Agbodzi, B.; Duodu, S.; Dela, H.; Kumordjie, S.; Yeboah, C.; Behene, E.; Ocansey, K.; Yanney, J.N.; Boateng-Sarfo, G.; Kwofie, S.K.; et al. Whole genome analysis and antimicrobial resistance of Neisseria gonorrhoeae isolates from Ghana. Front. Microbiol. 2023, 14, 1163450. [Google Scholar] [CrossRef]
- Mitchev, N.; Singh, R.; Allam, M.; Kwenda, S.; Ismail, A.; Garrett, N.; Ramsuran, V.; Niehaus, A.J.; Mlisana, K.P. Antimicrobial Resistance Mechanisms, Multilocus Sequence Typing, and NG-STAR Sequence Types of Diverse Neisseria gonorrhoeae Isolates in KwaZulu-Natal, South Africa. Antimicrob. Agents Chemother. 2021, 65, e0075921. [Google Scholar] [CrossRef]
- Unemo, M.S.W. Antimicrobial Resistance Expressed by Neisseria gonorrhoeae: A Major Global Public Health Problem in the 21st Century. Microbiol. Spectr. 2016, 4, 213–237. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Molecular Typing of Neisseria Gonorrhoeae—Results from a Pilot Study 2010–2011; European Centre for Disease Prevention and Control: Solna, Sweden, 2012. [Google Scholar] [CrossRef]
- Zimbro, M.J. Difco & BBL Manual: Manual of Microbiological Culture Media; BD Diagnostic: Franklin Lakes, NJ, USA, 2013. [Google Scholar]
- Carroll, K.C. Manual of Clinical Microbiology, 12th ed.; ASM Press: Washington, DC, USA, 2019. [Google Scholar]
- Protect Microorganism Preservation System Store It, Preserve It, Protect It. Available online: http://www.tscswabs.co.uk/uploads/images/PDF-Downloads/TSC_ProtectFlyerV5.pdf (accessed on 26 February 2025).
- Ng, L.K.; Martin, I.E. The laboratory diagnosis of Neisseria gonorrhoeae. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 15–25. [Google Scholar] [CrossRef]
- ETEST, Trusted Leader in MIC Gradient Strips Technology. Available online: https://www.biomerieux.com/content/dam/biomerieux-com/03----our-offer/clinical/in-hospital--in-lab/products/etest%C2%AE-telavancin/documents/prn_056750_rev_03.a_etest_brochure_final_art_2.pdf.coredownload.pdf (accessed on 26 February 2025).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 26 February 2025).
- National Surveillance of Antimicrobial Susceptibilities of Neisseria Gonorrhoeae Annual Summary 2019. Government of Canada. Published Online 2021. Available online: https://www.canada.ca/en/services/health/publications/drugs-health-products/national-surveillance-antimicrobial-susceptibilities-neisseria-gonorrhoeae-annual-summary-2019.html (accessed on 26 February 2025).
- Nextera® DNA Library Prep Reference Guide. Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_nextera/nexteradna/nextera-dna-library-prep-reference-guide-15027987-01.pdf (accessed on 26 February 2025).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Published Online 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 26 February 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Chemtob, D. A national strategic plan for reducing the burden of sexually transmitted infections in Israel by the year 2025. Isr. J. Health Policy Res. 2017, 6, 23. [Google Scholar] [CrossRef]
- Green, M.; Anis, E.; Gandacu, D.; Grotto, I. The fall and rise of gonorrhoea incidence in Israel: An international phenomenon? Sex. Transm. Infect. 2003, 79, 116–118. [Google Scholar] [CrossRef]
- Kridin, K.; Ingram, B.; Becker, D.; Shiloah, N.; Azrad, M.; Habib, S.; Peretz, A. Sexually Transmitted Diseases in Northern Israel: Insights From a Large Referral Laboratory. J. Low. Genit. Tract Dis. 2023, 27, 51–55. [Google Scholar]
- Dan, M.; Mor, Z.; Gottliev, S.; Sheinberg, B.; Shohat, T. Trends in Antimicrobial Susceptibility of Neisseria gonorrhoeae in Israel, 2002 to 2007, With Special Reference to Fluoroquinolone Resistance. Sex. Transm. Dis. 2010, 37, 451–453. [Google Scholar] [PubMed]
- Walker, C.K.; Sweet, R.L. Gonorrhea infection in women: Prevalence, effects, screening, and management. Int. J. Women’s Health 2011, 3, 197–206. [Google Scholar] [CrossRef]
- Martín-Sánchez, M. Clinical presentation of asymptomatic and symptomatic women who tested positive for genital gonorrhoea at a sexual health service in Melbourne, Australia. Epidemiol. Infect. 2020, 148, 240. [Google Scholar] [CrossRef]
- Levi, I.; Mor, Z.; Anis, E.; Maayan, S.; Leshem, E.; Pollack, S.; Chowers, M.; Mor, O.; Riesenberg, K.; Sthoeger, Z.; et al. Men Who Have Sex With Men, risk behavior and HIV infection: Integrative analysis of clinical, epidemiological and laboratory databases. Clin. Infect. Dis. 2011, 51, 1363–1370. [Google Scholar] [CrossRef]
- Meyer, T.; Buder, S. The Laboratory Diagnosis of Neisseria gonorrhoeae: Current Testing and Future Demands. Pathogens 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.G. Clinical Manifestations and Diagnosis of Neisseria Gonorrhoeae Infection in Adults and Adolescents. Available online: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-neisseria-gonorrhoeae-infection-in-adults-and-adolescents (accessed on 26 February 2025).
- WHO. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021. Available online: https://iris.who.int/bitstream/handle/10665/246296/WHO-RHR-16.09-eng.pdf?sequence=1 (accessed on 26 February 2025).
- Chidiac, O.; AlMukdad, S.; Harfouche, M.; Harding-Esch, E.; Abu-Raddad, L.J. Epidemiology of gonorrhoea: Systematic review, meta-analyses, and meta-regressions, World Health Organization European Region, 1949 to 2021. Euro Surveill. 2024, 29, 2300226. [Google Scholar] [CrossRef]
- Clifton, S.; Bolt, H.; Mohammed, H.; Town, K.; Furegato, M.; Cole, M.; Campbell, O.; Fifer, H.; Hughes, G. Prevalence of and factors associated with MDR Neisseria gonorrhoeae in England and Wales between 2004 and 2015: Analysis of annual cross-sectional surveillance surveys. J. Antimicrob. Chemother. 2018, 73, 923–932. [Google Scholar] [CrossRef]
- Harris, S.R.; Cole, M.J.; Spiteri, G.; Sánchez-Busó, L.; Golparian, D.; Jacobsson, S.; Goater, R.; Abudahab, K.; Yeats, C.A.; Bercot, B.; et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: A genomic survey. Lancet Infect. Dis. 2018, 18, 758–768. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of gonorrhoea: A global perspective. Sex. Health 2019, 16, 401–411. [Google Scholar] [CrossRef]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.A.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 17, e1002344. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.A.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex. Health 2019, 16, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; Chen, M.Y. Emerging and Reemerging Sexually Transmitted Infections. N. Engl. J. Med. 2020, 382, 2023–2032. [Google Scholar] [CrossRef]
- Cole. The European gonococcal antimicrobial surveillance program (Euro-GASP) appropriately reflects the antimicrobial resistance situation for Neisseria gonorrhoeae in the European Union/ European Economic Area. BMC Infect. Dis. 2019, 19, 1040. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae. Published Online 2012. Available online: https://iris.who.int/bitstream/handle/10665/44863/9789241503501_eng.pdf?sequence=1&isAllowed=y (accessed on 26 February 2025).
- World Health Organization. Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP): General Protocol. Published Online 2021. Available online: https://iris.who.int/bitstream/handle/10665/341333/9789240021341-eng.pdf?sequence=1&isAllowed=y (accessed on 26 February 2025).
- Kirkcaldy, R.D.; Kidd, S.; Weinstock, H.S.; Papp, J.R.; Bolan, G.A. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: The Gonococcal Isolate Surveillance Project (GISP), January 2006–June 2012. Sex. Transm. Infect. 2013, 89, 5–10. [Google Scholar] [CrossRef]
- Lahra, M.M.; Hogan, T.R.; Shoushtari, M.; Armstrong, B.H. Australian Gonococcal Surveillance Programme Annual Report, 2020. Commun. Intell. 2018, 45, 20220121566. [Google Scholar] [CrossRef]
- García, U.I. Epidemiological surveillance study of gonococcal infection in Northern Spain. Enferm. Infecc. Microbiol. Clın. 2020, 38, 59–64. [Google Scholar] [CrossRef]
- Murray-Watson, R.E.; Grad, Y.H.; St Cyr, S.B.; Yaesoubi, R. Personalizing the empiric treatment of gonorrhea using machine learning models. PLoS Digit. Health 2024, 3, e0000549. [Google Scholar] [CrossRef]
- Buonsenso, D.; Sodero, G.; Mariani, F.; Lazzareschi, I.; Proli, F.; Zampino, G.; Pierantoni, L.; Valentini, P.; Rendeli, C. Comparison between short therapy and standard therapy in pediatric patients hospitalized with urinary tract infection: A single center retrospective analysis. Children 2022, 9, 1647. [Google Scholar] [CrossRef]
- Martin, I.; Sawatzky, P.; Allen, V. Multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae in Canada, 2012–2016. Can Commun Rep. 2019, 45, 45–53. [Google Scholar] [CrossRef]
- Assessment Rapidrisk. European Centre for Disease Prevention and Control. Extensively Drug-Resistant (XDR) Neisseria Gonorrhoeae in the United Kingdom and Australia—7 May 2018; European Centre for Disease Prevention and Control: Solna, Sweden, 2018. [Google Scholar]
- Maubaret, C.; Caméléna, F.; Mrimèche, M.; Braille, A.; Liberge, M.; Mainardis, M.; Guillaume, C.; Noel, F.; Bébéar, C.; Molina, J.M.; et al. Two cases of extensively drug-resistant (XDR) Neisseria gonorrhoeae infection combining ceftriaxone-resistance and high-level azithromycin resistance. Eurosurveillance 2022, 28, 2300456. [Google Scholar] [CrossRef]
- Młynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Młynarczyk, G.; Majewski, S. Multiresistant Neisseria gonorrhoeae: A new threat in second decade of the XXI century. Med. Microbiol. Immunol. 2020, 209, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kandinov, I.; Dementieva, E.; Kravtsov, D.; Chestkov, A.; Kubanov, A.; Solomka, V.; Deryabin, D.; Gryadunov, D.; Shaskolskiy, B. Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types. Pathogens 2020, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Gonococcal Isolate Surveillance Project (GISP) and Enhanced GISP (eGISP), 2021. Published Online 2021. Available online: https://www.federalregister.gov/documents/2021/03/08/2021-04671/proposed-data-collection-submitted-for-public-comment-and-recommendations (accessed on 26 February 2025).
- Kirkcaldy, R.D.; Harvey, A.; Papp, J. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill Summ. 2016, 65, 1–19. [Google Scholar] [CrossRef]
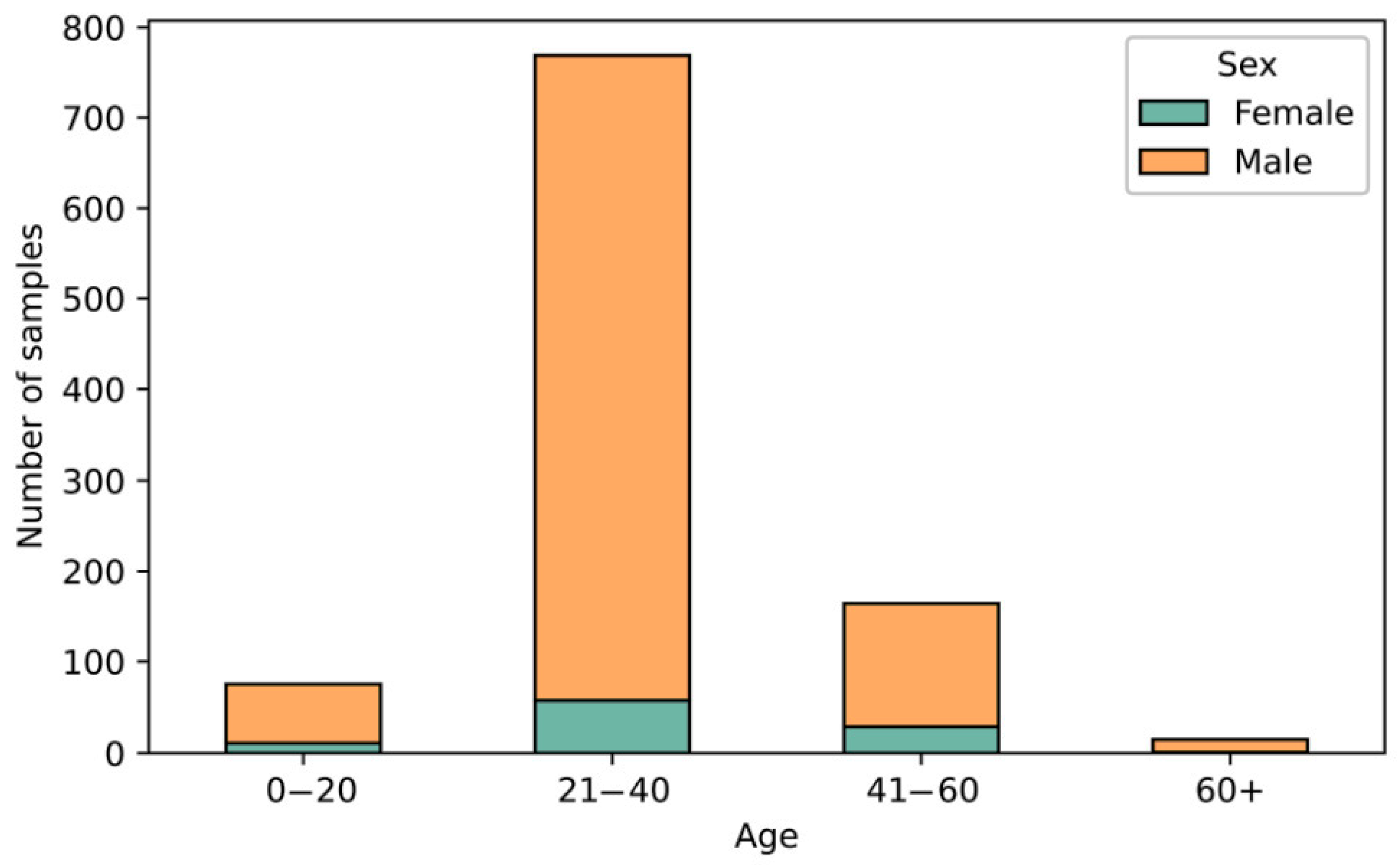
| Year | Number of Isolates | Ratio—Male to Female | Median Age—Female/Male |
|---|---|---|---|
| 2016 | 111 | 14.7 | 27/29 |
| 2017 | 228 | 6.8 | 31/30 |
| 2018 | 157 | 5.8 | 31/29 |
| 2019 | 200 | 14.6 | 41/31 |
| 2020 | 143 | 16.1 | 46/29 |
| 2021 | 152 | 8.9 | 33/28.5 |
| 2022 | 214 | 15.3 | 30/32 |
| All years | 1205 | 10 * | 33 */30 * |
| 12.3 ** | 32/30 ** |
| Year | Number of Isolates | Resistance * | ||||||
|---|---|---|---|---|---|---|---|---|
| CIX | AZI | TET | BEN | SPE | CIP | CTR | ||
| 2016 | 111 | 20 (18.0%) | 56 (50.5%) | 50 (46.3%) | 37 (33.3%) | 0 | 79 (71.2%) | 0 |
| 2017 | 228 | 38 (16.7%) | 76 (33.3%) | 58 (25.4%) | 54 (23.7%) | 0 | 105 (46.1%) | 0 |
| 2018 | 157 | 18 (11.5%) | 52 (33.1%) | 36 (22.9%) | 17 (10.8%) | 0 | 62 (39.5%) | 0 |
| 2019 | 200 | 14 (7.0%) | 75 (37.5%) | 56 (28.0%) | 35 (17.5%) | 0 | 109 (54.5%) | 0 |
| 2020 | 143 | 11 (7.7%) | 64 (44.8%) | 43 (30.1%) | 19 (13.3%) | 0 | 105 (73.4%) | 0 |
| 2021 | 152 | 2 (1.3%) | 68 (44.7%) | 36 (23.7%) | 11 (7.2%) | 0 | 85 (55.9%) | 0 |
| 2022 | 214 | 2 (0.9%) | 106 (49.5%) | 53 (24.8%) | 18 (8.4%) | 0 | 111 (51.9%) | 0 |
| Total (Ave% ± SD) | 1205 | 105 (8.7% ± 6.3) | 497 (41.2% ± 7.1) | 332 (27.6% ± 8.7) | 191 (15.9% ± 9.6) | 0 | 656 (54.4% ± 13.4) | 0 |
| Year | Number of Isolates | Number of NG-MAST STs | Most Common NG-MAST STs (Number of Isolates) * |
|---|---|---|---|
| 2017 | 78 | 72 | ST4269 (13), ST2318 (7), ST2997 (7), ST5441 (6), ST5049 (5), ST292 (4), ST5624 (4) |
| 2018 | 77 | 72 | ST292 (12), ST5441 (6), ST16169 (6), ST2992 (5), ST11547 (5), ST11461 (4) |
| 2022 | 124 | 72 | ST19665 (31), ST11461 (25), 14994 (10), 19762 (10), 17972 (8), 14764 (5) |
| Total | 279 | 72 | ST19665 (31), ST11461 (30), ST4269 (16), ST292 (16), ST14994 (14), ST5441 (13), ST19762 (10), ST2318 (9), ST17972 (8), ST2992 (7), ST11547 (7), ST2997 (7), ST16169 (6), ST5049 (5), ST9208 (5), ST14764 (5), ST3935 (4), ST14760 (4), ST14051 (4), ST5624 (4), ST12302 (4) |
| NG-MAST | n * | MDR,% | XDR,% | CIP ** | BEN ** | TET ** | AZI ** | CIX ** |
|---|---|---|---|---|---|---|---|---|
| ST19665 | 31 | 0 | 0 | S(96.8%) R(3.2%) | I | S(3.2%) I(93.5%) R(3.2%) | S(9.7%) R(90.3%) | S(96.8%) R(3.2%) |
| ST11461 | 30 | 0 | 0 | S(83.3%) R(16.7%) | I | S(3.3%) I(13.3%) R(83.3%) | S(86.7%) R(13.3%) | S |
| ST292 | 16 | 0 | 0 | S | I | S(25.0%) I(75.0%) | S(87.5%) I(6.2%) R(6.2%) | S |
| ST4269 | 16 | 29.4 | 64.7 | R | I(17.6%) R(82.4%) | I(5.9%) R(94.1%) | I(5.9%) R(94.1%) | S(29.4%) R(70.6%) |
| ST14994 | 14 | 0 | 0 | R | I(92.9%) R(7.1%) | S(50.0%) I(50.0%) | S(85.7%) I(7.1%) R(7.1%) | S |
| ST5441 | 13 | 0 | 0 | S | S(7.7%) I(92.3%) | S | S(30.8%) I(30.8%) R(38.5%) | S |
| ST19762 | 10 | 10 | 0 | R | I | S(10.0%) I(80.0%) R(10.0%) | S(20.0%) R(80.0%) | S |
| ST2318 | 9 | 45.5 | 9.1 | S(9.1%) R(90.9%) | I(81.8%) R(18.2%) | I(18.2%) R(81.8%) | S(27.3%) R(72.7%) | S(90.9%) R(9.1%) |
| ST17972 | 8 | 12.5 | 0 | R | I | I(87.5%) R(12.5%) | S(25.0%) R(75.0%) | S |
| ST2997 | 7 | 0 | 0 | S | I | I(71.4%) R(28.6%) | I(14.3%) R(85.7%) | S |
| ST2992 | 7 | 0 | 0 | S(85.7%) I(14.3%) | I | S(14.3%) I(85.7%) | I(28.6%) R(71.4%) | S |
| ST11547 | 7 | 14.3 | 0 | R | I(85.7%) R(14.3%) | S(28.6%) I(71.4%) | S(71.4%) I(14.3%) R(14.3%) | S(14.3%) R(85.7%) |
| ST16169 | 6 | 0 | 0 | R | I | S(33.3%) I(66.7%) | S(66.7%) R(33.3%) | S |
| ST9208 | 5 | 0 | 0 | S | I | S(40.0%) I(60.0%) | S(40.0%) I(20.0%) R(40.0%) | S |
| ST5049 | 5 | 0 | 0 | S | I | I(80.0%) R(20.0%) | R | S |
| ST14764 | 5 | 0 | 0 | R | I | I | R | S |
| ST14760 | 4 | 0 | 0 | S | S(25.0%) I(50.0%) R(25.0%) | S(50.0%) I(50.0%) | S | S |
| ST12302 | 4 | 50 | 0 | I(25.0%) R(75.0%) | I | I(50.0%) R(50.0%) | R | S |
| ST14051 | 4 | 50 | 0 | R | I | I(25.0%) R(75.0%) | S(50.0%) R(50.0%) | S |
| ST5624 | 4 | 50 | 0 | R | I(50.0%) R(50.0%) | I | R | S |
| ST3935 | 4 | 0 | 0 | S | I | I | R | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dveyrin, Z.; Alon, T.; Makhon, A.; Nissan, I.; Mor, Z.; Rorman, E. Neisseria gonorrhoeae—Susceptibility Trends and Basic Molecular Mapping of Isolates Collected in Israel in 2016–2022. Microorganisms 2025, 13, 750. https://doi.org/10.3390/microorganisms13040750
Dveyrin Z, Alon T, Makhon A, Nissan I, Mor Z, Rorman E. Neisseria gonorrhoeae—Susceptibility Trends and Basic Molecular Mapping of Isolates Collected in Israel in 2016–2022. Microorganisms. 2025; 13(4):750. https://doi.org/10.3390/microorganisms13040750
Chicago/Turabian StyleDveyrin, Zeev, Tal Alon, Andrei Makhon, Israel Nissan, Zohar Mor, and Efrat Rorman. 2025. "Neisseria gonorrhoeae—Susceptibility Trends and Basic Molecular Mapping of Isolates Collected in Israel in 2016–2022" Microorganisms 13, no. 4: 750. https://doi.org/10.3390/microorganisms13040750
APA StyleDveyrin, Z., Alon, T., Makhon, A., Nissan, I., Mor, Z., & Rorman, E. (2025). Neisseria gonorrhoeae—Susceptibility Trends and Basic Molecular Mapping of Isolates Collected in Israel in 2016–2022. Microorganisms, 13(4), 750. https://doi.org/10.3390/microorganisms13040750






