Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
2.2. Random Amplification of Polymorphic DNA (RAPD)–PCR Analysis
2.3. Unweighted Pair–Group Method with Arithmetic Mean (UPGMA) Analysis
2.4. Growth Curve Analysis
2.5. Biofilm-Growth Evaluation
2.6. Invasion of A549 Cells
2.7. Fluorescence Microscopy
2.8. Motility Assays
2.9. Determining the Tobramycin Susceptibility of the Strains
2.10. Phenotypic Tests on the Effects of Sub-MICs of Tobramycin
2.11. Viability of Bacteria Cultivated in the Presence of Quarter or Half MIC of Tobramycin
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis
3. Results and Discussion
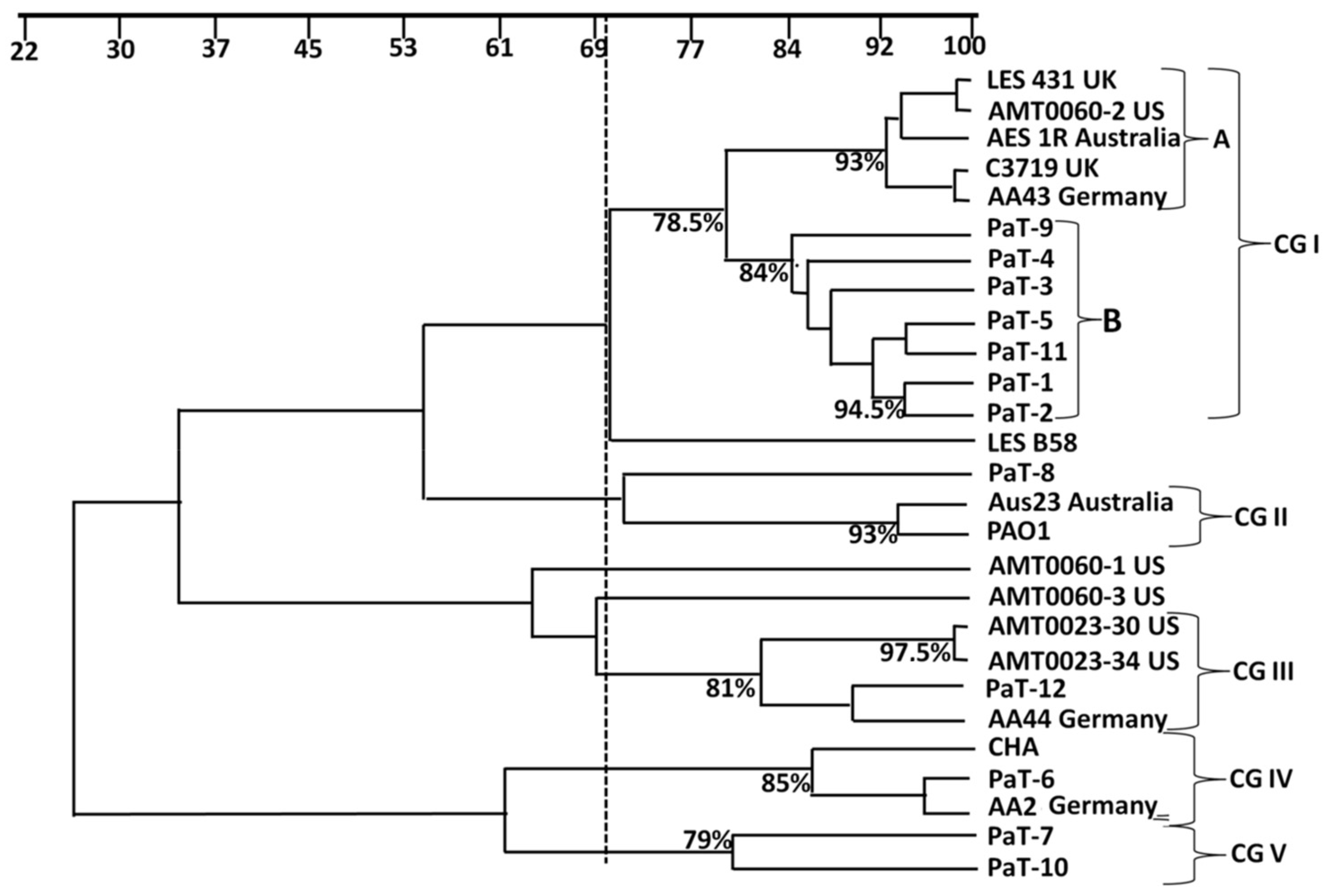
3.1. Molecular Epidemiological Typing by RAPD-PCR
3.2. Growth Parameters
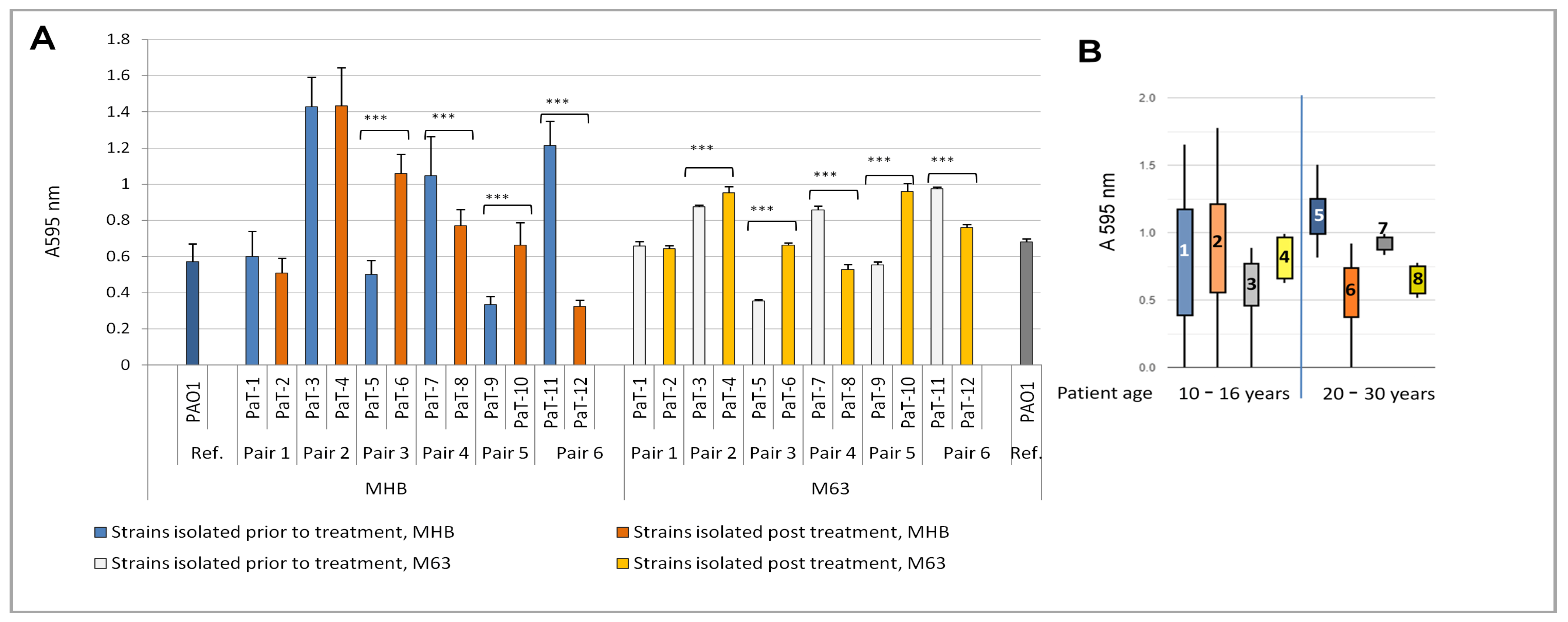
3.3. Biofilm Growth
3.4. Invasion and Intracellular Reproduction of the Bacteria in Cultured A549 Cells
3.5. Motility
3.6. Tobramycin Susceptibility of the Paired Strains
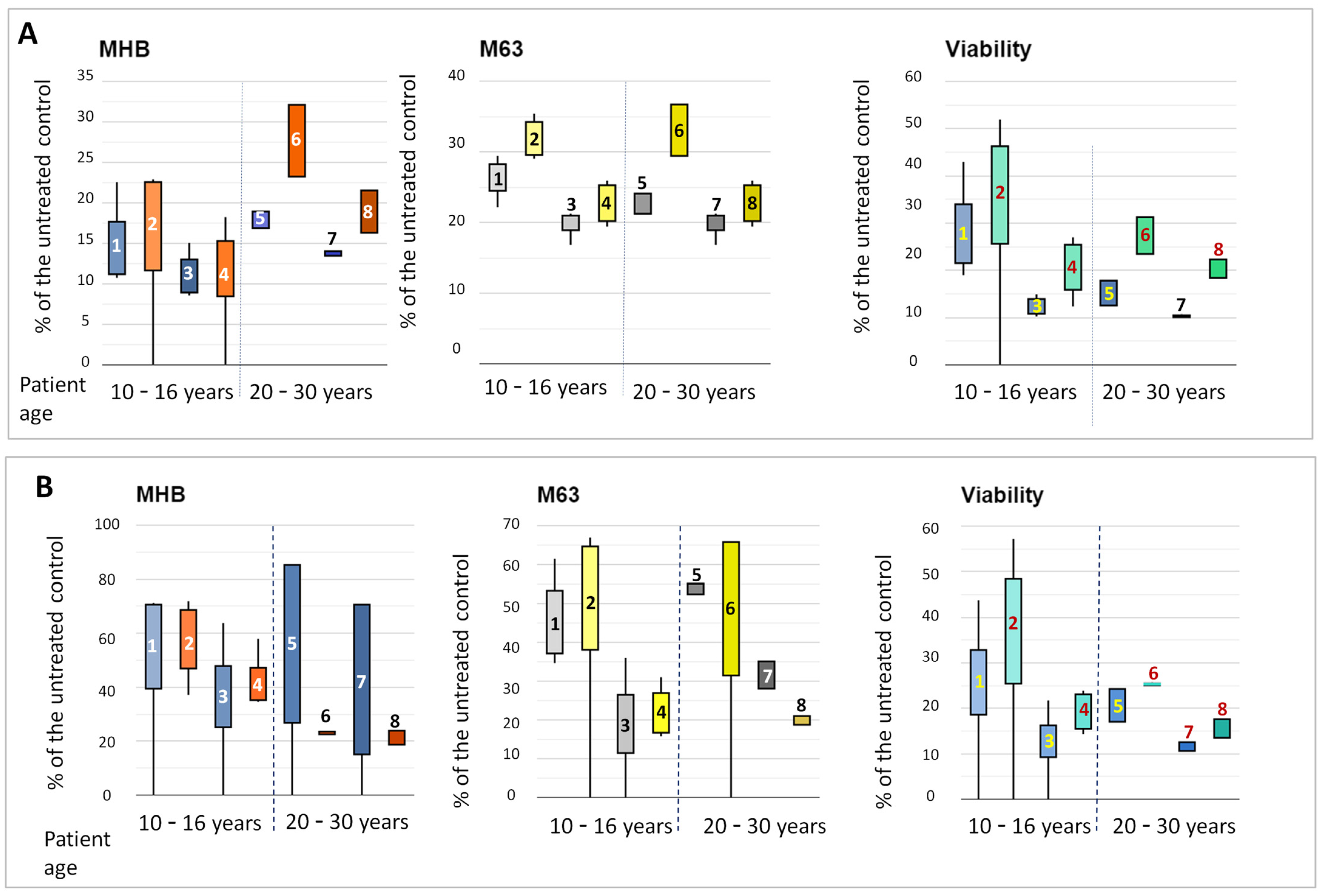
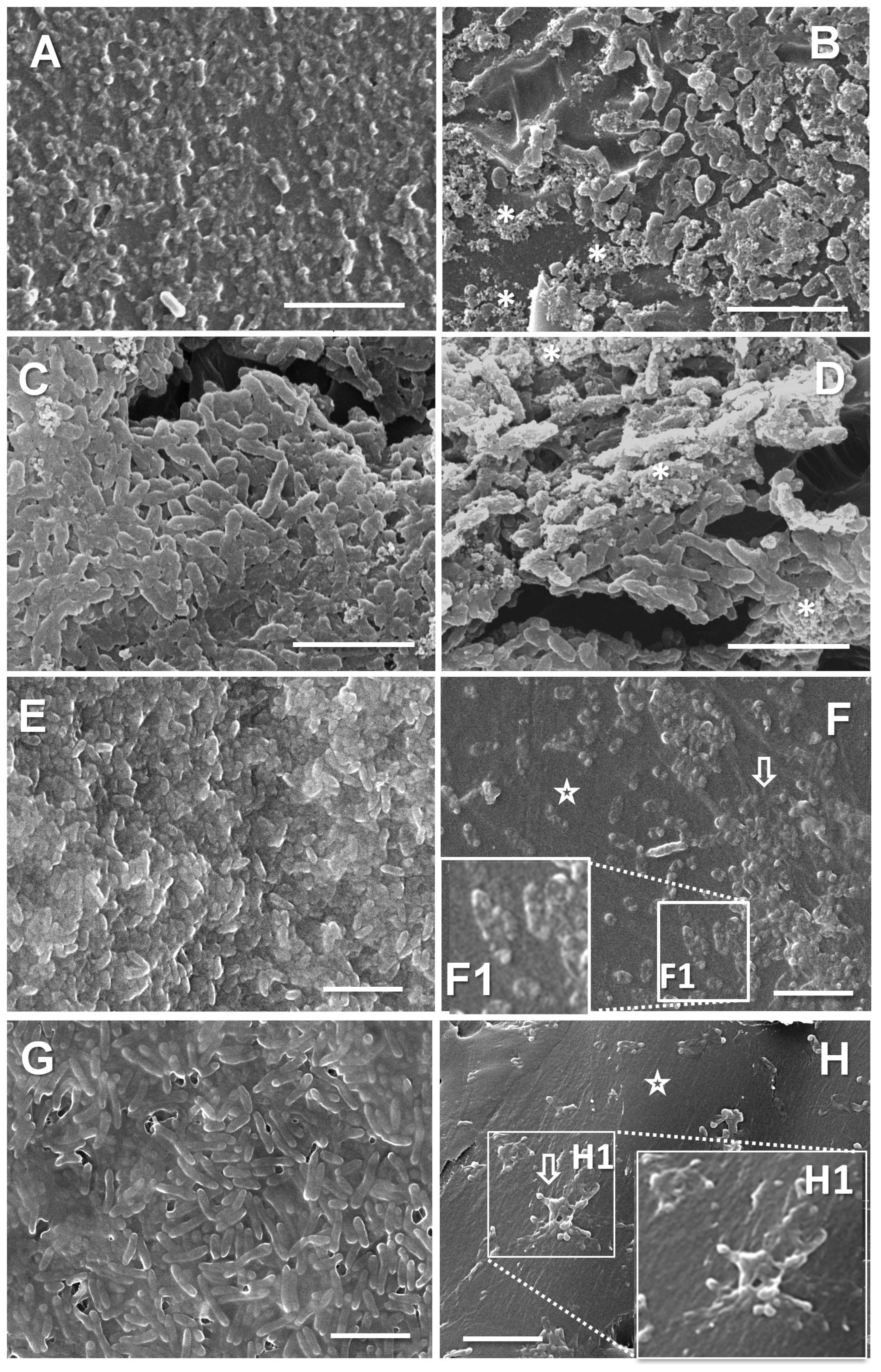
3.7. Phenotypic Shifts in the Strains in the Presence of Sub-MICs of Tobramycin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, C.A.; Winstanley, C. Persistent and Aggressive Bacteria in the Lungs of Cystic Fibrosis Children. Br. Med. Bull. 2002, 61, 81–96. [Google Scholar] [CrossRef]
- Ciofu, O.; Mandsberg, L.F.; Bjarnsholt, T.; Wassermann, T.; Høiby, N. Genetic Adaptation of Pseudomonas aeruginosa during Chronic Lung Infection of Patients with Cystic Fibrosis: Strong and Weak Mutators with Heterogeneous Genetic Backgrounds Emerge in mucA and/or lasR Mutants. Microbiology 2010, 156, 1108–1119. [Google Scholar] [CrossRef]
- Cramer, N.; Klockgether, J.; Tümmler, B. Microevolution of Pseudomonas aeruginosa in the Airways of People with Cystic Fibrosis. Curr. Opin. Immunol. 2023, 83, 102328. [Google Scholar] [CrossRef]
- Hansen, C.M.E.; Breukelman, A.J.; Van Den Bemt, P.M.L.A.; Zwitserloot, A.M.; Van Dijk, L.; Van Boven, J.F.M. Medication Adherence to CFTR Modulators in Patients with Cystic Fibrosis: A Systematic Review. Eur. Respir. Rev. 2024, 33, 240060. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Manzanares, D.; Li, Y.; Wang, X.; Li, Z.; Terreau, S.; He, Z.; Lyu, J.; Wang, W.; Lara-Sáez, I. Highly Branched Poly β-Amino Ester/CpG-Depleted CFTR Plasmid Nanoparticles for Non-Viral Gene Therapy in Lung Cystic Fibrosis Disease. Mol. Ther.—Methods Clin. Dev. 2024, 32, 101292. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yeoh, E.; Fitzgerald, D.A.; Selvadurai, H. A Systematic Review on the Use of Bacteriophage in Treating Staphylococcus aureus and Pseudomonas aeruginosa Infections in Cystic Fibrosis. Paediatr. Respir. Rev. 2023, 48, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cocorullo, M.; Stelitano, G.; Chiarelli, L.R. Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 8321. [Google Scholar] [CrossRef]
- Hahn, A.; Sami, I.; Chaney, H.; Koumbourlis, A.C.; Del Valle Mojica, C.; Cochrane, C.; Chan, B.K.; Koff, J.L. Bacteriophage Therapy for Pan-Drug-Resistant Pseudomonas aeruginosa in Two Persons With Cystic Fibrosis. J. Investig. Med. High Impact Case Rep. 2023, 11, 23247096231188243. [Google Scholar] [CrossRef]
- Alipour-Khezri, E.; Skurnik, M.; Zarrini, G. Pseudomonas aeruginosa Bacteriophages and Their Clinical Applications. Viruses 2024, 16, 1051. [Google Scholar] [CrossRef]
- Li, D.; Schneider-Futschik, E.K. Current and Emerging Inhaled Antibiotics for Chronic Pulmonary Pseudomonas aeruginosa and Staphylococcus aureus Infections in Cystic Fibrosis. Antibiotics 2023, 12, 484. [Google Scholar] [CrossRef]
- Jones, A.; Beisty, J.; McKenna, D.; Clough, D.; Webb, K.; Morris, J.; Keevil, B. Monitoring of Tobramycin Levels in Patients with Cystic Fibrosis by Finger-Prick Sampling. Eur. Respir. J. 2012, 39, 1537–1538. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Taccetti, G.; Burgel, P.-R.; Mulrennan, S. Tobramycin Safety and Efficacy Review Article. Respir. Med. 2022, 195, 106778. [Google Scholar] [CrossRef]
- Islam, N.; Reid, D. Inhaled Antibiotics: A Promising Drug Delivery Strategies for Efficient Treatment of Lower Respiratory Tract Infections (LRTIs) Associated with Antibiotic Resistant Biofilm-Dwelling and Intracellular Bacterial Pathogens. Respir. Med. 2024, 227, 107661. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.; Fischer, S.; Hedtfeld, S.; Dorda, M.; Tümmler, B. Intraclonal Competitive Fitness of Longitudinal Cystic Fibrosis Pseudomonas aeruginosa Airway Isolates in Liquid Cultures. Environ. Microbiol. 2020, 22, 2536–2549. [Google Scholar] [CrossRef]
- Pelegrin, A.C.; Palmieri, M.; Mirande, C.; Oliver, A.; Moons, P.; Goossens, H.; van Belkum, A. Pseudomonas aeruginosa: A Clinical and Genomics Update. FEMS Microbiol. Rev. 2021, 45, fuab026. [Google Scholar] [CrossRef]
- Espaillat, A.; Colque, C.A.; Rago, D.; La Rosa, R.; Molin, S.; Johansen, H.K. Adaptive Evolution of Pseudomonas aeruginosa in Human Airways Shows Phenotypic Convergence Despite Diverse Patterns of Genomic Changes. Mol. Biol. Evol. 2024, 41, msae022. [Google Scholar] [CrossRef]
- Drevinek, P.; Canton, R.; Johansen, H.K.; Hoffman, L.; Coenye, T.; Burgel, P.-R.; Davies, J.C. New Concepts in Antimicrobial Resistance in Cystic Fibrosis Respiratory Infections. J. Cyst. Fibros. 2022, 21, 937–945. [Google Scholar] [CrossRef]
- Alcala-Franco, B.; Montanari, S.; Cigana, C.; Bertoni, G.; Oliver, A.; Bragonzi, A. Antibiotic Pressure Compensates the Biological Cost Associated with Pseudomonas aeruginosa Hypermutable Phenotypes in Vitro and in a Murine Model of Chronic Airways Infection. J. Antimicrob. Chemother. 2012, 67, 962–969. [Google Scholar] [CrossRef]
- Van Den Bossche, S.; De Broe, E.; Coenye, T.; Van Braeckel, E.; Crabbé, A. The Cystic Fibrosis Lung Microenvironment Alters Antibiotic Activity: Causes and Effects. Eur. Respir. Rev. 2021, 30, 210055. [Google Scholar] [CrossRef]
- Planet, P.J. Adaptation and Evolution of Pathogens in the Cystic Fibrosis Lung. J. Pediatr. Infect. Dis. Soc. 2022, 11, S23–S31. [Google Scholar] [CrossRef]
- Fischer, S.; Klockgether, J.; Gonzalez Sorribes, M.; Dorda, M.; Wiehlmann, L.; Tümmler, B. Sequence Diversity of the Pseudomonas aeruginosa Population in Loci That Undergo Microevolution in Cystic Fibrosis Airways. Access Microbiol. 2021, 3, 000286. [Google Scholar] [CrossRef]
- Feliziani, S.; Marvig, R.L.; Luján, A.M.; Moyano, A.J.; Di Rienzo, J.A.; Krogh Johansen, H.; Molin, S.; Smania, A.M. Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable Pseudomonas aeruginosa in Long-Term Cystic Fibrosis Infections. PLoS Genet. 2014, 10, e1004651. [Google Scholar] [CrossRef]
- Geller, D.E. Aerosol Antibiotics in Cystic Fibrosis. Respir. Care 2009, 54, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Bartell, J.A.; Sommer, L.M.; Haagensen, J.A.J.; Loch, A.; Espinosa, R.; Molin, S.; Johansen, H.K. Evolutionary Highways to Persistent Bacterial Infection. Nat. Commun. 2019, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, J.L.; Mowat, E.; Ledson, M.J.; Walshaw, M.J.; Winstanley, C. Fluctuations in Phenotypes and Genotypes within Populations of Pseudomonas aeruginosa in the Cystic Fibrosis Lung during Pulmonary Exacerbations. J. Med. Microbiol. 2010, 59, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barat, L.; Ciofu, O.; Kragh, K.N.; Pressler, T.; Johansen, U.; Motos, A.; Torres, A.; Hoiby, N. Phenotypic Shift in Pseudomonas aeruginosa Populations from Cystic Fibrosis Lungs after 2-Week Antipseudomonal Treatment. J. Cyst. Fibros. 2017, 16, 222–229. [Google Scholar] [CrossRef]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.A.; et al. Developing an International Pseudomonas aeruginosa Reference Panel. MicrobiologyOpen 2013, 2, 1010–1023. [Google Scholar] [CrossRef]
- Clark, S.T.; Guttman, D.S.; Hwang, D.M. Diversification of Pseudomonas aeruginosa within the Cystic Fibrosis Lung and Its Effects on Antibiotic Resistance. FEMS Microbiol. Lett. 2018, 365, fny026. [Google Scholar] [CrossRef]
- Bos, A.C.; Passé, K.M.; Mouton, J.W.; Janssens, H.M.; Tiddens, H.A.W.M. The Fate of Inhaled Antibiotics after Deposition in Cystic Fibrosis: How to Get Drug to the Bug? J. Cyst. Fibros. 2017, 16, 13–23. [Google Scholar] [CrossRef]
- Davies, E.V.; James, C.E.; Brockhurst, M.A.; Winstanley, C. Evolutionary Diversification of Pseudomonas aeruginosa in an Artificial Sputum Model. BMC Microbiol. 2017, 17, 3. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Campbell, M.E.; Foster, J.; Lam, J.S.; Speert, D.P. Random Amplified Polymorphic DNA Typing of Pseudomonas aeruginosa Isolates Recovered from Patients with Cystic Fibrosis. J. Clin. Microbiol. 1996, 34, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.J.; Towner, K.J.; Dijkshoorn, L.; Gerner-Smidt, P.; Maher, M.; Seifert, H.; Vaneechoutte, M. Multicenter Study Using Standardized Protocols and Reagents for Evaluation of Reproducibility of PCR-Based Fingerprinting of Acinetobacter spp. J. Clin. Microbiol. 1997, 35, 3071–3077. [Google Scholar] [CrossRef]
- Cullen, L.; Weiser, R.; Olszak, T.; Maldonado, R.F.; Moreira, A.S.; Slachmuylders, L.; Brackman, G.; Paunova-Krasteva, T.S.; Zarnowiec, P.; Czerwonka, G.; et al. Phenotypic Characterization of an International Pseudomonas aeruginosa Reference Panel: Strains of Cystic Fibrosis (CF) Origin Show Less in Vivo Virulence than Non-CF Strains. Microbiology 2015, 161, 1961–1977. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Velkova, V.; Borisova, D.; Stoitsova, S. Intracellular Survival of Pseudomonas aeruginosa Pao1 in A549 Cells; l’Université de Sofia: Sofia, Bulgaria, 2016; Volume 104. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0. 2025. Available online: https://eucast.org (accessed on 20 January 2025).
- Paunova-Krasteva, T.; Haladjova, E.; Petrov, P.; Forys, A.; Trzebicka, B.; Topouzova-Hristova, T.; Stoitsova, S.R. Destruction of Pseudomonas aeruginosa Pre-Formed Biofilms by Cationic Polymer Micelles Bearing Silver Nanoparticles. Biofouling 2020, 36, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Andrighetto, C.; Zampese, L.; Lombardi, A. RAPD-PCR Characterization of Lactobacilli Isolated from Artisanal Meat Plants and Traditional Fermented Sausages of Veneto Region (Italy). Lett. Appl. Microbiol. 2001, 33, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Herbel, S.R.; Vahjen, W.; Wieler, L.H.; Guenther, S. Timely Approaches to Identify Probiotic Species of the Genus Lactobacillus. Gut Pathog. 2013, 5, 27. [Google Scholar] [CrossRef]
- Freschi, L.; Bertelli, C.; Jeukens, J.; Moore, M.P.; Kukavica-Ibrulj, I.; Emond-Rheault, J.-G.; Hamel, J.; Fothergill, J.L.; Tucker, N.P.; McClean, S.; et al. Genomic Characterisation of an International Pseudomonas aeruginosa Reference Panel Indicates That the Two Major Groups Draw upon Distinct Mobile Gene Pools. FEMS Microbiol. Lett. 2018, 365, fny120. [Google Scholar] [CrossRef]
- Hall, A.J.; Fothergill, J.L.; Kaye, S.B.; Neal, T.J.; McNamara, P.S.; Southern, K.W.; Winstanley, C. Intraclonal Genetic Diversity amongst Cystic Fibrosis and Keratitis Isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2013, 62, 208–216. [Google Scholar] [CrossRef][Green Version]
- Jarzynka, S.; Makarewicz, O.; Weiss, D.; Minkiewicz-Zochniak, A.; Iwańska, A.; Skorupa, W.; Padzik, M.; Augustynowicz-Kopeć, E.; Olędzka, G. The Impact of Pseudomonas aeruginosa Infection in Adult Cystic Fibrosis Patients—A Single Polish Centre Study. Pathogens 2023, 12, 1440. [Google Scholar] [CrossRef]
- Gutiérrez-Santana, J.C.; Gerónimo-Gallegos, A.; Martínez-Corona, M.B.; López-López, M.; Toscano-Garibay, J.D.; Cuevas-Schacht, F.; Coria-Jiménez, V.R. High Rates of Extensively Drug-Resistant Pseudomonas aeruginosa in Children with Cystic Fibrosis. Curr. Microbiol. 2022, 79, 353. [Google Scholar] [CrossRef]
- Boushra, M.R.; Gad, G.F.M.; Hassuna, N.A.; Waly, N.G.F.; Ibrahem, R.A. Phenotypic and Genotypic Assessment of Fluoroquinolones and Aminoglycosides Resistances in Pseudomonas aeruginosa Collected from Minia Hospitals, Egypt during COVID-19 Pandemic. BMC Infect. Dis. 2024, 24, 763. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Sleiman, A.; Frem, J.A.; Doumat, G.; Gharamti, A.; El Hafi, B.; Doumith, M.; AlGhoribi, M.F.; Kanj, S.S.; Araj, G.F.; et al. Molecular Characterization and Differential Effects of Levofloxacin and Ciprofloxacin on the Potential for Developing Quinolone Resistance among Clinical Pseudomonas aeruginosa Isolates. Front. Microbiol. 2023, 14, 1209224. [Google Scholar] [CrossRef]
- Diaz Iglesias, Y.; Van Bambeke, F. Activity of Antibiotics against Pseudomonas aeruginosa in an In Vitro Model of Biofilms in the Context of Cystic Fibrosis: Influence of the Culture Medium. Antimicrob. Agents Chemother. 2020, 64, e02204-19. [Google Scholar] [CrossRef]
- Bové, M.; Kolpen, M.; Lichtenberg, M.; Bjarnsholt, T.; Coenye, T. Adaptation of Pseudomonas aeruginosa Biofilms to Tobramycin and the Quorum Sensing Inhibitor C-30 during Experimental Evolution Requires Multiple Genotypic and Phenotypic Changes. Microbiology 2023, 169, 001278. [Google Scholar] [CrossRef]
- Wardell, S.J.T.; Gauthier, J.; Martin, L.W.; Potvin, M.; Brockway, B.; Levesque, R.C.; Lamont, I.L. Genome Evolution Drives Transcriptomic and Phenotypic Adaptation in Pseudomonas aeruginosa during 20 Years of Infection. Microb. Genom. 2021, 7, 000681. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.H.; Simões, F.B.; Pogrebnyakov, I.; Welch, M.; Johansen, H.K.; Molin, S.; La Rosa, R. Metabolic Specialization Drives Reduced Pathogenicity in Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients. PLoS Biol. 2024, 22, e3002781. [Google Scholar] [CrossRef]
- Schultz, D.; Kishony, R. Optimization and Control in Bacterial Lag Phase. BMC Biol. 2013, 11, 120. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Shi, H.; Yin, H. The Importance of Lag Time Extension in Determining Bacterial Resistance to Antibiotics. Analyst 2016, 141, 3059–3067. [Google Scholar] [CrossRef]
- Simsek, E.; Kim, M. Power-Law Tail in Lag Time Distribution Underlies Bacterial Persistence. Proc. Natl. Acad. Sci. USA 2019, 116, 17635–17640. [Google Scholar] [CrossRef]
- Hamill, P.G.; Stevenson, A.; McMullan, P.E.; Williams, J.P.; Lewis, A.D.R.; S, S.; Stevenson, K.E.; Farnsworth, K.D.; Khroustalyova, G.; Takemoto, J.Y.; et al. Microbial Lag Phase Can Be Indicative of, or Independent from, Cellular Stress. Sci. Rep. 2020, 10, 5948. [Google Scholar] [CrossRef]
- Bertrand, R.L. Lag Phase Is a Dynamic, Organized, Adaptive, and Evolvable Period That Prepares Bacteria for Cell Division. J. Bacteriol. 2019, 201, e00697-18. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, R.; Rossi, E.; Feist, A.M.; Johansen, H.K.; Molin, S. Compensatory Evolution of Pseudomonas aeruginosa’s Slow Growth Phenotype Suggests Mechanisms of Adaptation in Cystic Fibrosis. Nat. Commun. 2021, 12, 3186. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Das, T.; Manoharan, A.; Whiteley, G.; Glasbey, T.; Manos, J. Pseudomonas aeruginosa Biofilms and Infections: Roles of Extracellular Molecules. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–46. ISBN 978-0-444-64279-0. [Google Scholar]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorghe, A.G.; et al. Bacterial Biofilms Predominate in Both Acute and Chronic Human Lung Infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [PubMed]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Jones, C.J.; Wozniak, D.J. Psl Produced by Mucoid Pseudomonas aeruginosa Contributes to the Establishment of Biofilms and Immune Evasion. mBio 2017, 8, e00864-17. [Google Scholar] [CrossRef]
- Guillaume, O.; Butnarasu, C.; Visentin, S.; Reimhult, E. Interplay between Biofilm Microenvironment and Pathogenicity of Pseudomonas aeruginosa in Cystic Fibrosis Lung Chronic Infection. Biofilm 2022, 4, 100089. [Google Scholar] [CrossRef]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas aeruginosa Aggregates in Cystic Fibrosis Sputum Produce Exopolysaccharides That Likely Impede Current Therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef]
- Liang, Z.; Rybtke, M.; Kragh, K.N.; Johnson, O.; Schicketanz, M.; Zhang, Y.E.; Andersen, J.B.; Tolker-Nielsen, T. Transcription of the Alginate Operon in Pseudomonas aeruginosa Is Regulated by C-Di-GMP. Microbiol. Spectr. 2022, 10, e00675-22. [Google Scholar] [CrossRef]
- Cao, B.; Christophersen, L.; Kolpen, M.; Jensen, P.Ø.; Sneppen, K.; Høiby, N.; Moser, C.; Sams, T. Diffusion Retardation by Binding of Tobramycin in an Alginate Biofilm Model. PLoS ONE 2016, 11, e0153616. [Google Scholar] [CrossRef]
- Høiby, N.; Moser, C.; Ciofu, O. Pseudomonas aeruginosa in the Frontline of the Greatest Challenge of Biofilm Infection—Its Tolerance to Antibiotics. Microorganisms 2024, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Yau, Y.C.W.; Park, S.; Eisha, S.; McDonald, N.; Parsek, M.R.; Howell, P.L.; Hoffman, L.R.; Nguyen, D.; DiGiandomenico, A.; et al. Pseudomonas aeruginosa Aggregation and Psl Expression in Sputum Is Associated with Antibiotic Eradication Failure in Children with Cystic Fibrosis. Sci. Rep. 2022, 12, 21444. [Google Scholar] [CrossRef]
- Morris, A.J.; Jackson, L.; Cw Yau, Y.; Reichhardt, C.; Beaudoin, T.; Uwumarenogie, S.; Guttman, K.M.; Lynne Howell, P.; Parsek, M.R.; Hoffman, L.R.; et al. The Role of Psl in the Failure to Eradicate Pseudomonas aeruginosa Biofilms in Children with Cystic Fibrosis. npj Biofilms Microbiomes 2021, 7, 63. [Google Scholar] [CrossRef]
- Thomsen, K.; Høiby, N.; Jensen, P.Ø.; Ciofu, O.; Moser, C. Immune Response to Biofilm Growing Pulmonary Pseudomonas aeruginosa Infection. Biomedicines 2022, 10, 2064. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-P.; Chou, C.-F. Morphological Plasticity of Bacteria—Open Questions. Biomicrofluidics 2016, 10, 031501. [Google Scholar] [CrossRef] [PubMed]
- Farnia, P.; Farnia, P.; Ghanavi, J.; Zhavnerko, G.; Poleschuyk, N.; Velayati, A. A Review on the Shape Changes in Pathogenic Bacteria with Emphasis on Mycobacterium tuberculosis. Biomed. Biotechnol. Res. J. 2018, 2, 242. [Google Scholar] [CrossRef]
- Cendra, M.d.M.; Torrents, E. Pseudomonas aeruginosa Biofilms and Their Partners in Crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef]
- Del Mar Cendra, M.; Christodoulides, M.; Hossain, P. Effect of Different Antibiotic Chemotherapies on Pseudomonas aeruginosa Infection In Vitro of Primary Human Corneal Fibroblast Cells. Front. Microbiol. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Harmer, C.J.; Triccas, J.A.; Hu, H.; Rose, B.; Bye, P.; Elkins, M.; Manos, J. Pseudomonas aeruginosa Strains from the Chronically Infected Cystic Fibrosis Lung Display Increased Invasiveness of A549 Epithelial Cells over Time. Microb. Pathog. 2012, 53, 37–43. [Google Scholar] [CrossRef]
- Lorè, N.I.; Cigana, C.; De Fino, I.; Riva, C.; Juhas, M.; Schwager, S.; Eberl, L.; Bragonzi, A. Cystic Fibrosis-Niche Adaptation of Pseudomonas aeruginosa Reduces Virulence in Multiple Infection Hosts. PLoS ONE 2012, 7, e35648. [Google Scholar] [CrossRef]
- Malet, J.K.; Hennemann, L.C.; Hua, E.M.-L.; Faure, E.; Waters, V.; Rousseau, S.; Nguyen, D. A Model of Intracellular Persistence of Pseudomonas aeruginosa in Airway Epithelial Cells. Cell. Microbiol. 2022, 2022, 5431666. [Google Scholar] [CrossRef]
- Del Mar Cendra, M.; Torrents, E. Differential Adaptability between Reference Strains and Clinical Isolates of Pseudomonas aeruginosa into the Lung Epithelium Intracellular Lifestyle. Virulence 2020, 11, 862–876. [Google Scholar] [CrossRef]
- Winton; Wan; Cannell; Gruenert; Thompson, J.; Garrod; Stewart; Robinson. Cell Lines of Pulmonary and Non-pulmonary Origin as Tools to Study the Effects of House Dust Mite Proteinases on the Regulation of Epithelial Permeability. Clin. Exp. Allergy 1998, 28, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, A.; Coraux, C.; Guillard, T. Current Concepts on Pseudomonas aeruginosa Interaction with Human Airway Epithelium. PLoS Pathog. 2023, 19, e1011221. [Google Scholar] [CrossRef]
- Deligianni, E.; Pattison, S.; Berrar, D.; Ternan, N.G.; Haylock, R.W.; Moore, J.E.; Elborn, S.J.; Dooley, J.S. Pseudomonas aeruginosa Cystic Fibrosis Isolates of Similar RAPD Genotype Exhibit Diversity in Biofilm Forming Ability in Vitro. BMC Microbiol. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Imburgia, T.A.; Seagren, R.M.; Christensen, H.; Lasarev, M.R.; Bogenschutz, M.C. Review of Tobramycin Dosing in Pediatric Patients With Cystic Fibrosis. J. Pediatr. Pharmacol. Ther. 2023, 28, 63–70. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Jaulhac, B.; Prévost, G.; Bernardi, T.; Jehl, F. Tobramycin and Amikacin Delay Adhesion and Microcolony Formation in Pseudomonas aeruginosa Cystic Fibrosis Isolates. Front. Microbiol. 2017, 8, 1289. [Google Scholar] [CrossRef]
- Tahrioui, A.; Duchesne, R.; Bouffartigues, E.; Rodrigues, S.; Maillot, O.; Tortuel, D.; Hardouin, J.; Taupin, L.; Groleau, M.-C.; Dufour, A.; et al. Extracellular DNA Release, Quorum Sensing, and PrrF1/F2 Small RNAs Are Key Players in Pseudomonas aeruginosa Tobramycin-Enhanced Biofilm Formation. npj Biofilms Microbiomes 2019, 5, 15. [Google Scholar] [CrossRef]
- Ramsay, K.A.; McTavish, S.M.; Wardell, S.J.T.; Lamont, I.L. The Effects of Sub-Inhibitory Antibiotic Concentrations on Pseudomonas aeruginosa: Reduced Susceptibility Due to Mutations. Front. Microbiol. 2021, 12, 789550. [Google Scholar] [CrossRef]
- Ghoul, M.; Andersen, S.B.; Marvig, R.L.; Johansen, H.K.; Jelsbak, L.; Molin, S.; Perron, G.; Griffin, A.S. Long-Term Evolution of Antibiotic Tolerance in Pseudomonas aeruginosa Lung Infections. Evol. Lett. 2023, 7, 389–400. [Google Scholar] [CrossRef]
- Higazy, D.; Pham, A.D.; Van Hasselt, C.; Høiby, N.; Jelsbak, L.; Moser, C.; Ciofu, O. In Vivo Evolution of Antimicrobial Resistance in a Biofilm Model of Pseudomonas aeruginosa Lung Infection. ISME J. 2024, 18, wrae036. [Google Scholar] [CrossRef] [PubMed]
- Mangiaterra, G.; Cedraro, N.; Vaiasicca, S.; Citterio, B.; Galeazzi, R.; Laudadio, E.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F. Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics 2020, 9, 399. [Google Scholar] [CrossRef]
- Law, J.P.; Wood, A.J.; Friman, V.-P. The Effects of Antibiotic Combination Treatments on Pseudomonas aeruginosa Tolerance Evolution and Coexistence with Stenotrophomonas Maltophilia. Microbiol. Spectr. 2022, 10, e01842-22. [Google Scholar] [CrossRef] [PubMed]
- Stoitsova, S.R.; Paunova-Krasteva, T.S.; Borisova, D.B. Modulation of Biofilm Growth by Sub-Inhibitory Amounts of Antibacterial Substances. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; InTech: Rjeka, Croatia, 2016; ISBN 978-953-51-2435-1. [Google Scholar]
- De Bleeckere, A.; Van Den Bossche, S.; De Sutter, P.-J.; Beirens, T.; Crabbé, A.; Coenye, T. High Throughput Determination of the Biofilm Prevention Concentration for Pseudomonas aeruginosa Biofilms Using a Synthetic Cystic Fibrosis Sputum Medium. Biofilm 2023, 5, 100106. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K.; Weber, C.A.; Pettit, G.R. Application of a High Throughput Alamar Blue Biofilm Susceptibility Assay to Staphylococcus aureus Biofilms. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 28. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic Reduction of Resazurin; Location within the Cell for Cytotoxicity Assays. Biotech. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef]
- Valentin, J.D.P.; Straub, H.; Pietsch, F.; Lemare, M.; Ahrens, C.H.; Schreiber, F.; Webb, J.S.; Van Der Mei, H.C.; Ren, Q. Role of the Flagellar Hook in the Structural Development and Antibiotic Tolerance of Pseudomonas aeruginosa Biofilms. ISME J. 2022, 16, 1176–1186. [Google Scholar] [CrossRef]
- Schick, A.; Kassen, R. Rapid Diversification of Pseudomonas aeruginosa in Cystic Fibrosis Lung-like Conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 10714–10719. [Google Scholar] [CrossRef]
- Camus, L.; Vandenesch, F.; Moreau, K. From Genotype to Phenotype: Adaptations of Pseudomonas aeruginosa to the Cystic Fibrosis Environment. Microb. Genom. 2021, 7, 000513. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, R.; Johansen, H.K.; Molin, S. Adapting to the Airways: Metabolic Requirements of Pseudomonas aeruginosa during the Infection of Cystic Fibrosis Patients. Metabolites 2019, 9, 234. [Google Scholar] [CrossRef] [PubMed]


| Pair | Strain | Patient Age at the Time of Strain Isolation (Years) | Number of Inhaled Tobramycin Cycles |
|---|---|---|---|
| Pair 1 | PaT-1 | 15 | 0 |
| PaT-2 | 16 | 3 | |
| Pair 2 | PaT-3 | 15 | 0 |
| PaT-4 | 16 | 3 | |
| Pair 3 | PaT-5 | 13 | 0 |
| PaT-6 | 16 | 15 | |
| Pair 4 | PaT-7 | 25 | 0 |
| PaT-8 | 27 | 2 | |
| Pair 5 | PaT-9 | 10 | 0 |
| PaT-10 | 11 | 2 | |
| Pair 6 | PaT-11 | 20 | 0 |
| PaT-12 | 21 | 3 |
| Strain | Swimming Motility (cm) | Swarming Motility (cm) | Twitching Motility (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Quarter MIC | Half MIC | Control | Quarter MIC | Half MIC | Control | Quarter MIC | Half MIC | |
| PaT-1 | 2.77 ± 0.08 | - | - | 0.5 ± 0.08 | - | - | - | - | - |
| PaT-2 | 2.8 ± 0.29 | - | - | 0.375 ± 0.09 | - | - | 1 ± 0 | 0.15 ± 0.05 | - |
| PaT-3 | 2.6 ± 0.33 | - | - | 0.6 ± 0.08 | - | - | 1.075 ± 0.09 | - | - |
| PaT-4 | 2.7 ± 0.12 | - | - | 0.6 ± 0.08 | - | - | 1.125 ± 0.05 | 1.125 ± 0.05 | 0.825 ± 0.12 |
| PaT-5 | 2.1 ± 0.21 | - | - | 0.65 ± 0.12 | 0.4 ± 0.8 | - | - | - | - |
| PaT-6 | 3.5 ± 0.32 | - | - | 0.75 ± 0.23 | - | - | - | - | - |
| PaT-7 | 3.75 ± 0.16 | - | - | 0.825 ± 0.05 | 0.6 ± 0.08 | - | - | - | - |
| PaT-8 | 2.6 ± 0.12 | - | - | 0.625 ± 0.17 | - | - | - | - | - |
| PaT-9 | 2.1 ± 0.27 | - | - | 0.45 ± 0.13 | - | - | - | - | - |
| PaT-10 | 1.47 ± 0.08 | - | - | 0.125 ± 0.05 | - | - | - | - | - |
| PaT-11 | 2.95 ± 0.1 | - | - | 0.85 ± 0.21 | - | - | - | - | - |
| PaT-12 | 1.95 ± 0.20 | - | - | 0.374 ± 0.15 | 0.275 ± 0.05 | 0.2 ± 0.08 | - | - | - |
| PA O1 | 2.5 ± 0.43 | - | - | 1.25 ± 0.28 | - | - | 2 ± 0.16 | - | - |
| Strain | MIC (µg mL−1) | Susceptibility | |
|---|---|---|---|
| Pair 1 | PaT-1 | 0.25 | S |
| PaT-2 | 0.25 | S | |
| Pair 2 | PaT-3 | 0.50 | S |
| PaT-4 | 0.75 | S | |
| Pair 3 | PaT-5 | 0.50 | S |
| PaT-6 | 1.00 | S | |
| Pair 4 | PaT-7 | 0.38 | S |
| PaT-8 | 0.75 | S | |
| Pair 5 | PaT-9 | 1.50 | S |
| PaT-10 | 1.50 | S | |
| Pair 6 | PaT-11 | 0.75 | S |
| PaT-12 | 1.50 | S | |
| Reference | PAO1 | 1.5 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisova, D.; Strateva, T.; Dimov, S.G.; Atanassova, B.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Danova, S.T.; Tropcheva, R.; Stoitsova, S. Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates. Microorganisms 2025, 13, 730. https://doi.org/10.3390/microorganisms13040730
Borisova D, Strateva T, Dimov SG, Atanassova B, Paunova-Krasteva T, Topouzova-Hristova T, Danova ST, Tropcheva R, Stoitsova S. Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates. Microorganisms. 2025; 13(4):730. https://doi.org/10.3390/microorganisms13040730
Chicago/Turabian StyleBorisova, Dayana, Tanya Strateva, Svetoslav G. Dimov, Borjana Atanassova, Tsvetelina Paunova-Krasteva, Tanya Topouzova-Hristova, Svetla T. Danova, Rositsa Tropcheva, and Stoyanka Stoitsova. 2025. "Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates" Microorganisms 13, no. 4: 730. https://doi.org/10.3390/microorganisms13040730
APA StyleBorisova, D., Strateva, T., Dimov, S. G., Atanassova, B., Paunova-Krasteva, T., Topouzova-Hristova, T., Danova, S. T., Tropcheva, R., & Stoitsova, S. (2025). Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates. Microorganisms, 13(4), 730. https://doi.org/10.3390/microorganisms13040730








