Current Risks and Prevention Strategies Against Vector-Borne Diseases in Cyprus
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
| Etiological Agent | Prevalence, Seropositivity, Case | Study Year | Reference |
|---|---|---|---|
| Viruses | |||
| Dengue Virus | 2 cases of imported dengue cases in Cyprus. | 2024 | [12] |
| West Nile Virus | First neuroinvasive human case of WNV infection in Cyprus. | 2017 | [4] |
| Complete genome sequence of the first human neuroinvasive WNV infection, placing it into genetic lineage 1, clade 1a, cluster 2. | 2017 | [13] | |
| Seroprevalence rate for anti-WNV IgG of 5%; anti-WNV IgM 17 out of the 127 patients with symptoms. | 2019 | [14] | |
| 2 (0.3%) IgM+ and 31 (4.1%) IgG+ cases out of 760 sera screened. | 2020 | [15] | |
| 1.3% seropositivity rate detected out of 836 avian blood samples from 44 migratory and local bird species. | 2021 | [16] | |
| WNV RNA detected in Culex pipiens mosquitoes from Nicosia (Figure 1) (2019). | 2022 | [17] | |
| Phleboviruses | SFSV, SNV, and TOSV seropositivity associated with symptomatic disease (2007) | 2007 | [18] |
| Identification of TOSV in sandflies from Northern Cyprus | 2014 | [19] | |
| Neutralizing antibodies against TOSV, SFSV, Arbia, and Adana viruses (Salehabad viruses) in dogs. | 2016 | [20] | |
| SFSV antibodies detected in a 45-year-old tourist with associated symptoms. | 2018 | [21] | |
| Zika virus | Absence of seropositivity among blood donors in North Cyprus. | 2021 | [22] |
| Bacteria | |||
| Rickettsia spp. | First detection of Rickettsia felis in Ctenocephalides felis fleas in rats in Cyprus. | 2006 | [23] |
| A novel, uncultured Rickettsia species was identified in ticks in Cyprus (Rickettsia species strain Tselenti). | 2013 | [24] | |
| Cases until 2017 reviewed by Tsioutis et al. (2017) as follows: 21 pediatric cases in Cyprus from 2000 to 2006 with R. typhi infection and a case during pregnancy by Koliou et al. [25], and 193 human cases of R. typhi infection Cyprus from 2000 to 2008 by Psaroulaki et al., 2012 [26]. | 2017 | [27] | |
| Ectoparasites of 161 dogs and 59 cats in Cyprus carried Rickettsia massiliae, Rickettsia conorii, Rickettsia felis (ticks), and Rickettsia felis, Rickettsia spp. (fleas). | 2022 | [28] | |
| Ricketsial IgG seropositivity of 2% (6 total) when sera from 300 hunters were screened between 2017 and 2018 from Kyrenia and Rizokarpaso (Figure 1). | 2022 | [29] | |
| Anaplasma spp. | 2 (4%) dogs positive for Anaplasma platys DNA detected in 47 dogs with clinical leishmaniosis and 3 (3%) in 87 healthy control dogs. | 2018 | [30] |
| Anti-Anaplasma phagocytophilum/Anaplasma platys antibodies in 5 out of 134 dogs. | 2018 | [30] | |
| 3 ticks from 161 dogs in Cyprus had Anaplasma platys. | 2022 | [28] | |
| Ehrlichia spp. | 6 (12%) dogs positive for Ehrlichia canis DNA detected in 47 dogs with Clinical leishmaniosis and 1 (1%) in 87 healthy control dogs. | 2018 | [30] |
| Ehrlichia ewingii antibodies in 17 out of 134 tested dogs. | 2018 | [30] | |
| Increased risk for E. canis/ E. ewingii seropositivity in dogs with clinical leishmaniasis compared to healthy dogs. | 2018 | [30] | |
| Borrelia spp. | 47 dogs with clinical leishmaniosis were screened for anti-Borrelia burgdorferii antibodies, but no seropositivity was detected. | 2018 | [30] |
| Protozoan Parasites | |||
| Leishmania spp. | Patient diagnosed with L. donovani complex cutaneous leishmaniasis after a 3-day visit to north Cyprus. | 2015 | [31] |
| Animal and human cases along with seropositivity rates detailed by Schou et al., between 1998 and 2018. | 2020 | [32] | |
| Since 2018, 47 dogs were identified with leishmaniosis. | 2018 | [30] | |
| 1 P. papatasi sandfly identified as positive for L. major from Cyprus using PCR-based detection methods. | 2022 | [33] | |
| L. infantum IgG positivity was 4.7% (14/300) in healthy donors from north Cyprus. | 2022 | [34] | |
| Plasmodium spp. | Three cases of vivax malaria in individuals returning to UK from Cyprus were reported in 2017. | 2020 | [35] |
| 13 patients were diagnosed with malaria between 2016 and 2019, and Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale species were identified, revealing a significant increase in imported cases in 2019. | 2021 | [22] |
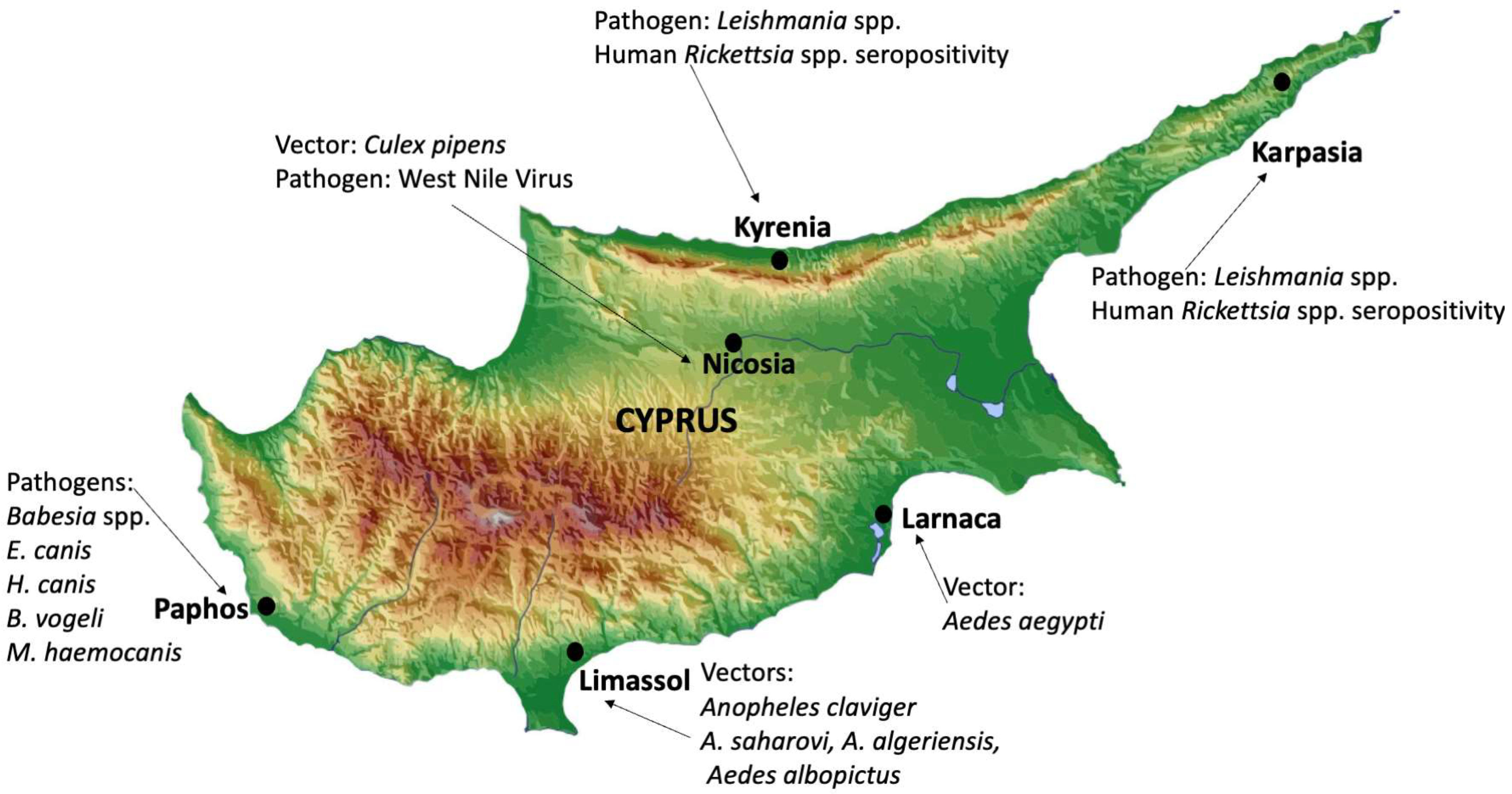
3.1. Viruses
3.1.1. Dengue Virus
| Study | Method Used | Prediction/Identification |
|---|---|---|
| Vasques et al. [40] | Morphological and molecular identification | Identified Ae. albopictus and Ae. aegypti in Larnaka and Limassol (Figure 1) |
| Proestos et al. [41] and Georgiades et al. [48] | Machine learning and simulation modeling | Predicted 2.4 billion people exposed to Ae. albopictus by 2050 within 20 million km2 |
| Erguler et al. [43] | Large-scale environmentally driven mathematical model | Hypothesized the survival of Ae. albopictus through harsh winters |
3.1.2. West Nile Virus
3.1.3. Phleboviruses
3.2. Bacteria
3.2.1. Rickettsia spp.
3.2.2. Other Members of the Order Rickettsiales: Anaplasma & Ehrlichia spp.
3.3. Protozoan Parasites
3.3.1. Plasmodium spp.
3.3.2. Leishmania spp.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lange, M.A. Impacts of Climate Change on the Eastern Mediterranean and the Middle East and North Africa Region and the Water–Energy Nexus. Atmosphere 2019, 10, 455. [Google Scholar] [CrossRef]
- Pavia, G.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Marascio, N.; Quirino, A.; Gigliotti, S.; et al. The issue of climate change and the spread of tropical diseases in Europe and Italy: Vector biology, disease transmission, genome-based monitoring and public health implications. Infect. Dis. 2025, 57, 121–136. [Google Scholar] [CrossRef]
- Shelley, H. Mehmed Aziz Anopheles eradication in Cyprus. Br. Med. J. 1949, 1, 767. [Google Scholar] [CrossRef]
- Paphitou, N.I.; Tourvas, A.; Floridou, D.; Richter, J.; Tryfonos, C.; Christodoulou, C. The first human case of neuroinvasive West Nile virus infection identified in Cyprus. J. Infect. Public Health 2017, 10, 891–893. [Google Scholar] [CrossRef]
- Ruh, E.; Bostanci, A.; Kunter, V.; Tosun, O.; Imir, T.; Schallig, H.; Taylan-Ozkan, A. Leishmaniasis in northern Cyprus: Human cases and their association with risk factors. J. Vector Borne Dis. 2017, 54, 358–365. [Google Scholar] [CrossRef]
- Martinou, A.F.; Athanasiou, K.; Shawcross, K. Monitoring for Native and Invasive Mosquitoes at the Limassol Port in Cyprus. Med. Sci. Forum 2022, 13, 21. [Google Scholar] [CrossRef]
- Adam, M.; Nahzat, S.; Kakar, Q.; Assada, M.; Witkowski, B.; Tag Eldin Elshafie, A.; Abuobaida, D.; Safi, N.; Khan, M.A.; Nagi, M.; et al. Antimalarial drug efficacy and resistance in malaria-endemic countries in HANMAT-PIAM_net countries of the Eastern Mediterranean Region 2016-2020: Clinical and genetic studies. Trop. Med. Int. Health 2023, 28, 817–829. [Google Scholar] [CrossRef]
- Piccinno, R.; Fiorenza, G.; Vasquez, M.I.; Bouyer, J.; Notarides, G.; Gomulski, L.M.; Meletiou, S.; Akiner, M.; Michaelakis, A.; Forneris, F.; et al. On the tracks of an uninvited guest, the Asian tiger mosquito, Aedes albopictus in Cyprus. Parasites Vectors 2025, 18, 39. [Google Scholar] [CrossRef]
- Seyer-Cagatan, A.; Ruh, E.; Taylan-Ozkan, A. Vector-borne diseases in Cyprus: A detailed review of the literature. Trop. Biomed. 2024, 41, 328–344. [Google Scholar] [CrossRef]
- Abreu, F.V.S.d.; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; de Albuquerque Motta, M.; Barcellos, C.; Romão, A.R.; Magalhães, M.d.A.F.M.; et al. Ecological and environmental factors affecting transmission of sylvatic yellow fever in the 2017-2019 outbreak in the Atlantic Forest, Brazil. Parasites Vectors 2022, 15, 23. [Google Scholar] [CrossRef]
- Kiryluk, H.D.; Beard, C.B.; Holcomb, K.M. The use of environmental data in descriptive and predictive models of vector-borne disease in North America. J. Med. Entomol. 2024, 61, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Shkurko, J. Women Hospitalised with Dengue Fever ‘in Good Conditions’. Cyprus Mail, 27 November 2023. Available online: https://cyprus-mail.com/2023/11/27/women-hospitalised-with-dengue-fever-in-good-conditions/ (accessed on 5 December 2023).
- Richter, J.; Tryfonos, C.; Tourvas, A.; Floridou, D.; Paphitou, N.I.; Christodoulou, C. Complete Genome Sequence of West Nile Virus (WNV) from the First Human Case of Neuroinvasive WNV Infection in Cyprus. Genome Announc. 2017, 5, e01110-17. [Google Scholar] [CrossRef] [PubMed]
- Billioud, G.; Tryfonos, C.; Richter, J. The Prevalence of Antibodies against Sandfly Fever Viruses and West Nile Virus in Cyprus. J. Arthropod Borne Dis. 2019, 13, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Balaman, N.; Gazi, U.; Imir, T.; Sanlidag, T.; Ruh, E.; Tosun, O.; Ozkul, A.; Taylan-Ozkan, A. Serological screening of West Nile virus among blood donors in northern Cyprus. J. Med. Virol. 2020, 92, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Pallari, C.T.; Efstathiou, A.; Moysi, M.; Papanikolas, N.; Christodoulou, V.; Mazeris, A.; Koliou, M.; Kirschel, A.N.G. Evidence of West Nile virus seropositivity in wild birds on the island of Cyprus. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101592. [Google Scholar] [CrossRef]
- Pallari, C.T.; Christodoulou, V.; Koliou, M.; Kirschel, A.N.G. First detection of WNV RNA presence in field-collected mosquitoes in Cyprus. Acta Trop. 2022, 231, 106470. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Papa, A.; Antoniadis, A. Sandfly-fever outbreak in Cyprus: Are phleboviruses still a health problem? Travel. Med. Infect. Dis. 2007, 5, 239–242. [Google Scholar] [CrossRef]
- Ergunay, K.; Gunay, F.; Erisoz Kasap, O.; Oter, K.; Gargari, S.; Karaoglu, T.; Tezcan, S.; Cabalar, M.; Yildirim, Y.; Emekdas, G.; et al. Serological, molecular and entomological surveillance demonstrates widespread circulation of West Nile virus in Turkey. PLoS Negl. Trop. Dis. 2014, 8, e3028. [Google Scholar] [CrossRef]
- Alwassouf, S.; Christodoulou, V.; Bichaud, L.; Ntais, P.; Mazeris, A.; Antoniou, M.; Charrel, R.N. Seroprevalence of Sandfly-Borne Phleboviruses Belonging to Three Serocomplexes (Sandfly fever Naples, Sandfly fever Sicilian and Salehabad) in Dogs from Greece and Cyprus Using Neutralization Test. PLoS Negl. Trop. Dis. 2016, 10, e0005063. [Google Scholar] [CrossRef]
- Stahn, B.; Sudeck, H.; Frickmann, H.; Krüger, A.; Burchard, H.G.; Wiemer, D. Sandfly fever-a “neglected” disease. Hautarzt 2018, 69, 928–937. [Google Scholar] [CrossRef]
- Abushoufa, F.; Arikan, A.; Sanlidag, T.; Guvenir, M.; Guler, E.; Suer, K. Absence of Zika Virus Seroprevalence Among Blood Donors in Northern Cyprus. J. Infect. Dev. Ctries. 2021, 15, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Antoniou, M.; Papaeustathiou, A.; Toumazos, P.; Loukaides, F.; Tselentis, Y. First detection of Rickettsia felis in Ctenocephalides felis fleas parasitizing rats in Cyprus. Am. J. Trop. Med. Hyg. 2006, 74, 120–122. [Google Scholar] [PubMed]
- Sandalakis, V.; Chochlakis, D.; Ioannou, I.; Psaroulaki, A. Identification of a novel uncultured Rickettsia species strain (Rickettsia species strain Tselenti) in Cyprus. Am. J. Trop. Med. Hyg. 2013, 88, 698–700. [Google Scholar] [CrossRef]
- Koliou, M.; Psaroulaki, A.; Georgiou, C.; Ioannou, I.; Tselentis, Y.; Gikas, A. Murine typhus in Cyprus: 21 paediatric cases. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 491–493. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Christou, C.; Chochlakis, D.; Tsiligianni, I.; Sandalakis, V.; Georgalis, L.; Ioannou, I.; Giorgalas, G.; Tselentis, Y. Murine typhus in Cyprus: A 9-year survey. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 489–495. [Google Scholar] [CrossRef]
- Tsioutis, C.; Zafeiri, M.; Avramopoulos, A.; Prousali, E.; Miligkos, M.; Karageorgos, S.A. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: A systematic review. Acta Trop. 2017, 166, 16–24. [Google Scholar] [CrossRef]
- Diakou, A.; Sofroniou, D.; Paoletti, B.; Tamvakis, A.; Kolencik, S.; Dimzas, D.; Morelli, S.; Grillini, M.; Traversa, D. Ticks, Fleas, and Harboured Pathogens from Dogs and Cats in Cyprus. Pathogens 2022, 11, 1403. [Google Scholar] [CrossRef]
- Ruh, E.; Aras, S.; Gazi, U.; Celebi, B.; Tosun, O.; Sanlidag, T.; Imir, T.; Taylan-Ozkan, A. Seroprevalence of rickettsial infection in northern Cyprus: A study among hunters. Trop. Biomed. 2022, 39, 221–225. [Google Scholar] [CrossRef]
- Attipa, C.; Solano-Gallego, L.; Papasouliotis, K.; Soutter, F.; Morris, D.; Helps, C.; Carver, S.; Tasker, S. Association between canine leishmaniosis and Ehrlichia canis co-infection: A prospective case-control study. Parasit. Vectors 2018, 11, 184–188. [Google Scholar] [CrossRef]
- de Silva, T.I.; Debroy Kidambi, A.; Green, S.T.; Mahadeva, U.; Mcgregor, A.C.; Levy, M.; Hardcastle, N. Cutaneous leishmaniasis acquired during a brief visit to Cyprus. J. Infect. 2015, 70, 314–316. [Google Scholar] [CrossRef]
- Schou, C.; Filippova, M.; Quattrocchi, A.; Karanis, P. The Current Status of Protozoan Parasitic Diseases in Cyprus: A Narrative Literature Review. Environ. Sci. Proc. 2020, 2, 61. [Google Scholar] [CrossRef]
- Yetişmiş, K.; Mert, U.; Caner, A.; Nalçaci, M.; Töz, S.; Özbel, Y. Blood Meal Analysis and Molecular Detection of Leishmania DNA in Wild-Caught Sand Flies in Leishmaniasis Endemic Areas of Turkey and Northern Cyprus. Acta Parasitol. 2022, 67, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Özdoğaç, M.; Güler, E.; Güvenir, M.; Hürdoğanoğlu, U.; Kiraz, A.; Süer, K. Investigation of Leishmania infantum Seroprevalance and Leishmaniasis Knowledge Level in Northern Cyprus. Mikrobiyol. Bul. 2022, 56, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Emms, H.; Lee, R.; Thomas, A.; Doerholt, K.; Le Doare, K. Return of vivax malaria in Cyprus. Arch. Dis. Child. 2020, 105, 102–103. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Hombach, J.; Ferguson, N.; Selgelid, M.; O’Brien, K.; Vannice, K.; Barrett, A.; Ferdinand, E.; Flasche, S.; Guzman, M.; et al. Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 2019, 19, e31–e38. [Google Scholar] [CrossRef]
- Garcia-Rejon, J.E.; Navarro, J.; Cigarroa-Toledo, N.; Baak-Baak, C.M. An Updated Review of the Invasive Aedes albopictus in the Americas; Geographical Distribution, Host Feeding Patterns, Arbovirus Infection, and the Potential for Vertical Transmission of Dengue Virus. Insects 2021, 12, 967. [Google Scholar] [CrossRef]
- Chen, R.E.; Smith, B.K.; Errico, J.M.; Gordon, D.N.; Winkler, E.S.; VanBlargan, L.A.; Desai, C.; Handley, S.A.; Dowd, K.A.; Amaro-Carambot, E.; et al. Implications of a highly divergent dengue virus strain for cross-neutralization, protection, and vaccine immunity. Cell Host Microbe 2021, 29, 1634–1648.e5. [Google Scholar] [CrossRef]
- Rodríguez-Barraquer, I.; Salje, H.; Cummings, D.A. Dengue pre-vaccination screening and positive predictive values. Lancet Infect. Dis. 2019, 19, 132–134. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Notarides, G.; Meletiou, S.; Patsoula, E.; Kavran, M.; Michaelakis, A.; Bellini, R.; Toumazi, T.; Bouyer, J.; Petrić, D. Two invasions at once: Update on the introduction of the invasive species Aedes aegypti and Aedes albopictus in Cyprus—A call for action in Europe. Parasite 2023, 30, 41. [Google Scholar] [CrossRef]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130554. [Google Scholar] [CrossRef]
- Tippelt, L.; Werner, D.; Kampen, H. Low temperature tolerance of three Aedes albopictus strains (Diptera: Culicidae) under constant and fluctuating temperature scenarios. Parasite Vectors 2020, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Erguler, K.; Smith-Unna, S.E.; Waldock, J.; Proestos, Y.; Christophides, G.K.; Lelieveld, J.; Parham, P.E. Large-Scale Modelling of the Environmentally-Driven Population Dynamics of Temperate Aedes albopictus (Skuse). PLoS ONE 2016, 11, e0149282. [Google Scholar] [CrossRef]
- Nuclear Technique Used in Europe for First time to Battle Yellow Fever Mosquito Found in Cyprus. Available online: https://www.iaea.org/newscenter/pressreleases/nuclear-technique-used-in-europe-for-first-time-to-battle-yellow-fever-mosquito-found-in-cyprus (accessed on 20 December 2023).
- Martinou, A.F.; Fawcett, J.; Georgiou, M.; Angelidou, I.; Philippou, M.; Schaffner, F. Occurrence of Aedes cretinus in Cyprus based on information collected by citizen scientists. J. Eur. Mosq. Control Assoc. 2021, 39, 31–38. [Google Scholar] [CrossRef]
- Drakou, K.; Nikolaou, T.; Vasquez, M.; Petric, D.; Michaelakis, A.; Kapranas, A.; Papatheodoulou, A.; Koliou, M. The Effect of Weather Variables on Mosquito Activity: A Snapshot of the Main Point of Entry of Cyprus. Int. J. Environ. Res. Public Health 2020, 17, 1403. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Shi, P.; Li, J.; Niu, J.; Chen, J.; Wang, G.; Wu, L.; Chen, L.; Yang, Z.; et al. A naturally isolated symbiotic bacterium suppresses flavivirus transmission by Aedes mosquitoes. Science 2024, 384, eadn9524. [Google Scholar] [CrossRef]
- Georgiades, P.; Proestos, Y.; Lelieveld, J.; Erguler, K. Machine Learning Modeling of Aedes albopictus Habitat Suitability in the 21st Century. Insects 2023, 14, 447. [Google Scholar] [CrossRef]
- Mbonde, A.A.; Gritsch, D.; Harahsheh, E.Y.; Kasule, S.N.; Hasan, S.; Parsons, A.M.; Zhang, N.; Butterfield, R.; Shiue, H.; Norville, K.A.; et al. Neuroinvasive West Nile Virus Infection in Immunosuppressed and Immunocompetent Adults. JAMA Netw Open 2024, 7, e244294. [Google Scholar] [CrossRef]
- Bohers, C.; Vazeille, M.; Bernaoui, L.; Pascalin, L.; Meignan, K.; Mousson, L.; Jakerian, G.; Karch, A.; de Lamballerie, X.; Failloux, A. Aedes albopictus is a competent vector of five arboviruses affecting human health, greater Paris, France, 2023. Euro Surveill 2024, 29, 2400271. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Silverj, A.; Diagne, M.M.; Curini, V.; Teodori, L.; Di Domenico, M.; Mbaye, R.; Leone, A.; Marcacci, M.; et al. Spatial and temporal dynamics of West Nile virus between Africa and Europe. Nat. Commun. 2023, 14, 6440–6447. [Google Scholar] [CrossRef]
- Yeniduzen Batı Nil Virüsü Nedeniyle 1 Kişi Hayatını Kaybetti. 2023. Available online: https://www.yeniduzen.com/bati-nil-virusu-nedeniyle-1-kisi-hayatini-kaybetti-166942h.htm (accessed on 4 December 2023).
- Moriconi, M.; Rugna, G.; Calzolari, M.; Bellini, R.; Albieri, A.; Angelini, P.; Cagarelli, R.; Landini, M.P.; Charrel, R.N.; Varani, S. Phlebotomine sand fly-borne pathogens in the Mediterranean Basin: Human leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017, 11, e0005660. [Google Scholar] [CrossRef]
- Eitrem, R.; Vene, S.; Niklasson, B. Incidence of sand fly fever among Swedish United Nations soldiers on Cyprus during 1985. Am. J. Trop. Med. Hyg. 1990, 43, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Psaroulaki, A.; Antoniou, M.; Toumazos, P.; Ioannou, I.; Mazeris, A.; Chochlakis, D.; Tselentis, Y. Rickettsia typhi and Rickettsia felis in Xenopsylla cheopis and Leptopsylla segnis parasitizing rats in Cyprus. Am. J. Trop. Med. Hyg. 2010, 83, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Antoniou, M.; Toumazos, P.; Mazeris, A.; Ioannou, I.; Chochlakis, D.; Christophi, N.; Loukaides, P.; Patsias, A.; Moschandrea, I.; et al. Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: A seroepidemiological study. Trans. R Soc. Trop. Med. Hyg. 2010, 104, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Chochlakis, D.; Ioannou, I.; Angelakis, E.; Tselentis, Y. Presence of Coxiella burnetii in fleas in Cyprus. Vector Borne Zoonotic Dis. 2014, 14, 685–687. [Google Scholar] [CrossRef]
- Ioannou, I.; Chochlakis, D.; Kasinis, N.; Anayiotos, P.; Lyssandrou, A.; Papadopoulos, B.; Tselentis, Y.; Psaroulaki, A. Carriage of Rickettsia spp., Coxiella burnetii and Anaplasma spp. by endemic and migratory wild birds and their ectoparasites in Cyprus. Clin. Microbiol. Infect 2009, 15 (Suppl. 2), 158–160. [Google Scholar] [CrossRef]
- Cantas, L.; Muwonge, A.; Sareyyupoglu, B.; Yardimci, H.; Skjerve, E. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Vet. Res. 2011, 7, 13. [Google Scholar] [CrossRef]
- Ioannou, I.; Sandalakis, V.; Kassinis, N.; Chochlakis, D.; Papadopoulos, B.; Loukaides, F.; Tselentis, Y.; Psaroulaki, A. Tick-borne bacteria in mouflons and their ectoparasites in Cyprus. J. Wildl. Dis. 2011, 47, 300–306. [Google Scholar] [CrossRef]
- Koliou, M.; Christoforou, C.; Soteriades, E.S. Murine typhus in pregnancy: A case report from Cyprus. Scand. J. Infect. Dis. 2007, 39, 625–628. [Google Scholar] [CrossRef]
- Amoros, J.; Fattar, N.; Buysse, M.; Louni, M.; Bertaux, J.; Bouchon, D.; Duron, O. Reassessment of the genetic basis of natural rifampin resistance in the genus Rickettsia. Microbiologyopen 2024, 13, e1431. [Google Scholar] [CrossRef]
- Chigwada, A.D.; Mapholi, N.O.; Ogola, H.J.O.; Mbizeni, S.; Masebe, T.M. Pathogenic and Endosymbiotic Bacteria and Their Associated Antibiotic Resistance Biomarkers in Amblyomma and Hyalomma Ticks Infesting Nguni Cattle (Bos spp.). Pathogens 2022, 11, 432. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Koliou, M.; Chochlakis, D.; Ioannou, I.; Mazeri, S.; Tselentis, Y. Anaplasma phagocytophilum infection in a child. Pediatr. Infect. Dis. J. 2008, 27, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Koliou, M.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Kawasaki disease and Anaplasma sp. infection of an infant in Cyprus. Int. J. Infect. Dis. 2009, 13, 71. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Sharif, L.; Kokkini, S.; Hristophi, N.; Dimitriou, T.; Tselentis, Y.; Psaroulaki, A. Prevalence of Anaplasma sp. in goats and sheep in Cyprus. Vector Borne Zoonotic. Dis. 2009, 9, 457–463. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Chochlakis, D.; Sandalakis, V.; Vranakis, I.; Ioannou, I.; Tselentis, Y. Phylogentic analysis of Anaplasma ovis strains isolated from sheep and goats using groEL and mps4 genes. Vet. Microbiol. 2009, 138, 394–400. [Google Scholar] [CrossRef]
- Bouzouraa, T.; René-Martellet, M.; Chêne, J.; Attipa, C.; Lebert, I.; Chalvet-Monfray, K.; Cadoré, J.; Halos, L.; Chabanne, L. Clinical and laboratory features of canine Anaplasma platys infection in 32 naturally infected dogs in the Mediterranean basin. Ticks Tick Borne Dis. 2016, 7, 1256–1264. [Google Scholar] [CrossRef]
- Attipa, C.; Hicks, C.A.E.; Barker, E.N.; Christodoulou, V.; Neofytou, K.; Mylonakis, M.E.; Siarkou, V.I.; Vingopoulou, E.I.; Soutter, F.; Chochlakis, D.; et al. Canine tick-borne pathogens in Cyprus and a unique canine case of multiple co-infections. Ticks Tick Borne Dis. 2017, 8, 341–346. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef]
- Attipa, C.; Solano-Gallego, L.; Leutenegger, C.M.; Papasouliotis, K.; Soutter, F.; Balzer, J.; Carver, S.; Buch, J.S.; Tasker, S. Associations between clinical canine leishmaniosis and multiple vector-borne co-infections: A case-control serological study. BMC Vet. Res. 2019, 15, 331–336. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Chochlakis, D.; Ioannou, I.; Florentia, A.; Gikas, A.; Tselentis, Y. Acute anaplasmosis in humans in Cyprus. Clin. Microbiol. Infect. 2009, 15 (Suppl. 2), 10–11. [Google Scholar] [CrossRef]
- Constantinou, K. Anopheles (malaria) eradication in Cyprus. Parassitologia 1998, 40, 131–135. [Google Scholar]
- Güler, E.; Özbilgin, A.; Çavuş, İ.; Şanlıdağ, T.; Süer, K. Evaluation of Imported Malaria Cases in Northern Cyprus between 2016 and 2019: First Data Series. Turkiye Parazitol. Derg. 2020, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Violaris, M.; Vasquez, M.I.; Samanidou, A.; Wirth, M.C.; Hadjivassilis, A. The mosquito fauna of the Republic of Cyprus: A revised list. J. Am. Mosq. Control Assoc. 2009, 25, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, M.; Rodgers, F.H.; Yerbanga, R.S.; Ouédraogo, J.B.; Basáñez, M.; Cohuet, A.; Christophides, G.K. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat. Commun. 2015, 6, 5921. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA—A One Health Response; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2022. [Google Scholar]
- Todeschini, R.; Musti, M.A.; Pandolfi, P.; Troncatti, M.; Baldini, M.; Resi, D.; Natalini, S.; Bergamini, F.; Galletti, G.; Santi, A.; et al. Re-emergence of human leishmaniasis in northern Italy, 2004 to 2022: A retrospective analysis. Euro. Surveill. 2024, 29, 2300190. [Google Scholar] [CrossRef]
- Yetismis, K.; Erguler, K.; Angelidou, I.; Yetismis, S.; Fawcett, J.; Foroma, E.; Jarraud, N.; Ozbel, Y.; Martinou, A.F. Establishing the Aedes watch out network, the first island-wide mosquito citizen-science initiative in Cyprus within the framework of the Mosquitoes Without Borders project. MBI 2022, 13, 798. [Google Scholar] [CrossRef]
- Ergunay, K.; Kasap, O.E.; Orsten, S.; Oter, K.; Gunay, F.; Yoldar, A.Z.A.; Dincer, E.; Alten, B.; Ozkul, A. Phlebovirus and Leishmania detection in sandflies from eastern Thrace and northern Cyprus. Parasit. Vectors 2014, 7, 575–576. [Google Scholar] [CrossRef]
- Mazeris, A.; Soteriadou, K.; Dedet, J.P.; Haralambous, C.; Tsatsaris, A.; Moschandreas, J.; Messaritakis, I.; Christodoulou, V.; Papadopoulos, B.; Ivovic, V.; et al. Leishmaniases and the Cyprus paradox. Am. J. Trop. Med. Hyg. 2010, 82, 441–448. [Google Scholar] [CrossRef]
- Léger, N.; Depaquit, J. Leishmania donovani leishmaniasis in Cyprus. Lancet Infect Dis 2008, 8, 402–404. [Google Scholar] [CrossRef]
- Svobodová, M.; Alten, B.; Zídková, L.; Dvorák, V.; Hlavacková, J.; Mysková, J.; Seblová, V.; Kasap, O.E.; Belen, A.; Votýpka, J.; et al. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int. J. Parasitol. 2009, 39, 251–256. [Google Scholar] [CrossRef]
- Sayili, A.; Ozkan, A.T.; Schallig, H.D.F.H. Pediatric Visceral Leishmaniasis Caused by Leishmania infantum in Northern Cyprus. Am. J. Trop. Med. Hyg. 2016, 95, 1386–1388. [Google Scholar] [CrossRef]
- Antoniou, M.; Haralambous, C.; Mazeris, A.; Pratlong, F.; Dedet, J.; Soteriadou, K. Leishmania donovani leishmaniasis in Cyprus. Lancet Infect. Dis. 2009, 9, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Koliou, M.G.; Antoniou, Y.; Antoniou, M.; Christodoulou, V.; Mazeris, A.; Soteriades, E.S. A cluster of four cases of cutaneous leishmaniasis by Leishmania donovani in Cyprus: A case series. J. Med. Case Rep. 2014, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, P.; Ascione, T. Pediatric Visceral Leishmaniasis Caused by Leishmania infantum in Northern Cyprus. Am. J. Trop. Med. Hyg. 2017, 96, 758. [Google Scholar] [CrossRef] [PubMed]
- Koliou, M.G.; Soteriades, E.S.; Ephros, M.; Mazeris, A.; Antoniou, M.; Elia, A.; Novelli, V. Hemophagocytic lymphohistiocytosis associated with Epstein Barr virus and Leishmania donovani coinfection in a child from Cyprus. J. Pediatr. Hematol. Oncol. 2008, 30, 704–707. [Google Scholar] [CrossRef]
- Poeppl, W.; Walochnik, J.; Pustelnik, T.; Auer, H.; Mooseder, G. Cutaneous leishmaniasis after travel to Cyprus and successful treatment with miltefosine. Am. J. Trop. Med. Hyg. 2011, 84, 562–565. [Google Scholar] [CrossRef]
- Alam, M.Z.; Haralambous, C.; Kuhls, K.; Gouzelou, E.; Sgouras, D.; Soteriadou, K.; Schnur, L.; Pratlong, F.; Schönian, G. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009, 11, 707–715. [Google Scholar] [CrossRef]
- Tsirigotakis, N.; Christodoulou, V.; Ntais, P.; Mazeris, A.; Koutala, E.; Messaritakis, I.; Antoniou, M. Geographical Distribution of MDR1 Expression in Leishmania Isolates, from Greece and Cyprus, Measured by the Rhodamine-123 Efflux Potential of the Isolates, Using Flow Cytometry. Am. J. Trop. Med. Hyg. 2016, 94, 987–992. [Google Scholar] [CrossRef]
- Attipa, C.; Papasouliotis, K.; Solano-Gallego, L.; Baneth, G.; Nachum-Biala, Y.; Sarvani, E.; Knowles, T.G.; Mengi, S.; Morris, D.; Helps, C.; et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit. Vectors 2017, 10, 130–132. [Google Scholar] [CrossRef]
- Beyhan, Y.E.; Çelebi, B.; Ergene, O.; Mungan, M. Seroprevalance of Leishmaniasis in Dogs from Hatay and Burdur Provinces of Turkey and Northern Cyprus. Turkiye Parazitol. Derg. 2016, 40, 9–12. [Google Scholar] [CrossRef]
- Töz, S.O.; Ertabaklar, H.; Göçmen, B.; Demir, S.; Karakuş, M.; Arserim, S.K.; Balcıoğlu, I.C.; Canakçı, T.; Ozbel, Y. An epidemiological study on canine leishmaniasis (CanL) and sand flies in Northern Cyprus. Turkiye Parazitol. Derg. 2013, 37, 107–112. [Google Scholar] [CrossRef]
- Çanakçı, T.; Kurtdede, A.; Paşa, S.; Töz Özensoy, S.; Özbel, Y. Seroprevalence of Canine Leishmaniasis in Northern Cyprus. Turkiye Parazitol. Derg. 2016, 40, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Grimm, F.; Papaprodromou, M.; Cavaliero, T.; Gramiccia, M.; Christofi, G.; Christofi, N.; Economides, P.; Eckert, J. Canine leishmaniosis in Cyprus due to Leishmania infantum MON 1. Acta Trop. 1998, 71, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Berriatua, E.; Pérez-Cutillas, P.; Vidal, A.G.; Briët, O.J.T. The spatial relationship between leishmaniases and sand flies in Europe and neighboring countries. Parasites Vectors 2024, 17, 404. [Google Scholar] [CrossRef] [PubMed]
| Year | Month | Place of Infection | Number of Cases ** |
|---|---|---|---|
| 2016 | 8 | Cyprus | <5 |
| 2018 | 9 | Cyprus | <5 |
| 2019 | 7 | Cyprus | <5 |
| 2019 | 8 | Cyprus | <5 |
| 2019 | 8 | Cyprus | 14 |
| 2019 | 9 | Cyprus | 5 |
| 2019 | 10 | Cyprus | <5 |
| 2021 | 7 | Cyprus | <5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkan, E.; Karanis, P. Current Risks and Prevention Strategies Against Vector-Borne Diseases in Cyprus. Microorganisms 2025, 13, 726. https://doi.org/10.3390/microorganisms13040726
Volkan E, Karanis P. Current Risks and Prevention Strategies Against Vector-Borne Diseases in Cyprus. Microorganisms. 2025; 13(4):726. https://doi.org/10.3390/microorganisms13040726
Chicago/Turabian StyleVolkan, Ender, and Panagiotis Karanis. 2025. "Current Risks and Prevention Strategies Against Vector-Borne Diseases in Cyprus" Microorganisms 13, no. 4: 726. https://doi.org/10.3390/microorganisms13040726
APA StyleVolkan, E., & Karanis, P. (2025). Current Risks and Prevention Strategies Against Vector-Borne Diseases in Cyprus. Microorganisms, 13(4), 726. https://doi.org/10.3390/microorganisms13040726







