Abstract
Probiotics are increasingly used to promote oral health, with Lacticaseibacillus rhamnosus demonstrating proven effectiveness. Additionally, Heyndrickxia coagulans shows promising potential in this field. Chewing gum has recently been proposed as an innovative delivery method for probiotics. This study aimed to evaluate the kinetics in saliva of Heyndrickxia coagulans SNZ1969® and Lacticaseibacillus rhamnosus GG in microencapsulated and non-microencapsulated forms (LGG®) following their administration via sugar-free chewing gums. A randomized cross-over trial was conducted involving 10 volunteers. Participants chewed gums containing one of the probiotic strains for 10 min. Saliva samples were collected at baseline (T0) and six subsequent time points over 2 h (T1–T6). Colony-forming units (CFUs) were identified and quantified. The Tukey’s range test was applied to make pairwise comparisons between different probiotics at every time point, between different time points of the same probiotic, and between the area under the curve describing the kinetics of different probiotics in saliva. At T1, all probiotics exhibited peak counts, followed by a gradual decline until T6. H. coagulans SNZ1969® achieved the highest counts at T1, T2, and T3 (mean log10 CFU/mL: 6.1 ± 0.5; 5.8 ± 0.5; 5.6 ± 0.5, respectively), while the non-microencapsulated form of LGG® peaked at T4, T5, and T6 (mean log10 CFU/mL: 4.0 ± 0.7; 3.8 ± 0.9; 3.3 ± 1.3, respectively). The participants reported no adverse effects. Probiotics were detectable in saliva up to 2 h post-administration via chewing gum, indicating its suitability as a delivery vehicle. However, significant variability was observed among participants.
1. Introduction
The oral cavity is intimately connected to other systems and organs, as evidenced by associations between oral health and conditions such as cardiovascular disease, diabetes, lung disease, and pregnancy [1]. These associations can be ascribed to several factors, including alterations in the community structure of the oral microbiota.
Probiotics have become widely used over the last two decades. They are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” [2]. Initially recognized for their gastrointestinal benefits, probiotics have been extensively studied for their ability to prevent and treat gastrointestinal diseases, enhance digestive well-being, and combat pathogens [3,4,5,6,7,8]. These benefits contributed to a growing awareness of their positive effects and a subsequent increase in their use [9]. Consequently, the global probiotics market is projected to grow from USD 71.2 billion in 2024 to USD 105.7 billion in 2029, at an annual growth rate of 8.2% expected over this period [10].
Beyond gastrointestinal health, probiotics have demonstrated potential benefits for oral health, including caries, halitosis, and periodontitis, in healthy individuals and those with systemic diseases [11,12,13]. Conditions such as dental caries and periodontal disease have been associated with an imbalance in the oral microbiota. This association is explained by the “ecological plaque hypothesis” [14] and probiotic administration has shown promise in addressing this imbalance. Some of the most studied and promising strains include Lactobacillus rhamnosus, Lactobacillus reuteri, Lactobacillus acidophilus, Lactobacillus salivarius, Lactobacillus casei and paracasei, Bifidobacterium lactis, and various others [15]. As evident from the above, all strains have demonstrated beneficial effects on the oral cavity. Lacticaseibacillus rhamnosus GG is one of the most extensively investigated, and it has shown its ability to counteract the most prevalent diseases of the gums and teeth, dental caries, and periodontal disease [16,17,18,19]. In addition, it has shown an inhibitory effect on halitosis-causing bacteria, such as Porphyromonas gingivalis, Tannerella forsythia, and Prevotella intermedia [20] and a strong anticandidal activity [21]. All these properties make it a remarkably versatile and effective microorganism for promoting oral health and preventing common oral diseases.
Spore-forming probiotics are experiencing an increase in popularity, attributable to their capacity to enhance survival and stability. In the field of functional food research related to human health, there is an increasing focus on Bacillus spp. due to their remarkable tolerance and survivability in the harsh conditions of the gastrointestinal tract. Furthermore, their superior stability during food and pharmaceutical processing and storage renders them ideal candidates for health-promoting formulations [22]. In contrast, vegetative probiotic species are more sensitive to these processes and often require refrigeration to maintain their potency [23]. Microencapsulation is proposed as an effective method for protecting probiotics, enhancing their viability during industrial processing, and extending their stability during storage and digestion [24].
Heyndrickxia coagulans (formerly Bacillus coagulans) has demonstrated antimicrobial, antioxidant, and immunomodulatory properties [25], and it has recently received considerable attention in dentistry [26,27]. Recent studies highlight its effectiveness in controlling dental caries by reducing Streptococcus mutans and Lactobacillus spp. counts in plaque and saliva [23,26]. Additionally, they have been shown to lower gingival index scores, reduce bleeding on probing, and combat gingival inflammation [28]. H. coagulans is listed by the European Food Safety Authority (EFSA) under the Qualified Presumption of Safety status for recommended biological agents. Its use has been approved due to the absence of acquired antimicrobial resistance genes to clinically relevant antibiotics and the lack of toxigenic activity [29], unlike other Bacillus spp. [30].
Sugar-free chewing gum positively affects oral health by stimulating saliva flow and promoting natural oral clearance mechanisms [31,32,33,34]. Moreover, it can serve as a delivery system for ingredients such as xylitol or fluoride, active in reducing dental plaque and the concentration of cariogenic bacteria, such as Streptococci mutans, and in remineralizing enamel, respectively [35,36]. The EFSA (European Food Safety Authority) has acknowledged the potential benefits of chewing sugar-free gum for oral health, including maintaining tooth mineralization, neutralizing plaque acids, and the reduction in oral dryness. To reap these benefits, the EFSA has recommended that individuals chew 2–3 g of sugar-free gum for at least 20 min, at least three times per day following mealtimes [37,38,39]. Sugar-free chewing gums are composed of a gum base, with the sugar being entirely replaced by alternative bulk sweeteners consisting of one or a combination of several polyols, such as sorbitol, mannitol, isomalt, maltitol, maltitol syrup, lactitol, xylitol, and erythritol. High-intensity sweeteners, including acesulfame K, aspartame, cyclamic acid and its sodium and calcium salts, saccharin and its sodium, potassium, and calcium salts, sucralose, thaumatin, neohesperidine dihydrochalcone, and the aspartame–acesulfame salt, are commonly used alone or in combination with other food additives and flavors [40]. Moreover, chewing gum is experiencing a surge in popularity as an oral drug delivery system, owing to its ease of use and palatability. Its applications extend to the domains of pain management, smoking cessation, and anti-emetic therapies. [41,42]. Chewing gum is particularly suitable for school children and adolescents, given the high compliance rates observed in these age groups [18].
The administration of probiotics through sugar-free chewing gum has demonstrated encouraging results in enhancing oral health by substantially reducing plaque accumulation, gingival scores, S. mutans counts, and bleeding on probing. Furthermore, a reduction in inflammatory mediator levels in gingival crevicular fluid, a major indicator of periodontal disease, has been observed. In addition to these benefits, probiotics have also been found to assist in the alleviation of halitosis (bad breath) [43,44,45,46,47]. The benefits mentioned above suggest that delivering probiotics via chewing gum could be an effective adjunct in managing oral conditions. Although studies have demonstrated the clinical efficacy of sugar-free probiotic chewing gum, it is imperative to ascertain the amount of the administered probiotic that remains in the oral cavity, as this directly influences its ability to colonize oral surfaces and ensure a lasting effect [48,49]. While the release and concentration of various active agents delivered via chewing gum have been investigated in both in vitro and in vivo studies [36,50], no research has evaluated the release kinetics of probiotics from chewing gum to date. Therefore, an in vivo microbiological study was designed to analyze the kinetics of probiotics in saliva in 2 h follow-up, following their administration through sugar-free chewing gum. The hypothesis to be tested is that the probiotic administered through chewing gum remains in saliva long enough for it to adhere to oral surfaces [51]. This study constitutes the initial phase of a more comprehensive research initiative. The subsequent stage of the project will entail an evaluation of the capacity of probiotics administered via sugar-free chewing gum to colonize oral surfaces and modify the microbial diversity of the oral microbiome.
2. Materials and Methods
2.1. Design of the Study
This randomized, cross-over microbiological study was designed and conducted at the Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy, following the principles of the Declaration of Helsinki. The Ethical Committee of the University of Milan approved the study (13 February 2024, no. 24/24). Recruitment of participants, intervention, and microbiological measurements were carried out between March and May 2024. This study represents the initial phase of a larger research project designed to evaluate the ability of probiotics delivered via chewing gum to colonize the oral cavity and dental plaque.
2.2. Sample Selection
The study was conducted on healthy adult volunteers selected from the staff of the Perfetti Van Melle S.p.A. (Lainate, Milan, Italy).
No prior studies with the same objective were identified, so a sample size calculation could not be performed. Therefore, conducting an in vivo microbiological study with 10 participants was decided. The inclusion criteria were adult subjects aged 18 to 64 years, at least 24 natural teeth (excluding third molars), gingival index and plaque index scores ≤ 2, and a stimulated salivary flow rate between 1.5 and 2.0 mL/min. Exclusion criteria included the presence of systemic diseases, pregnancy or lactation, history of drug abuse, smoking habits, use of fixed orthodontic appliances, and allergies to any ingredients in the chewing gums used. An email explaining the purpose of the study and inviting participation was sent to all staff at the PVM site in Lainate. Thirteen individuals who consented to participate were interviewed to assess their eligibility based on the inclusion and exclusion criteria. They were then examined by a calibrated dentist (SC) to obtain their gingival index scores [52], gingival index [53], and stimulated salivary flow rate. Ten eligible subjects were identified and enrolled. All study participants gave their written consent to participate.
2.3. Chewing Gums Production
All chewing gums used in the study were produced and supplied by PMV (Figure S2). The sugar-free chewing gums (weight 2.1 g) were formulated with gum base (Gum Base Co., Lainate, Milan, Italy), food-grade polyols, excluding xylitol (proprietary blend; manufactured by Roquette Frères S.A., Cassano Spinola, Alessandria, Italy and Cargill S.r.l., Milan, Italy), food-grade intensive sweeteners (Ajinomoto Co., Inc., Tokyo, Japan), flavors (Mondarom Selegroven AG, Bironico, Switzerland), and incorporated specific probiotic strains under investigation. These included Lacticaseibacillus rhamnosus GG provided either in its non-microencapsulated form (LGG®, DSMZ code: DSM 33156, supplied by Chr. Hansen, Boege Alle 10-12, 2970 Hoersholm, Denmark) or as microencapsulated cell (EncaptimusTM, ATCC 53103, provided by AnaBio Technologies Ltd., 11 Herbert Street, Dublin, D02 RW27, Ireland; containing Maltodextrin, Lactobacillus rhamnosus, Coconut Oil, Pea Protein Isolate, Polysorbate 20) [54], and Heyndrickxia coagulans SNZ1969 (Heyndrickxia coagulans SNZ1969®, provided by Sanzyme Biologics Ltd., Sattva Signature Tower, H. No. 8-2-472/1/A/B/SF-3, Road No. 1, Banjara Hills, Hyderabad, 500034 Telangana, India), which was added in its spore form. Therefore, three different chewing gums were produced.
The production process of the chewing gum is summarized in the Supplementary File (Supplementary Figure S1). Initially, the gum base is melted at 50 °C and combined with polyols, artificial intense sweeteners, and flavorings sequentially, achieving a homogeneous mixture. The freeze-dried probiotic biomass is incorporated as a final component below 50 °C to preserve its activity. After mixing, in the rolling and scoring system, a mass of gum is extruded into a thick slab, which is then worked into a thinner and thinner foil by a series of rollers. Finally, the foil is shaped into single pieces by one or more cutting rollers. These pieces undergo cooling in a conditioning room before being panned, and afterward, individual pieces are prepared for packaging [55].
At the end of the production process, the amount of Colony-Forming Units (CFUs) contained in the chewing gum was assessed for each probiotic strain according to the following protocol. The gum pieces in the bag were manually crushed and then processed in a Stomacher® (Stomacher® 3500 peristaltic homogenizer, Seward, West Sussex, UK) for 2 min. The resulting homogenized chewing gum mass was further serially diluted 1:10 in Maximum Recovery Diluent (MRD) buffer (GranuCult® prime Peptone salt solution—Maximum Recovery Diluent, Merck KGaA, Darmstadt, Germany). Aliquots of 100 μL were plated onto selective media: GYEA-agar (Glycerol 5 g/L, Yeast extract 2 g/L, K2HPO4 1 g/L, BromoCresol Green 10 mL/l from a stock of 5 g/L, Agar 15 g/L pH 5.5, Merck KGaA, Darmstadt, Germany) for H. coagulans SNZ1969® and RVB-MRS-agar (Lactobacilli MRS w/o Dextrose 3515 g/L, Condalab, Madrid, Spain; Rhamnose 20 g/L, Bromocresol green 10 mL/l from a stock of 5 g/L, Agar 15 g/L pH 5.5 after autoclave, Cysteine-HCl 0.05%, Vancomycin 50 mg/L, Merck KGaA, Darmstadt, Germany) for L. rhamnosus GG). For H. coagulans SNZ1969®, part of the samples underwent viable count after pasteurization (incubation in a water bath at 90° C for 10 min) to quantify bacterial spores. The plates were incubated at 37° C for 72 h for the evaluation of L. rhamnosus GG and at 55° C for 72 h in anaerobic conditions, established by incubating the plates in AnaeroJar Oxoid 2.5 L jars (Thermo Fisher Scientific™, Waltham, MA, USA) containing Anaerocult™ A (Merck KGaA, Darmstadt, Germany), for the assessment of H. coagulans SNZ1969® CFUs. The procedures described were repeated for each sample in triplicate. CFUs were identified by morphology and color and finally counted. The viable bacterial cell counts were expressed as colony-forming units/gram (CFU/g).
At the end of the production process, the mean counts of probiotics in one pellet of chewing gum were as follows:
- -
- 6 × 108 CFU of Lacticaseibacillus rhamnosus LGG® (non-microencapsulated form);
- -
- 2 × 108 CFU of Lacticaseibacillus rhamnosus GG (microencapsulated);
- -
- 5 × 108 CFU of Heyndrickxia coagulans SNZ1969®.
2.4. Use of Chewing Gum
Participants were instructed to chew a pellet of gum containing one of the probiotic strains. Following a washout period of one week, they were asked to chew a second pellet containing a different strain. After another one-week washout period, they chewed the third and final pellet. At each visit, each subject was administered only one formulation per session in a randomized cross-over design to ensure that the efficacy of each formulation was assessed independently. Each subject drew one of three chewing gums at the first appointment using a lottery system. They drew one of the two remaining gums at the second appointment, and the last remaining gum was administered at the third appointment. Administration occurred in the morning, at least 2 h after breakfast and oral hygiene routines.
Both participants and investigators were blinded to the specific probiotic strain in each gum. Volunteers were instructed to chew the gum for 10 min and to refrain from eating or drinking anything for the subsequent 2 h.
Saliva samples of at least 0.5 mL were collected from the floor of the mouth using sterile disposables at the following time points: before chewing gum use (T0), and at 1, 5, 10, and 20 min, as well as 1 and 2 h after the procedure began (T1–T6). Samples were stored at 4 °C, transported to the laboratory, and processed within 2 h.
An investigator (SC) conducted brief telephone interviews with the participants on the evening of the intervention day and one week later to record any side effects associated with the chewing gum administered.
The flow chart of the study design is presented in Figure 1. At the first appointment, each participant chewed one randomly selected chewing gum from the three gums tested in the study. At the second appointment, they chewed one of the two remaining gums, and at the final appointment, the last remaining gum. This process was repeated for each participant, ensuring that all three gums were tested in a randomized order across the different subjects. Each chewing gum was chewed 2 h after breakfast and the oral hygiene routine. Salivary samples were then collected over 2 h and analyzed microbiologically.
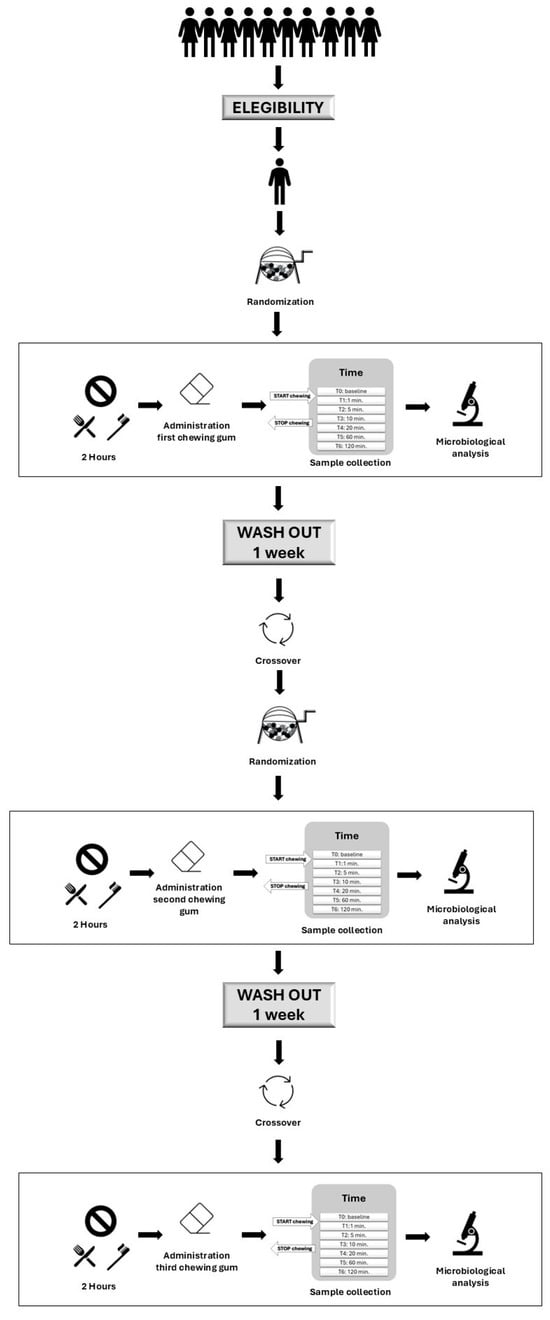
Figure 1.
Flow chart of the study design.
2.5. Microbiological Analyses
Aliquots of 300 μL of saliva were diluted in 600 μL of MDR buffer (GranuCult® prime Peptone salt solution—Maximum Recovery Diluent, Merck KGaA, Darmstadt, Germany). Samples were processed as it was explained for chewing gum analysis, but without any preventive treatment to break down the microcapsules. If different morphologies were detected, three colonies per type were selected and analyzed by colony polymerase chain reaction (PCR), picking the colony into a PCR reaction with the strain-specific primers PVM-Wc-1F 5′-TTGTCTTTGGATCAGTTACAG-3′ and PVM-Wc-1R 5′-GCATAGGAATACCTTGTGCA-3′ for H. coagulans SNZ1969® [56] and the primers GG I 5′-CAATCTGAATGAACAGTTGTC-3′ and GG II 5′-TATCTTGACCAAACTTGACG-3′ for L. rhamnosus GG [57]. Morphologies that revealed expected amplicons by agarose gel electrophoresis were confirmed as CFUs and included in the final count. Some of the amplicons obtained were confirmed by Sanger sequencing [58]. The amount of viable H. coagulans SNZ1969® (without spores) was obtained by subtracting the total H. coagulans colony count minus the colonies of pasteurized H. coagulans SNZ1969® (spores) for each interval.
2.6. Statistical Analysis
All data were transformed into a logarithmic scale to normalize the distribution. Values between 0 and 1 were rounded to 1 before the log transformation.
The ANOVA test was applied. When the variance, evaluated with Bartlet’s equal-variances test, was not equal, Welch’s t test was used to assess differences between probiotics. Tukey’s range test was applied to make pairwise comparisons between different probiotics at every time point, different time points of the same probiotic, and between the area under the curve of different probiotics. The area under the curve was calculated to quantify the overall exposure of the subject’s oral cavity to probiotic strains over time using the following STATA commands: mean_log_counts of the different probiotics and then the mean trapezoid_area, over the probiotic counts. Cuzick’s test with rank scores was used to describe trends within each strain during time. All data were analyzed using STATA® software (v18 for Mac). Statistical significance was set at α = 0.05 for all analyses.
3. Results
All included subjects, 6 females and 4 males, age range 23–52 years (mean age 36.4 ± 10.0), completed the study. No adverse effects were reported by the participants or noted by the investigators during the intervention.
At T0, none of the probiotics tested was detected in saliva. At T1, the highest counts’ value was registered for all probiotic strains, followed by a constant decrease until T6.
L. rhamnosus GG in microencapsulated form showed the lowest salivary counts at each time point (p < 0.01) (Supplementary Table S1; Figure 2).
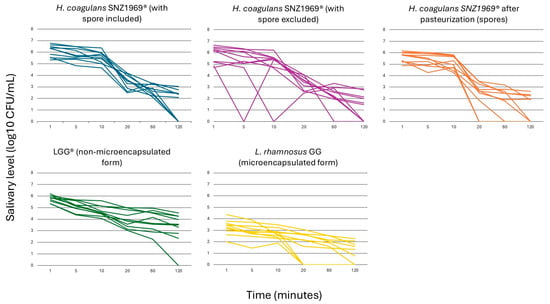
Figure 2.
Viable count kinetics of probiotic cells in salivary samples.
When comparing H. coagulans SNZ1969® and L. rhamnosus LGG® in non-microencapsulated form, the total viable counts of H. coagulans SNZ1969® (i.e., spores + vegetative cells) were higher at T1, T2, and T3. In comparison, the viable counts of L. rhamnosus LGG® were higher at T4, T5, and T6. However, no significant differences were observed between the two probiotic strains (p = 0.64 at T1; p = 0.52 at T2; p = 0.24 at T3; p = 0.67 at T4; p = 0.08 at T5; p = 0.02 at T6) (Supplementary Table S1; Figure 2).
At T1, T4, T5, and T6, the proportion of H. coagulans SNZ1969® in vegetative form was higher than that in spore form, but a significant difference was observed at T4, 20 min after the start of chewing (3.3 ± 0.6 log10 CFU/mL vs. 2.3 ± 1.2 log10 CFU/mL; p = 0.01) (Supplementary Table S1; Figure 2).
A high variability in the salivary counts among participants was observed for all the probiotic strains tested (Figure 2; Supplementary Figure S3).
All probiotic strains exhibited significant declines in salivary counts from T1 to T6 (p < 0.01) (Supplementary Table S1). Notably, the patterns differed between L. rhamnosus GG and H. coagulans SNZ1969®. For both microencapsulated and non-microencapsulated forms of L. rhamnosus GG, the counts’ differences between consecutive time points were not statistically significant, indicating a consistent linear decrease (Supplementary Table S1). In contrast, the total counts of H. coagulans SNZ1969® (counts of vegetative forms + spores) and its pasteurized form (spore counts) showed a rapid decline between T3 and T4 (p < 0.01). The total counts of SNZ1969® also exhibited a significant drop between T5 and T6 (p < 0.01) (Supplementary Table S1). However, no significant differences were observed at each time point for H. coagulans SNZ1969® with spores excluded (Supplementary Table S1).
Considering the area under the curve, a significant difference was detected between L. rhamnosus LGG® in non-microencapsulated form, L. rhamnosus GG in microencapsulated form, and H. coagulans SNZ1969® (i.e., spores + vegetative cells) (p < 0.01). Otherwise, significant differences between L. rhamnosus LGG® and H. coagulans SNZ1969® (i.e., spores + vegetative cells) were not detected (p = 0.90) (Figure 3).
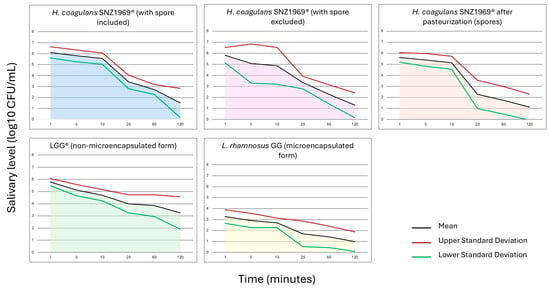
Figure 3.
Area under the curve of probiotic cells in salivary samples.
4. Discussion
This viable microbiological study investigates the kinetics of L. rhamnosus GG and H. coagulans SNZ1969® released from sugar-free chewing gums. Despite considerable variability in salivary counts among participants, the highest bacterial amount was attained after 1 min of chewing, with levels persisting at relatively high counts for up to 10 min. In some subjects, probiotics were still detectable in saliva approximately 2 h after chewing commenced.
Although there is a paucity of research in this area, studies have been conducted on the oral health benefits of probiotics administered via chewing gum. However, these studies have not specifically examined the kinetics of probiotics in saliva, focusing on their effects on oral biofilm, such as reducing S. mutans counts in saliva or dental plaque [45,59].
Few studies have evaluated the kinetics of probiotics obtained with different vehicles. Lozenges were shown to expose the oral environment to probiotics for an estimated period of tens of minutes [45,60]. The utilization of a mucoadhesive lipogel comprising probiotics resulted in a substantial and consistent release for 5 to 8 h in an in vitro model [61]. In the present study, chewing gum appears to promote the presence of probiotics in saliva for a longer time than lozenges but shorter than those obtained by lipogel in vitro. As anticipated, the probiotic load diminished following the gum mastication, yet it remained quantifiable in most subjects 2 h post-ingestion. This comparatively protracted residence time in the oral environment may play a pivotal role in plaque colonization by the delivered bacteria.
Active agents administered via sugar-free chewing gum exhibited analogous kinetic trends in saliva. The investigation focused on the salivary concentration of xylitol and fluoride produced by sugar-free chewing gum [36,62], which showed a salivary peak in the first 5 min, followed by a rapid decrease in the next 5 min and a slow decline in the tens of minutes to follow. The salivary counts of the probiotics tested in this study initially peaked analogous to that of xylitol and fluoride. However, this was followed either by a gradual decrease, as seen with L. rhamnosus GG, or by a later but rapid decline (after 10 min), as observed with H. coagulans SNZ1969®. The decline in probiotic counts is mainly due to swallowing, which is intensified by the increased salivary flow stimulated by chewing gum. Additionally, the potential bactericidal action of salivary molecules cannot be ruled out [31,32,33,34].
The significant variability observed in the salivary counts of the probiotics among the ten study participants is a matter of concern. Despite the inclusion criterion of normal salivary flow, it can be hypothesized that such substantial inter-individual differences are not related to the efficacy of chewing gum in releasing probiotic strains. Instead, they are likely to be attributable to the individual characteristics of the participants, such as the strength and number of chewing movements per unit of time [63]. Additionally, the different stimulation of saliva among subjects caused by the fruit flavoring in the chewing gum could further explain the observed variability [64].
One aspect that has been the focus of academic inquiry is microencapsulation’s protective effect on chewing gum’s bacterial survival. The study revealed that microencapsulation significantly enhanced the survival of Limosilactobacillus reuteri in chewing gum over 21 days. The protective role of inulin and lecithin in this process has been postulated [65]. In a recent study, microencapsulation improved the viability of Bifidobacterium animalis subsp. lactis but not of Levilactobacillus brevis [61,66]. Although the encapsulated form may offer advantages in terms of microbial survival within the product, the results of the present study indicate that L. rhamnosus GG in its microencapsulated form produced a lower salivary count than the non-microencapsulated form, and by far the lowest among the probiotics tested. This finding suggests that the enhanced survival observed in the chewing gum product is counterbalanced by a diminished capacity to release the strain in its non-microencapsulated form. Consequently, probiotics in microencapsulated form may be advantageous for gastrointestinal effects but may not be as effective for promoting oral health.
There are certain limitations to this study. The first limitation is the limited sample size, which limits the generalizability of the results to a larger population [67,68]. Probiotic studies designed and conducted for gastrointestinal application usually had a low number of participants as they focus solely on the microbiological performance of the probiotic strain, independent of any direct effects on the host. The present research aligns with this established approach, as its primary objective was to quantify probiotic cells’ presence in saliva after chewing gum administration. Given this specific focus, the sample size is fully consistent with existing literature. Numerous published works in this domain have employed similar sample sizes, as the primary endpoint (bacterial recovery) does not require the large cohorts typically associated with clinical efficacy trials. Furthermore, participants were selected according to rigorous criteria (e.g., number of teeth, salivary flow, and oral health status) to minimize uncontrolled variability, and the cross-over design enhances the reliability of the results of this study by allowing within-subject comparisons, thereby reducing inter-individual variability [66].
In the present study, it was ensured by verifying that none of the participants had detectable counts of the tested probiotic strains in their saliva before chewing the gum (T0 time point). A control group using a probiotic-free chewing gum would not provide additional meaningful information in this context, as this study does not aim to evaluate clinical effects or host responses but rather the presence of the administered probiotic in saliva. The presence or absence of probiotics in post-administration samples can be directly attributed to the intervention itself, as confirmed by the absence of these strains in baseline samples.
Further research involving larger cohorts is necessary to draw definitive conclusions regarding the kinetics of probiotics released from sugar-free chewing gum, as this factor may significantly influence their functionality within the oral environment. Furthermore, the considerable variability observed in the counts of probiotics among the study participants requires further investigation, as it could substantially impact their efficacy. The present research shows that some individuals displace bacteria more quickly than others, underscoring the necessity for personalized approaches when contemplating probiotic interventions, as individual differences can influence the effectiveness of such treatments.
5. Conclusions
This study demonstrated that a 10 min use of sugar-free chewing gum containing probiotics led to their detectable presence in saliva. L. rhamnosus GG and H. coagulans SNZ1969® exhibited comparable salivary counts, suggesting that both strains are well-suited for this mode of administration.
These findings represent the initial phase of a broader research initiative. It is well known that a single administration of probiotics is not capable of permanently modifying the composition of the oral bacterial flora [69]. Further investigations are needed to determine the ideal duration of the probiotic’s presence in the oral cavity to support colonization of the oral biofilm, thus improving its long-term efficacy. Moreover, the ability of probiotics administered via sugar-free chewing gum to influence microbial diversity following prolonged administration will be evaluated in the next part of this research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13040721/s1, Figure S1. The chewing gum production process; Figure S2. Chewing gum used in the study; Table S1. Salivary counts (log10 CFU/mL) at different time points of the probiotic strains; Figure S3. Viable count kinetics of probiotic cells in salivary samples of each subject.
Author Contributions
Conceptualization, S.C. and M.G.C.; methodology, S.D.G.; validation, S.C., C.S. and M.G.C.; microbiological analysis, V.M.; investigation, S.C.; data curation, C.S. and G.C.; writing—original draft preparation, S.C., C.S., V.M., N.K. and A.S.; writing—review and editing, M.G.C. and G.C.; supervision, M.G.C., G.C. and S.D.G.; funding acquisition, M.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Perfetti Van Melle S.p.A.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Milan (13 February 2024, no. 24/24) for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on reasonable request by contacting the corresponding author to not disclose information as we are patent pending.
Conflicts of Interest
A.S. and N.K. are employees of the company Perfetti Van Melle that funded the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EFSA | European Food Safety Authority |
| CFUs | Colony-Forming Units |
| MDR | Maximum Recovery Diluent |
References
- Fiorillo, L. Medicina Editorial Oral Health: The First Step to Well-Being. Medicina 2019, 55, 676. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Rasaei, N.; Heidari, M.; Esmaeili, F.; Khosravi, S.; Baeeri, M.; Tabatabaei-Malazy, O.; Emamgholipour, S. The Effects of Prebiotic, Probiotic or Synbiotic Supplementation on Overweight/Obesity Indicators: An Umbrella Review of the Trials’ Meta-Analyses. Front. Endocrinol. 2024, 15, 1277921. [Google Scholar] [CrossRef]
- Qing, Q.; Chen, Y.; Zheng, D.K.; Sun, M.L.; Xie, Y.; Zhang, S.H. Systematic Review with Meta-Analysis: Effects of Probiotic Fungi on Irritable Bowel Syndrome. Benef. Microbes 2023, 14, 303–315. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; He, Y.; Zhang, D.; Zhang, S. Probiotics for the Prevention of Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Biomol. Biomed. 2024, 24, 1092–1104. [Google Scholar] [CrossRef]
- Wei, K.; Liao, X.; Yang, T.; He, X.; Yang, D.; Lai, L.; Lang, J.; Xiao, M.; Wang, J. Efficacy of Probiotic Supplementation in the Treatment of Psoriasis-A Systematic Review and Meta-Analysis. J. Cosmet. Dermatol. 2024, 23, 2361–2367. [Google Scholar] [CrossRef]
- Peng, T.R.; Chen, S.M.; Lee, M.C. Effectiveness of Probiotic/Prebiotic/Synbiotic Treatments on Anxiety: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2024, 353, 36–37. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Liu, Z.; Gao, F. Effect of Supplementation with Probiotics or Synbiotics on Cardiovascular Risk Factors in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Endocrinol. 2024, 14, 1282699. [Google Scholar] [CrossRef]
- Prebiotic Ingredients Market Industry Leaders, Size, Share and Trends Report. Available online: https://www.marketsandmarkets.com/ResearchInsight/prebiotics-ingredients-market.asp (accessed on 1 May 2024).
- Probiotics Market Size, Share, Analysis and Growth Trends Report by Product Type (Funcional Food and Beverages (FnB), Dietary Supplements, and Feed), Ingredient (Bacteria and Yeast), End User (Human and Animal), Distribution Channel and Region—Global Forecast to 2029. Available online: https://www.marketsandmarkets.com/market-reports/probiotics-market-69.html (accessed on 10 January 2025).
- Lai, S.; Lingström, P.; Cagetti, M.G.; Cocco, F.; Meloni, G.; Arrica, M.A.; Campus, G. Effect of Lactobacillus Brevis CD2 Containing Lozenges and Plaque PH and Cariogenic Bacteria in Diabetic Children: A Randomised Clinical Trial. Clin. Oral Investig. 2021, 25, 115–123. [Google Scholar] [CrossRef]
- Motta, P.d.B.; Gonçalves, M.L.L.; Gallo, J.M.A.S.; Sobral, A.P.T.; Motta, L.J.; Mayer, M.P.A.; Kawamoto, D.; de Andrade, D.C.; Santos, E.M.; Fernandes, K.P.S.; et al. Short Term Effect of Antimicrobial Photodynamic Therapy and Probiotic L. Salivarius WB21 on Halitosis: A Controlled and Randomized Clinical Trial. PLoS ONE 2024, 19, e0297351. [Google Scholar] [CrossRef]
- Stuermer, E.K.; Bang, C.; Giessler, A.; Smeets, R.; Janke, T.M.; Seki, F.D.; Debus, E.S.; Franke, A.; Augustin, M. Effect of Oral Multispecies Probiotic on Wound Healing, Periodontitis and Quality of Life on Patients with Diabetes. J. Wound Care 2024, 33, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Are Dental Diseases Examples of Ecological Catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Dutt, R.; Sharma, A.; Bhagat, N.; Dhar, M.S. A Critical Appraisal of the Effects of Probiotics on Oral Health. J. Funct. Foods 2020, 70, 103985. [Google Scholar] [CrossRef]
- Lopes, P.C.; Gomes, A.T.P.C.; Mendes, K.; Blanco, L.; Correia, M.J. Unlocking the Potential of Probiotic Administration in Caries Management: A Systematic Review. BMC Oral Health 2024, 24, 216. [Google Scholar] [CrossRef]
- Yli-Knuuttila, H.; Snäll, J.; Kari, K.; Meurman, J.H. Colonization of Lactobacillus Rhamnosus GG in the Oral Cavity. Oral Microbiol. Immunol. 2006, 21, 129–131. [Google Scholar] [CrossRef]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus Rhamnosus and Bifidobacterium Lactis on Gingival Health, Dental Plaque, and Periodontopathogens in Adolescents: A Randomised Placebocontrolled Clinical Trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of Orally Administered Lozenges with Lactobacillus Rhamnosus GG and Bifidobacterium Animalis Subsp. Lactis BB-12 on the Number of Salivary Mutans Streptococci, Amount of Plaque, Gingival Inflammation and the Oral Microbiome in Healthy Adults. Clin. Oral Investig. 2015, 19, 77–83. [Google Scholar] [CrossRef]
- Patil, A.V.; Shetty, S.S.; Padhye, A.M. Comparative Evaluation of the Inhibitory Effect of Lactobacillus Rhamnosus on Halitosis-Causing Bacteria: An Invitro Microbiological Study. Cureus 2023, 15, e38568. [Google Scholar] [CrossRef]
- Chantanawilas, P.; Pahumunto, N.; Thananimit, S.; Teanpaisan, R. Anticandidal Activity of Various Probiotic Lactobacillus Strains and Their Efficacy Enhanced by Prebiotic Supplementation. Curr. Microbiol. 2024, 81, 271. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Ratna Sudha, M.; Neelamraju, J.; Surendra Reddy, M.; Kumar, M. Evaluation of the Effect of Probiotic Bacillus Coagulans Unique IS2 on Mutans Streptococci and Lactobacilli Levels in Saliva and Plaque: A Double-Blind, Randomized, Placebo-Controlled Study in Children. Int. J. Dent. 2020, 2020, 8891708. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni Suef Univ. J. Basic. Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Jebin, A.A.; Suresh, A. Oral Microbial Shift Induced by Probiotic Bacillus Coagualans along with Its Clinical Perspectives. J. Oral Biol. Craniofac. Res. 2023, 13, 398–402. [Google Scholar]
- Jindal, G.; Pandey, R.K.; Agarwal, J.; Singh, M. A Comparative Evaluation of Probiotics on Salivary Mutans Streptococci Counts in Indian Children. Eur. Arch. Paediatr. Dent. 2011, 12, 211–215. [Google Scholar] [CrossRef]
- Koopaie, M.; Fatahzadeh, M.; Jahangir, S.; Bakhtiari, R. Comparison of the Effect of Regular and Probiotic Cake (Bacillus coagulans) on Salivary Ph and Streptococcus Mutans Count. Dent. Med. Probl. 2019, 56, 33–38. [Google Scholar] [CrossRef]
- Jagadeesh, K.M.; Shenoy, N.; Talwar, A.; Shetty, S. Clinical Effect of Pro-Biotic Containing Bacillus Coagulans on Plaque Induced Gingivitis: A Randomised Clinical Pilot Study. J. Health Allied Sci. NU 2017, 7, 007–012. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; de Cesare, A.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Update of the List of Qualified Presumption of Safety (QPS) Recommended Microorganisms Intentionally Added to Food or Feed as Notified to EFSA. EFSA J. 2023, 21, 7747. [Google Scholar] [CrossRef]
- Alanber, M.N.; Alharbi, N.S.; Khaled, J.M. Evaluation of Multidrug-Resistant Bacillus Strains Causing Public Health Risks in Powdered Infant Milk Formulas. J. Infect. Public Health 2020, 13, 1462–1468. [Google Scholar] [CrossRef]
- Beiswanger, B.B.; Boneta, A.E.; Mau, M.S.; Katz, B.P.; Proskin, H.M.; Stookey, G.K. The Effect of Chewing Sugar-Free Gum after Meals on Clinical Caries Incidence. J. Am. Dent. Assoc. 1998, 129, 1623–1626. [Google Scholar] [CrossRef]
- Szöke, J.; Bánóczy, J.; Proskin, H.M. Effect of After-Meal Sucrose-Free Gum-Chewing on Clinical Caries. J. Dent. Res. 2005, 60, 1725–1729. [Google Scholar] [CrossRef]
- Dawes, C.; Macpherson, L.M. Effects of Nine Different Chewing-Gums and Lozenges on Salivary Flow Rate and PH. Caries Res. 1992, 26, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, X.; Ma, H.; Yin, W.; Que, K.; Hu, D.; Dodds, M.; Tian, M. Assessment of Chewing Sugar-Free Gums for Oral Debris Reduction: A Randomized Controlled Crossover Clinical Trial. Am. J. Dent. 2012, 25, 118–122. [Google Scholar] [PubMed]
- Cocco, F.; Cagetti, M.G.; Majdub, O.; Campus, G. Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study. Appl. Sci. 2020, 10, 2900. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Brambilla, E.; Fadini, L.; Strohmenger, L. Comparative Study of Salivary and Urinary Fluoride Levels and Clearance Patterns between Fluoridated Chewing Gum and Fluoride Tablets in Children. Eur. J. Paediatr. Dent. 2002, 3, 27–32. [Google Scholar]
- European Food Safety Authority (EFSA). Outcome of a Public Consultation on the Scientific Opinion of the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) on the Appropriate Age Range for Introduction of Complementary Feeding into an Infant’s Diet†. EFSA Support. Publ. 2019, 16, 1686E. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the Re-evaluation of Butylated Hydroxytoluene BHT (E 321) as a Food Additive. EFSA J. 2012, 10, 2588. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to Sugar Free Chewing Gum and Neutralisation of Plaque Acids Which Reduces the Risk of Dental Caries Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1776. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the Substantiation of a Health Claim Related to Sugar Free Chewing Gum and Reduction of Tooth Demineralisation Which Reduces the Risk of Dental Caries Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1775. [Google Scholar] [CrossRef]
- Surana, A.S. Chewing Gum: A Friendly Oral Mucosal Drug Delivery System. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 68–71. [Google Scholar]
- Aslani, A.; Rostami, F. Medicated Chewing Gum, a Novel Drug Delivery System. J. Res. Med. Sci. 2015, 20, 403. [Google Scholar] [CrossRef]
- Krasse, P.; Carlsson, B.; Dahl, C.; Paulsson, A.; Nilsson, A.; Sinkiewicz, G. Decreased Gum Bleeding and Reduced Gingivitis by the Probiotic Lactobacillus reuteri. Swed. Dent. J. 2006, 30, 55–60. [Google Scholar] [PubMed]
- Kaur, K.; Nekkanti, S.; Madiyal, M.; Choudhary, P. Effect of Chewing Gums Containing Probiotics and Xylitol on Oral Health in Children: A Randomized Controlled Trial. J. Int. Oral Health 2018, 10, 237–243. [Google Scholar] [CrossRef]
- Çaglar, E.; Kavaloglu, S.C.; Kuscu, O.O.; Sandalli, N.; Holgerson, P.L.; Twetman, S. Effect of Chewing Gums Containing Xylitol or Probiotic Bacteria on Salivary Mutans Streptococci and Lactobacilli. Clin. Oral Investig. 2007, 11, 425–429. [Google Scholar] [CrossRef]
- Twetman, S.; Derawi, B.; Keller, M.; Ekstrand, K.; Yucel-Lindberg, T.; Stecksén-Blicks, C. Short-Term Effect of Chewing Gums Containing Probiotic Lactobacillus reuteri on the Levels of Inflammatory Mediators in Gingival Crevicular Fluid. Acta Odontol. Scand. 2009, 67, 19–24. [Google Scholar] [CrossRef]
- Keller, M.K.; Bardow, A.; Jensdottir, T.; Lykkeaa, J.; Twetman, S. Effect of Chewing Gums Containing the Probiotic Bacterium Lactobacillus reuteri on Oral Malodour. Acta Odontol. Scand. 2012, 70, 246–250. [Google Scholar] [CrossRef]
- Aslani, A.; Rafiei, S. Design, Formulation and Evaluation of Nicotine Chewing Gum. Adv. Biomed. Res. 2012, 1, 57. [Google Scholar] [CrossRef]
- Aslani, A.; Ghannadi, A.; Khalafi, Z. Design, Formulation and Evaluation of Green Tea Chewing Gum. Adv. Biomed. Res. 2014, 3, 142. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Stasio, M.D.; Volpe, M.G. In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums. Molecules 2016, 21, 1008. [Google Scholar] [CrossRef]
- Heller, D.; Helmerhorst, E.J.; Gower, A.C.; Siqueira, W.L.; Paster, B.J.; Oppenheim, F.G. Microbial Diversity in the Early In Vivo -Formed Dental Biofilm. Appl. Environ. Microbiol. 2016, 82, 1881–1888. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy. II. Correlation Between Oral Hygiene and Periodontal Condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of Microbial Cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Hartel, R.W.; von Elbe, J.H. Confectionery Science and Technology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Perotti, S.; Mantegazza, G.; Pierallini, E.; Kirika, N.; Duncan, R.; Telesca, N.; Sarrica, A.; Guglielmetti, S. Human In Vivo Assessment of the Survival and Germination of Heyndrickxia Coagulans SNZ1969 Spores Delivered via Gummy Candies. Curr. Res. Food Sci. 2024, 9, 100793. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Alatossava, T. Specific Identification of Certain Probiotic Lactobacillus rhamnosus Strains with PCR Primers Based on Phage-Related Sequences. Int. J. Food Microbiol. 2003, 84, 189–196. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Rehder, J.; Gelbrich, G.; Jockel-Schneider, Y. Consumption of Lactobacillus reuteri-Containing Lozenges Improves Periodontal Health in Navy Sailors at Sea: A Randomized Controlled Trial. J. Periodontol. 2020, 91, 1328–1338. [Google Scholar] [CrossRef]
- Giannini, G.; Ragusa, I.; Nardone, G.N.; Soldi, S.; Elli, M.; Valenti, P.; Rosa, L.; Marra, E.; Stoppoloni, D.; Merlo Pich, E. Probiotics-Containing Mucoadhesive Gel for Targeting the Dysbiosis Associated with Periodontal Diseases. Int. J. Dent. 2022, 2022, 5007930. [Google Scholar] [CrossRef]
- Alemzadeh, K.; Jones, S.B.; Davies, M.; West, N. Development of a Chewing Robot with Built-in Humanoid Jaws to Simulate Mastication to Quantify Robotic Agents Release From Chewing Gums Compared to Human Participants. IEEE Trans. Biomed. Eng. 2021, 68, 492–504. [Google Scholar] [CrossRef]
- Nishigawa, K.; Suzuki, Y.; Matsuka, Y. Masticatory Performance Alters Stress Relief Effect of Gum Chewing. J. Prosthodont. Res. 2015, 59, 262–267. [Google Scholar] [CrossRef]
- Karami-Nogourani, M.; Kowsari-Isfahan, R.; Hosseini-Beheshti, M. The Effect of Chewing Gum’s Flavor on Salivary Flow Rate and PH. Dent. Res. J. 2011, 8, S71–S75. [Google Scholar]
- Qaziyani, S.D.; Pourfarzad, A.; Gheibi, S.; Nasiraie, L.R. Effect of Encapsulation and Wall Material on the Probiotic Survival and Physicochemical Properties of Synbiotic Chewing Gum: Study with Univariate and Multivariate Analyses. Heliyon 2019, 5, e02144. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.B.; Massaut, K.B.; Vitola, H.R.S.; Siqueira, M.F.F.; da Silva, W.P.; Fiorentini, Â.M. Antagonistic Activity of Lactobacillus Spp. and Bifidobacterium Spp. against Cariogenic Streptococcus mutans In Vitro and Viability When Added to Chewing Gum during Storage. Braz. J. Microbiol. 2023, 54, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Arioli, S.; Koirala, R.; Taverniti, V.; Fiore, W.; Guglielmetti, S. Quantitative Recovery of Viable Lactobacillus paracasei CNCM I-1572 (L. Casei DG®) After Gastrointestinal Passage in Healthy Adults. Front. Microbiol. 2018, 9, 1720. [Google Scholar] [CrossRef]
- Radicioni, M.; Koirala, R.; Fiore, W.; Leuratti, C.; Guglielmetti, S.; Arioli, S. Survival of L. Casei DG® (Lactobacillus paracasei CNCMI1572) in the Gastrointestinal Tract of a Healthy Paediatric Population. Eur. J. Nutr. 2019, 58, 3161–3170. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).