Cocoa Pod Husk Valorization Through Rhizopus stolonifer Solid-State Fermentation: Enhancement in Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cocoa Pod Husk Preparation
2.2. Physicochemical Characterization
2.3. Solid-State Fermentation
2.4. Extraction Methods
2.5. Total Phenolic Content
2.6. Oxygen Radical Absorbance Capacity Assay and TEAC Calculation
2.7. DPPH Radical Scavenging Activity and TEAC Calculation
2.8. Volatile Compounds Determination by GC-MS
2.9. Statistical Analysis
3. Results
3.1. CPH Characterization
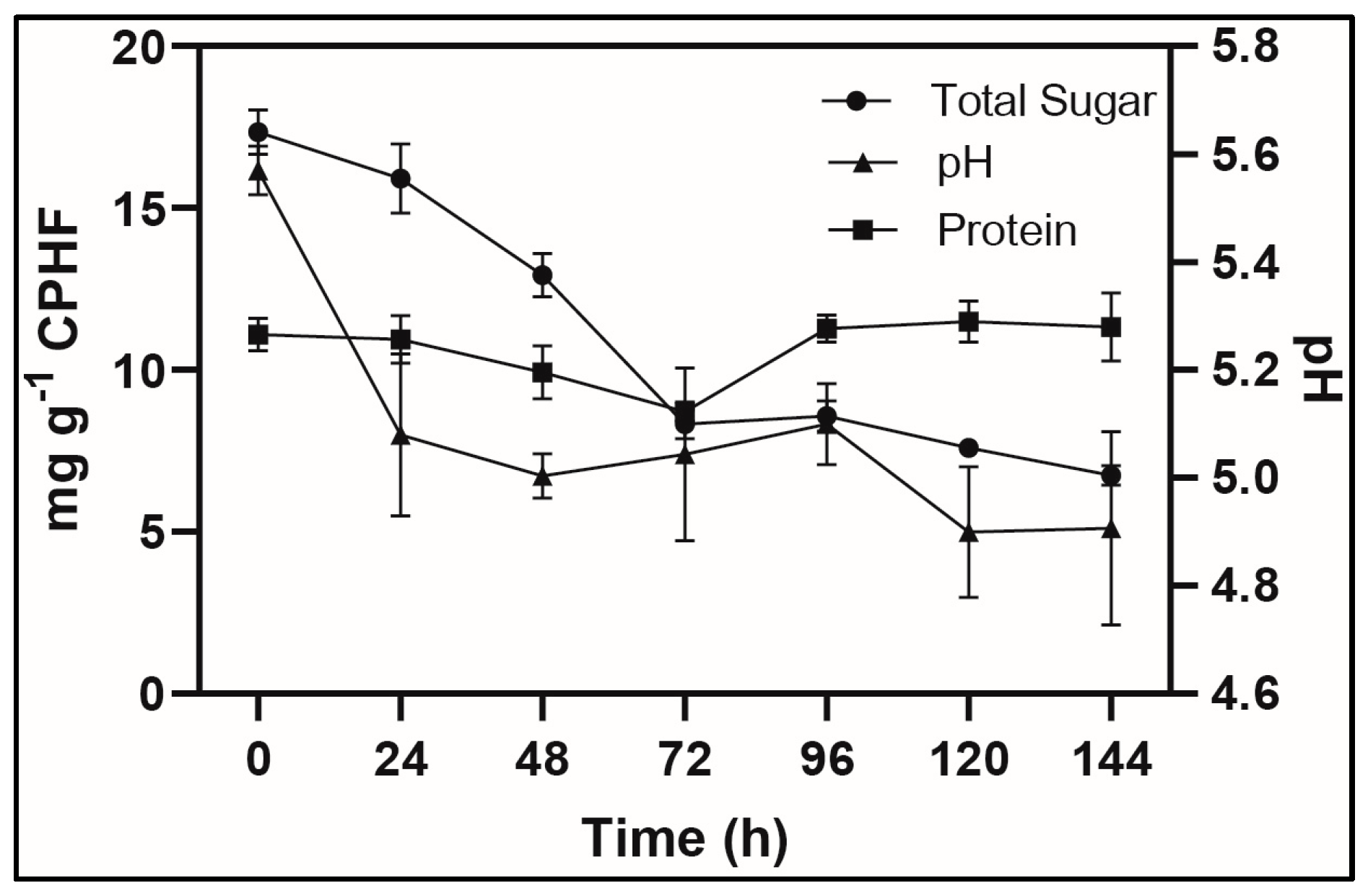
3.2. SSF Kinetics
3.3. TPC
3.4. ORAC
3.5. TEAC
3.6. GC-MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ICCO Quarterly Bulletin of Cocoa Statistics. Cocoa Year 2023/24, Volume L, No. 3. Available online: https://www.icco.org/wp-content/uploads/Production_QBCS-L-No.-3.pdf (accessed on 11 November 2024).
- AIPC—Associação Nacional das Indústrias Processadoras de Cacau. Available online: https://aipc.com.br/estatisticas/recebimento/ (accessed on 5 August 2024).
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao Pod Husks (Theobroma cacao L.): Composition and Hot-Water-Soluble Pectins. Ind. Crops Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. The Potential of Using Cocoa Pod Husks as Green Solid Base Catalysts for the Transesterification of Soybean Oil into Biodiesel: Effects of Biodiesel on Engine Performance. Chem. Eng. J. 2013, 220, 395–401. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological Potential of Agro-Industrial Residues. I: Sugarcane Bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Elhussieny, N.I.; El-Refai, H.A.; Mohamed, S.S.; Shetaia, Y.M.; Amin, H.A.; Klöck, G. Rhizopus Stolonifer Biomass Catalytic Transesterification Capability: Optimization of Cultivation Conditions. Microb. Cell Fact. 2023, 22, 154. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 17 March 2025).
- Instituto Adolfo Lutz (São Paulo); Zenebon, O.; Pascuet, N.S.; Tiglea, P. Métodos Físico-Químicos Para Análise de Alimentos. São Paulo, Brazil. 2008. Available online: https://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf (accessed on 17 March 2025).
- Lennartsson, P.R.; Taherzadeh, M.J.; Edebo, L. Rhizopus. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 284–290. ISBN 9780123847331. [Google Scholar]
- Counet, C.; Collin, S. Effect of the Number of Flavanol Units on the Antioxidant Activity of Procyanidin Fractions Isolated from Chocolate. J. Agric. Food Chem. 2003, 51, 6816–6822. [Google Scholar] [CrossRef]
- Gonçalves, E.; Gonçalves, C. Avaliação Do Impacto Da Fermentação, Secagem E Torração Sobre O Perfil Dos Compostos Fenólicos E Da Capacidade Antioxidante de Sementes de Cacau (Theobroma cacao Var. Forasteiro) Produzidos No Estado Do Pará. Master’s Thesis, Universidade Federal do Pará, Belém, Brazil, 2016. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.; Castilho, P.C. Antioxidant Potential of Artemisia Argentea L’Hér Alcoholic Extract and Its Relation with the Phenolic Composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- De Carvalho Neto, D.P.; De Melo Pereira, G.V.; Tanobe, V.O.A.; Thomaz Soccol, V.; G. da Silva, B.J.; Rodrigues, C.; Soccol, C.R. Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- Turgeman, T.; Shatil-Cohen, A.; Moshelion, M.; Teper-Bamnolker, P.; Skory, C.D.; Lichter, A.; Eshel, D. The Role of Aquaporins in pH-Dependent Germination of Rhizopus Delemar Spores. PLoS ONE 2016, 11, e0150543. [Google Scholar] [CrossRef]
- Balgobin, T.; Brandam, C.; Joannis-Cassan, C. Influence of Process Parameters on the Environmental Impact of 2,3-Butanediol Production by Fermentation. Biochem. Eng. J. 2024, 209, 109374. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Ibrahim, N.A. Benzyl alcohol. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 17–21. ISBN 9780323854344. [Google Scholar]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with Pichia Fermentans and Pediococcus Acidilactici on Metabolite Produced during Fermentation and Volatile Composition of Coffee Beans. Fermentation 2019, 5, 67. [Google Scholar] [CrossRef]
- Kinnunen, T.; Koskela, M. Antibacterial and Antifungal Properties of Propylene Glycol, Hexylene Glycol, and 1,3-Butylene Glycol In Vitro. Acta Derm. Venereol. 1991, 71, 148–150. [Google Scholar] [CrossRef]
- Mo, E.K.; Sung, C.K. Phenylethyl Alcohol (PEA) Application Slows Fungal Growth and Maintains Aroma in Strawberry. Postharvest Biol. Technol. 2007, 45, 234–239. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on α-Methylbenzyl Alcohol. Food Chem. Toxicol. 2012, 50 (Suppl. S2), S124–S129. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, F.; Buss, G.K.; Leal, W.S. 1-Octen-3-Ol—The Attractant That Repels. F1000Research 2015, 4, 156. [Google Scholar] [CrossRef]
- Chowdhury, L.; Croft, C.J.; Goel, S.; Zaman, N.; Tai, A.C.-S.; Walch, E.M.; Smith, K.; Page, A.; Shea, K.M.; Hall, C.D.; et al. Differential Potency of 2,6-Dimethylcyclohexanol Isomers for Positive Modulation of GABAA Receptor Currents. J. Pharmacol. Exp. Ther. 2016, 357, 570–579. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. Fragrance Material Review on 2-Ethyl-1-Hexanol. Food Chem. Toxicol. 2010, 48 (Suppl. S4), S115–S129. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Qin, Y.-L.; Li, S.-F.; Lv, Y.-Y.; Zhai, H.-C.; Hu, Y.-S.; Cai, J.-P. Antifungal Mechanism of 1-Nonanol against Aspergillus flavus Growth Revealed by Metabolomic Analyses. Appl. Microbiol. Biotechnol. 2021, 105, 7871–7888. [Google Scholar] [CrossRef]
- Wyeth, R.C.; Croll, R.P.; Willows, A.O.D.; Spencer, A.N. 1-Phenoxy-2-Propanol Is a Useful Anaesthetic for Gastropods Used in Neurophysiology. J. Neurosci. Methods 2009, 176, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ocak, E.; Javidipour, I.; Tuncturk, Y. Volatile Compounds of Van Herby Cheeses Produced with Raw and Pasteurized Milks from Different Species. J. Food Sci. Technol. 2015, 52, 4315–4323. [Google Scholar] [CrossRef]
- Dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, L.; An, P.; Qi, J.; Yu, X.; Lu, J.; Ren, X. Antifungal Mechanisms of α-Terpineol and Terpene-4-Alcohol as the Critical Components of Melaleuca alternifolia Oil in the Inhibition of Rot Disease Caused by Aspergillus ochraceus in Postharvest Grapes. J. Appl. Microbiol. 2019, 126, 1161–1174. [Google Scholar] [CrossRef]
- Mirata, M.-A.; Wüst, M.; Mosandl, A.; Schrader, J. Fungal Biotransformation of (+/−)-Linalool. J. Agric. Food Chem. 2008, 56, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, J.C.; Willemen, H.M. Biotransformation of Linalool to Furanoid and Pyranoid Linalool Oxides by Aspergillus Niger. Phytochemistry 1998, 47, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, J.C.; Adams, A.; Vanoverschelde, J.; De Kimpe, N. Biotransformation of (S)-(+)-Linalool by Aspergillus Niger: An Investigation of the Culture Conditions. J. Agric. Food Chem. 2001, 49, 5895–5901. [Google Scholar] [CrossRef]
- Ahmed, M.; Khan, K.-U.-R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.-U.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R.; et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum Candollei: An Insight into Potential for Natural Products Development. Molecules 2022, 27, 4113. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Alatar, A.A. Nutraceutical Potential of Mesembryanthemum Forsskaolii Hochst. Ex Bioss.: Insights into Its Nutritional Composition, Phytochemical Contents, and Antioxidant Activity. Open Chem. 2024, 22. [Google Scholar] [CrossRef]
- Rahman, M.A.; Uddin, M.N.; Babteen, N.A.; Alnajeebi, A.M.; Zakaria, Z.A.; Aboelenin, S.M. Natural Compounds from Hatikana Extract Potentiate Antidiabetic Actions as Displayed by in Vivo Assays and Verified by Network Pharmacological Tools. Biomed Res. Int. 2021, 2021, 6978450. [Google Scholar] [CrossRef]
- Mawa, J.; Rahman, M.A.; Hashem, M.A.; Juwel Hosen, M. Leea macrophylla Root Extract Upregulates the mRNA Expression for Antioxidative Enzymes and Repairs the Necrosis of Pancreatic β-Cell and Kidney Tissues in Fructose-Fed Type 2 Diabetic Rats. Biomed. Pharmacother. 2019, 110, 74–84. [Google Scholar] [CrossRef]
- Ma, W.; Johnson, E.T. Natural Flavour (E,E)-2,4-Heptadienal as a Potential Fumigant for Control of Aspergillus flavus in Stored Peanut Seeds: Finding New Antifungal Agents Based on Preservative Sorbic Acid. Food Control 2021, 124, 107938. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Hu, Y.-S.; Cai, J.-P. Heptanal Inhibits the Growth of Aspergillus flavus through Disturbance of Plasma Membrane Integrity, Mitochondrial Function and Antioxidant Enzyme Activity. Lebenson. Wiss. Technol. 2022, 154, 112655. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, Methoxycitronellal, CAS Registry Number 3613-30-7. Food Chem. Toxicol. 2022, 165 (Suppl. S1), 113133. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Lv, Y.; Wei, S.; Hu, Y. Synergistic Antifungal Mechanism of Cinnamaldehyde and Nonanal against Aspergillus flavus and Its Application in Food Preservation. Food Microbiol. 2024, 121, 104524. [Google Scholar] [CrossRef]
- Hpoo, M.K.; Mishyna, M.; Prokhorov, V.; Arie, T.; Takano, A.; Oikawa, Y.; Fujii, Y. Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum F. Sp. lycopersici. Sustainability 2020, 12, 9334. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Johnson, E.T.; Xie, Y.; Zhang, M. Natural Food Flavour (E)-2-Hexenal, a Potential Antifungal Agent, Induces Mitochondria-Mediated Apoptosis in Aspergillus flavus Conidia via a ROS-Dependent Pathway. Int. J. Food Microbiol. 2022, 370, 109633. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; Che, J.X.; Zhang, Y.; Ouyang, Q.; Tao, N. (E)-2-Octenal Suppresses the Growth of a Prochloraz-Resistant Penicillium italicum Strain and Its Potential Antifungal Mechanisms. Postharvest Biol. Technol. 2023, 205, 112515. [Google Scholar] [CrossRef]
- Ferreira, V.; de la Fuente, A.; Sáenz-Navajas, M.P. Wine Aroma Vectors and Sensory Attributes. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–39. ISBN 9780081020678. [Google Scholar]
- Wang, X.; Cao, H.; Zhu, Y.; Zhou, T.; Teng, F.; Tao, Y. β-Cyclocitral Induced Rapid Cell Death of Microcystis Aeruginosa. Environ. Pollut. 2024, 348, 123824. [Google Scholar] [CrossRef]
- Contreras, C.M.; Gutiérrez-García, A.G. 2-Heptanone Reduces Inhibitory Control of the Amygdala over the Prelimbic Region in Rats. Neurosci. Lett. 2021, 764, 136201. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, A.; Huang, W.; Chen, J.; Lin, X.; Yao, J.; Pan, L.; Khan, W.; Sun, B.; Liu, S.; et al. Metabolic Profiling Reveal Changes in Shoots and Roots of Nitrogen-Deficient Tea Plants (Camellia sinensis Cv. Jinxuan). Sci. Hortic. 2024, 337, 113528. [Google Scholar] [CrossRef]
- Soucy, N.V. Acetophenone. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 43–45. ISBN 9780123864550. [Google Scholar]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Guo, S.-S.; Pang, X.; Zhang, D.; Geng, Z.-F.; Du, S.-S. Insecticidal and Repellent Efficacy against Stored-Product Insects of Oxygenated Monoterpenes and 2-Dodecanone of the Essential Oil from Zanthoxylum planispinum Var. dintanensis. Environ. Sci. Pollut. Res. Int. 2019, 26, 24988–24997. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shimura, H.; Watanabe, N.; Tamai, M.; Takahashi, A.; Tanaka, Y.; Arai, I.; Hanada, K. 2-Acetylpyrrole, a Hepatoprotective Compound from Streptomyces sp. A-5071. Agric. Biol. Chem. 1991, 55, 2117–2121. [Google Scholar] [CrossRef]
- Pegnyemb, D.E.; Mbing, J.N.; de Théodore Atchadé, A.; Tih, R.G.; Sondengam, B.L.; Blond, A.; Bodo, B. Antimicrobial Biflavonoids from the Aerial Parts of Ouratea sulcata. Phytochemistry 2005, 66, 1922–1926. [Google Scholar] [CrossRef]
- Mathure, S.V.; Jawali, N.; Thengane, R.J.; Nadaf, A.B. Comparative Quantitative Analysis of Headspace Volatiles and Their Association with BADH2 Marker in Non-Basmati Scented, Basmati and Non-Scented Rice (Oryza sativa L.) Cultivars of India. Food Chem. 2014, 142, 383–391. [Google Scholar] [CrossRef]
- Plyuta, V.A.; Sidorova, D.E.; Koksharova, O.A.; Khmel, I.A.; Gnuchikh, E.Y.; Melkina, O.E. The Effect of β-Ionone on Bacterial Cells: The Use of Specific Lux-Biosensors. Res. Microbiol. 2024, 175, 104214. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Tang, X.; Peng, L.; Li, X.; Zhao, G.; Zhong, L. Chemical Composition, Antimicrobial and Antioxidant Activities of the Flower Volatile Oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum cymosum. Molecules 2018, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Rynjah, D.; Browne, R.B.; Gogoi, M.; Roy, J.D. Ethyl Acetate Fraction of Rhododendron arboreum as Potential Therapeutic against Drug-Resistant Bacterial Isolates through Experimental and in Silico Approach. Proc. Indian Natl. Sci. Acad. 2024, 90, 786–798. [Google Scholar] [CrossRef]
- Anyanwu, G.O.; Anzaku, D.; Donwell, C.C.; Usunobun, U.; Adegbegi, A.J.; Ofoha, P.C.; Rauf, K. Chemical Composition and In Vitro Antiobesity and In Vivo Anti-Hyperlipidemic Effects of Ceratotheca Sesamoides, Jatropha Tanjorensis, Mucuna Flagellipes, Pterocarpus Mildbraedii and Piper Guineense. Phytomed. Plus 2021, 1, 100042. [Google Scholar] [CrossRef]
- Schweikl, H.; Altmannberger, I.; Hanser, N.; Hiller, K.-A.; Bolay, C.; Brockhoff, G.; Spagnuolo, G.; Galler, K.; Schmalz, G. The Effect of Triethylene Glycol Dimethacrylate on the Cell Cycle of Mammalian Cells. Biomaterials 2005, 26, 4111–4118. [Google Scholar] [CrossRef]
- Pyrzynska, K. Ferulic acid—A Brief Review of Its Extraction, Bioavailability and Biological Activity. Sep. Technol. 2024, 11, 204. [Google Scholar] [CrossRef]
- Yin, H.; Shen, J.; Qian, X.; Zhai, L.; Guan, Q.; Shen, H.; Wang, G. Dimethyl Phthalate Exposure Induces Cognitive Impairment via COX2-Mediated Neuroinflammation. Ecotoxicol. Environ. Saf. 2024, 284, 117039. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; EL-Swaify, Z.A.S.E.-S.; Maaty, D.A.M.; Youssef, M.M. Phytochemistry and Antiviral Properties of Two Lotus Species Growing in Egypt: Antimicrobial Activity of Two Lotus Species. Rev. Vitae 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Thakur, R.S.; Ahirwar, B. A Steroidal Derivative from Trigonella Foenum graecum L. That Induces Apoptosis In Vitro and In Vivo. J. Food Drug Anal. 2019, 27, 231–239. [Google Scholar] [CrossRef]
- Moharregh-Khiabani, D.; Linker, R.A.; Gold, R.; Stangel, M. Fumaric Acid and Its Esters: An Emerging Treatment for Multiple Sclerosis. Curr. Neuropharmacol. 2009, 7, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Miles, S. Methyl salicylate. In xPharm: The Comprehensive Pharmacology Reference; POISONDEX® SystemMartindale: The Complete Drug Reference The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals Physicians’ Desk Reference Committee for Veterinary Medicinal Products: Salicylic Acid, Sodium Salicylate, Aluminum Salicylate, Basic and Methylsalicylate. Summary Report; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–6. ISBN 9780080552323. [Google Scholar]
- Al-Mussawii, M.A.Y.; AL-Sultan, E.Y.A.; AL-Hamdani, M.A.; Ramadhan, U.H. Antibacterial Activity of Alkaloid Compound Methoxy Phenyl–Oxime (CHN0) Isolated and Purified from Leaf of Conocarpus lancifolius Engl. Available online: https://faculty.uobasrah.edu.iq/uploads/publications/1696613228.pdf (accessed on 29 October 2024).
- Son, J.; Lee, H.; Lee, T.; Sohn, H.; Jae Park, S.; Na, J.-G.; Woo Seo, S.; Wook Lee, J.; Young Yoo, H.; Park, C. Novel Synthetic Pathway for Methyl 3-Hydroxybutyrate from β-Hydroxybutyric Acid and Methanol by Enzymatic Esterification. J. Ind. Eng. Chem. 2023, 123, 355–360. [Google Scholar] [CrossRef]
- Takano, E. Gamma-Butyrolactones: Streptomyces Signalling Molecules Regulating Antibiotic Production and Differentiation. Curr. Opin. Microbiol. 2006, 9, 287–294. [Google Scholar] [CrossRef]
- Du, Z.-H.; Lv, X.-X.; Liu, N.; Chen, F.; Yuan, M.; Da, C.-S. Asymmetric Synthesis of Pantolactone: Recent Advances. Eur. J. Org. Chem. 2023, 26, e202300918. [Google Scholar] [CrossRef]
- Edwards, P.A.; Lan, S.F.; Tanaka, R.D.; Fogelman, A.M. Mevalonolactone Inhibits the Rate of Synthesis and Enhances the Rate of Degradation of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Rat Hepatocytes. J. Biol. Chem. 1983, 258, 7272–7275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, W.; He, K.; Lin, C.; Du, S.; Kou, Y.; Nie, B. Inhibition of ACOX1 Enhances the Therapeutic Efficacy of Obeticholic Acid in Treating Non-Alcoholic Fatty Liver Disease and Mitigates Its Lipotoxicity. Front. Pharmacol. 2024, 15, 1366479. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Crowe, M.S.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Branched Short-Chain Fatty Acid Isovaleric Acid Causes Colonic Smooth Muscle Relaxation via cAMP/PKA Pathway. Dig. Dis. Sci. 2019, 64, 1171–1181. [Google Scholar] [CrossRef]
- Álvarez-Torres, J.N.; Ramírez-Bribiesca, J.E.; Bautista-Martínez, Y.; Crosby-Galván, M.M.; Granados-Rivera, L.D.; Ramírez-Mella, M.; Ruiz-González, A. Stability and Effects of Protected Palmitic Acid on in Vitro Rumen Degradability and Fermentation in Lactating Goats. Fermentation 2024, 10, 110. [Google Scholar] [CrossRef]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and Repellent Activity of Tetradecanoic Acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera:Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef]
- Gogoi, R.; Sarma, N.; Begum, T.; Chanda, S.K.; Lekhak, H.; Sastry, G.N.; Lal, M. Agarwood (Aquilaria malaccensis L.) a Quality Fragrant and Medicinally Significant Plant Based Essential Oil with Pharmacological Potentials and Genotoxicity. Ind. Crops Prod. 2023, 197, 116535. [Google Scholar] [CrossRef]
- Alansari, R.M.; Seleem, A.A.; Hussein, B.H.M. Recording Insect Death and Essential Oil Composition of Ferula communis L. Flowers in Al Ula, Kingdom of Saudi Arabia. Kuwait J. Sci. 2024, 51, 100171. [Google Scholar] [CrossRef]
- M’hamdi, Z.; Bouymajane, A.; Riffi, O.; Rhazi Filali, F.; Ettarchouch, M.; Elhourri, M.; Amechrouq, A. Chemical Composition and Antibacterial Activity of Essential Oil of Pelargonium graveolens and Its Fractions. Arab. J. Chem. 2024, 17, 105375. [Google Scholar] [CrossRef]
- Kurnianda, V.; Hu, H.-C.; Sung, P.-J.; Hayashi, Y.; Mori-Yasumoto, K.; Suetake, A.; Nakayama, H.; Yasumoto-Hirose, M.; Tsutsumi, Y.; Koja, G.; et al. Two Isoledene-Type Sesquiterpenoids from a Soft Coral Heteroxenia sp. Tetrahedron Lett. 2024, 151, 155323. [Google Scholar] [CrossRef]
- Takemoto, H.; Yagura, T.; Ito, M. Evaluation of Volatile Components from Spikenard: Valerena-4,7(11)-Diene Is a Highly Active Sedative Compound. J. Nat. Med. 2009, 63, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, H.; Yang, S.; Qi, Y.; Li, W.; Kang, S.; Hu, H.; Hua, Q.; Wu, Y.; Liu, Z. Antimicrobial Activity and Mechanism of α-Copaene against Foodborne Pathogenic Bacteria and Its Application in Beef Soup. Lebenson. Wiss. Technol. 2024, 195, 115848. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Wognin, E.L.; Kenne Kemene, T.; Genva, M.; Saive, M.; Tonzibo, Z.F.; Fauconnier, M.-L. A New Chemotype of Lantana Rhodesiensis Moldenke Essential Oil from Côte d’Ivoire: Chemical Composition and Biological Activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Branquinho, L.S.; Santos, J.A.; Cardoso, C.A.L.; Mota, J.d.S.; Junior, U.L.; Kassuya, C.A.L.; Arena, A.C. Anti-Inflammatory and Toxicological Evaluation of Essential Oil from Piper Glabratum Leaves. J. Ethnopharmacol. 2017, 198, 372–378. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, Y.; Yang, L.; Chen, A.; Zhang, Z.; Shen, G. Antibiofilm Activity of D-Limonene against Spoilage Bacillus amyloliquefaciens. Food Biosci. 2024, 61, 104568. [Google Scholar] [CrossRef]
- Boulebd, H. Are Thymol, Rosefuran, Terpinolene and Umbelliferone Good Scavengers of Peroxyl Radicals? Phytochemistry 2021, 184, 112670. [Google Scholar] [CrossRef]
- Azad, S.M.A.K.; Sayeed, M.A.; Meah, M.S.; Mawa, S.J.; Alam, S.; Hasan, M.N.; Hanif, M.A.; Khan, R.; Arman, M.; Kim, M.G. Unveiling the Therapeutic Potentialities and Chemical Characterization of Methanolic Merremia vitifolia (Burm.f) Hallier F. Stem Extract: A Multi-Faceted Investigation via In Vitro, In Vivo, and In Silico Approaches. Heliyon 2024, 10, e38449. [Google Scholar] [CrossRef]
- Ghaffari, T.; Kafil, H.S.; Asnaashari, S.; Farajnia, S.; Delazar, A.; Baek, S.C.; Hamishehkar, H.; Kim, K.H. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Pinus eldarica Grown in Northwestern Iran. Molecules 2019, 24, 3203. [Google Scholar] [CrossRef]
- Omer, E.; Elshamy, A.; Taher, R.; El-Kashak, W.; Shalom, J.; White, A.; Cock, I. Cakile Maritima Scop. Extracts Inhibit CaCo2 and HeLa Human Carcinoma Cell Growth: GC-MS Analysis of an Anti-Proliferative Extract. Pharmacogn. J. 2019, 11, 258–266. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kumar, R.S.; Chakraborty, B.; Nagaraja, S.K.; Gireesh Babu, K.; Nayaka, S. Eicosane: An Antifungal Compound Derived from Streptomyces Sp. KF15 Exhibits Inhibitory Potential against Major Phytopathogenic Fungi of Crops. Environ. Res. 2024, 251, 118666. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-K.; Li, H.-Y.; Zhu, Y.-L.; Xiong, M.-Q.; Zhong, J.-X. Neuroprotective and Anti-Inflammatory Effects of Eicosane on Glutamate and NMDA-Induced Retinal Ganglion Cell Injury. Int. J. Ophthalmol. 2024, 17, 638–645. [Google Scholar] [CrossRef]
- Vanitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Vidhya, E.; Praseetha, P.K. Heneicosane—A Novel Microbicidal Bioactive Alkane Identified from Plumbago zeylanica L. Ind. Crops Prod. 2020, 154, 112748. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, Octanenitrile, CAS Registry Number 124-12-9. Food Chem. Toxicol. 2022, 164, 113112. [Google Scholar] [CrossRef]
- Sztanke, M.; Kandefer-Szerszeń, M.; Sztanke, K. Biologically and Chemically Important Hydrazino-Containing Imidazolines as Antioxidant Agents. Free Radic. Res. 2018, 52, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; Carradori, S.; Amoroso, R.; Fernández, I.F. 2-Substituted Benzothiazoles as Antiproliferative Agents: Novel Insights on Structure-Activity Relationships. Eur. J. Med. Chem. 2020, 207, 112762. [Google Scholar] [CrossRef]
- Evans, J.; Richards, J.R.; Battisti, A.S. Caffeine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Modi, P.; Shah, B.M.; Patel, S. Interleukin-1β Converting Enzyme (ICE): A Comprehensive Review on Discovery and Development of Caspase-1 Inhibitors. Eur. J. Med. Chem. 2023, 261, 115861. [Google Scholar] [CrossRef]
- Köhler, A.; Finger-Baier, K.; Antunes, L. Anesthesia, Restraint and Analgesia in Laboratory Fishes. In Anesthesia and Analgesia in Laboratory Animals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 393–409. ISBN 9780128222157. [Google Scholar]
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. J. Braz. Chem. Soc. 2014, 25, 2102–2107. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; McQueen Mason, S.; Gomez, L.; et al. Valorisation Strategies for Cocoa Pod Husk and Its Fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary Fibre Composition, Antioxidant Capacity and Physico-Chemical Properties of a Fibre-Rich Product from Cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Yapo, K.D.; Ouffoue, S.K.; Okpekon, T.A.; Kouakou, T.H. Soil Effect on Polyphenols Content and Antioxidant Capacity of New Hybrid Variety of Cocoa from Côte d’Ivoire. Int. J. Bio. Chem. Sci 2013, 7, 1794–1803. [Google Scholar]
- Yapo, B.M.; Besson, V.; Koubala, B.; Koffi, K.L. Adding Value to Cacao Pod Husks as a Potential Antioxidant-Dietary Fiber Source. Am. J. Food Nutr. 2013, 1, 38–46. [Google Scholar]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 9781119135357. [Google Scholar]
- Zeng, J.; Deng, S.; Wang, Y.; Li, P.; Tang, L.; Pang, Y. Specific Inhibition of Acyl-CoA Oxidase-1 by an Acetylenic Acid Improves Hepatic Lipid and Reactive Oxygen Species (ROS) Metabolism in Rats Fed a High Fat Diet. J. Biol. Chem. 2017, 292, 3800–3809. [Google Scholar] [CrossRef]
- Shang, Z.; Gao, Y.; Xue, Y.; Zhang, C.; Qiu, J.; Qian, Y.; Fang, M.; Zhang, X.; Sun, X.; Kong, X.; et al. Shenge Formula Attenuates High-Fat Diet-Induced Obesity and Fatty Liver via Inhibiting ACOX1. Phytomedicine 2024, 123, 155183. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ling, X.; He, S.; Cui, H.; Yang, Z.; An, H.; Wang, L.; Zou, P.; Chen, Q.; Liu, J.; et al. PPARα/ACOX1 as a Novel Target for Hepatic Lipid Metabolism Disorders Induced by per- and Polyfluoroalkyl Substances: An Integrated Approach. Environ. Int. 2023, 178, 108138. [Google Scholar] [CrossRef]
- Teo, S.; Chuah, X.; Okechukwu, P.; Amini, F. Eicosane, Pentadecane and Palmitic Acid: The Effects in In Vitro Wound Healing Studies. Asian Pac. J. Trop. Biomed. 2018, 8, 490. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Dong, H.-W.; Wang, K.; Chang, X.-X.; Jin, F.-F.; Wang, Q.; Jiang, X.-F.; Liu, J.-R.; Wu, Y.-H.; Yang, C. Beta-Ionone-Inhibited Proliferation of Breast Cancer Cells by Inhibited COX-2 Activity. Arch. Toxicol. 2019, 93, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of Limonene by an Endophytic Fungus Using Synthetic and Orange Residue-Based Media. Fungal Biol. 2017, 121, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic Acids Enzymatic Lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Value in CPH |
|---|---|
| pH | 6.18 ± 0.01 |
| Aw | 0.283 ± 0.001 |
| Ashes Content (%) | 8.2 ± 0.04 |
| Humidity (%) | 3.4 ± 0.003 |
| Value (mg g−1) | |
| Total sugar | 17.347 ± 0.26 |
| Reducing sugar | 11.67 ± 0.15 |
| Protein | 18.30 ± 0.51 |
| F− | 0.114 ± 0.009 |
| Cl− | 0.318 ± 0.015 |
| Br− | 0.198 ± 0.008 |
| NO2− | 0.359 ± 0.005 |
| SO4−2 | 0.649 ± 0.029 |
| PO4−3 | 0.335 ± 0.010 |
| Na+ | 0.022 ± 0.003 |
| NH4+ | 0.080 ± 0.010 |
| K+ | 0.200 ± 0.006 |
| Mg+ | 0.440 ± 0.021 |
| Ca+ | 0.032 ± 0.009 |
| Fermentation Time (h) | TPC (mg GAE. g−1 Sample) | |
|---|---|---|
| EAE | AAE | |
| Dried CPH | 125.00 ± 0.53 e* | 204.48 ± 1.27 g |
| 0 h | 73.17 ± 0.84 a* | 82.39 ± 2.64 a |
| 24 h | 87.69 ± 1.06 c* | 134.18 ± 2.11 e |
| 48 h | 85.26 ± 2.10 c* | 115.52 ± 0.63 c |
| 72 h | 114.70 ± 0.23 d* | 188.21 ± 0.53 f |
| 96 h | 71.08 ± 1.16 a* | 122.84 ± 2.32 d |
| 120 h | 82.09 ± 1.48 bc* | 137.61 ± 1.16 e |
| 144 h | 75.90 ± 0.11 ab* | 92.69 ± 0.74 b |
| Fermentation Time (h) | ORAC (µmol TE. g−1 Sample) |
|---|---|
| Dried CPH | 47,723.04 ± 2796.90 a |
| 48 h | 50,312.11 ± 485.19 b |
| 72 h | 50,391.40 ± 4501.17 bc |
| 96 h | 50,535.57 ± 800.70 bc |
| 120 h | 51,645.00 ± 76.93 cd |
| 144 h | 51,676.48 ± 347.99 d |
| Fermentation Time (h) | TEAC (µmol TE. g−1 Sample) | |
|---|---|---|
| EAE | AAE | |
| Dried CPH | 6.52 ± 0.03 a* | 6.60 ± 0.03 a |
| 0 | 6.55 ± 0.05 a* | 6.72 ± 0.01 b |
| 24 | 6.56 ± 0.05 a* | 6.95 ± 0.02 c |
| 48 | 6.64 ± 0.08 ab* | 7.10 ± 0.02 d |
| 72 | 6.73 ± 0.09 bc* | 7.15 ± 0.01 de |
| 96 | 7.09 ± 0.05 e* | 7.25 ± 0.04 f |
| 120 | 6.98 ± 0.01 de* | 7.17 ± 0.05 ef |
| 144 | 6.93 ± 0.09 cd* | 7.09 ± 0.03 d |
| Solid-State | EAE | AAE | Bioactivity | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Formula | Mol Wt | CPH | CPHF | CPH | 48 h | 96 h | 144 h | CPH | 48 h | 96 h | 144 h | ||
| 2,3-Butanediol | C4H10O2 | 90 | - | + | - | - | - | - | - | - | - | - | Fuel additive | [23] |
| 1-Hexanol | C6H14O | 102 | + | - | - | - | - | - | - | - | - | - | Aroma | [24] |
| (2S,4S)-(+)-Pentanediol | C5H12O2 | 104 | + | + | - | - | - | - | - | - | - | - | N/A | |
| 1,2-Pentanediol | C5H12O2 | 104 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 1-Methoxy-2-butanol | C5H12O2 | 104 | + | + | - | - | - | - | - | - | - | - | N/A | |
| 2-Ethoxy-1-propanol | C5H12O2 | 104 | - | - | - | - | - | - | + | - | - | - | N/A | |
| Benzyl alcohol | C7H8O | 108 | - | + | - | - | - | - | - | - | - | - | Antimicrobial | [25] |
| 3,4-Dimethylpent-2-en-1-ol | C7H14O | 114 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 5-Methyl-2-hexanol | C7H16O | 116 | - | + | - | - | - | - | - | - | - | - | Aroma | [26] |
| 2-Methyl-3-hexanol | C7H16O | 116 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 2-Ethyl-3-pentanol | C7H16O | 116 | + | - | - | - | - | - | - | - | - | - | N/A | |
| Hexylene glycol | C6H14O2 | 118 | - | - | + | - | + | + | - | - | - | + | Antibacterial & Antifungal | [27] |
| Phenylethyl Alcohol | C8H10O | 122 | + | + | - | - | - | - | - | - | - | - | Antifungal & Aroma | [28] |
| α-Methylbenzyl alcohol | C8H10O | 122 | - | + | - | - | - | - | - | - | - | - | Aroma | [29] |
| 1-Octen-3-ol | C8H16O | 128 | + | - | - | - | - | - | - | - | - | - | Food additive | [30] |
| 2,6-Dimethylcyclohexanol | C8H16O | 128 | + | - | - | - | - | - | - | - | - | - | Anesthetic | [31] |
| 2-Ethyl-1-hexanol | C8H18O | 130 | + | - | - | - | - | - | - | - | - | - | Aroma | [32] |
| 1-Nonanol | C9H20O | 144 | + | - | - | - | - | - | - | - | - | - | Antifungal & Aroma | [33] |
| 1-Phenoxy-2-propanol | C9H12O2 | 152 | - | - | - | - | + | - | - | - | - | - | Anesthetic | [34] |
| 3-Phenoxy-1-propanol | C9H12O2 | 152 | - | - | - | - | + | - | - | - | - | - | Aroma | [35] |
| Linalool | C10H18O | 154 | + | - | - | - | - | - | - | - | - | - | Anti-inflammatory & Anticonvulsant | [36] |
| α-Terpineol | C10H18O | 154 | + | - | - | - | - | - | - | - | - | - | Antifungal | [37] |
| [5-Hydroxymethyl)-1,3-dioxolan-4-yl]methanol | C5H10O4 | 134 | - | - | - | - | - | + | + | - | - | - | N/A | |
| Cis-Linalool oxide | C10H18O2 | 170 | + | + | - | - | - | - | - | - | - | - | Aroma | [38] |
| Linalool oxide pyranoid | C10H18O2 | 170 | + | + | - | - | - | - | - | - | - | - | Aroma | [39] |
| Trans-furanoid linalool oxide | C10H18O | 170 | + | - | - | - | - | - | - | - | - | - | Aroma | [40] |
| 11-Methyldodecanol | C13H28O | 200 | + | - | - | - | - | - | - | - | - | - | N/A | |
| 1-Tridecanol | C13H28O | 200 | + | - | - | - | - | - | - | - | - | - | N/A | |
| 1-Methoxy-3-(2-hydroxyethyl)nonane | C12H26O2 | 202 | - | - | + | - | - | - | - | - | - | - | Antioxidant & Antifungal | [41,42] |
| 2,2-Dimethyl-6-methylene-1-[3,5-dihydroxy-1-pentenyl]cyclohexan-1-perhydrol | C14H24O4 | 256 | - | - | - | - | - | - | - | - | + | - | Antioxidant, Anti-inflammatory, Antidiabetic, Antitumor, etc | [43,44] |
| Aldehyde | ||||||||||||||
| (E,E)-2,4-Heptadienal | C7H10O | 110 | + | - | - | - | - | - | - | - | - | - | Antifungal & Aroma | [45] |
| Heptanal | C7H14O | 114 | + | - | - | - | - | - | - | - | - | - | Antifungal | [46] |
| Methoxycitronellal | C11H22O2 | 186 | - | + | - | - | - | - | - | - | - | - | Aroma | [47] |
| Nonanal | C9H18O | 142 | + | + | - | - | - | - | - | - | - | - | Antifungal | [48] |
| Octanal | C8H16O | 128 | + | + | - | - | - | - | - | - | - | - | Antifungal | [49] |
| (E)-2-hexenal | C6H10O | 98 | + | - | - | - | - | - | - | - | - | - | Antifungal | [50] |
| (E)-2-Octenal | C8H14O | 126 | + | - | - | - | - | - | - | - | - | - | Antifungal | [51] |
| Phenylacetaldehyde | C8H8O | 120 | + | + | - | - | - | - | - | - | - | - | Aroma | [52] |
| β-Cyclocitral | C10H16O | 152 | + | - | - | - | - | - | - | - | - | - | Antibacterial | [53] |
| Ketone | ||||||||||||||
| 2-Heptanone | C7H14O | 114 | + | - | - | - | - | - | - | - | - | - | Neuromodulation | [54] |
| Geranylacetone | C13H22O | 196 | + | - | - | + | - | - | - | - | - | - | Aroma | [55] |
| Acetophenone | C8H8O | 87 | + | - | - | - | - | - | - | - | - | - | Aroma & Food additive | [56] |
| 2-Dodecanone | C12H24O | 184 | + | - | - | - | - | - | - | - | - | - | Insecticidal & Repellent | [57] |
| 1,3-Diacetylbenzene | C10H10O2 | 162 | - | - | - | - | + | + | + | - | - | + | N/A | |
| 1,4-Diacetylbenzene | C10H10O2 | 162 | - | - | + | - | + | + | + | - | + | + | N/A | |
| 2-Acetylpyrrole | C6H7NO | 109 | - | + | - | - | - | - | - | - | - | - | Antioxidant & Hepatoprotective | [58] |
| Sulcatone | C8H14O | 126 | + | - | - | - | - | - | - | - | - | - | Antimicrobial | [59] |
| trans-3-Octen-2-one | C8H14O | 126 | + | - | - | - | - | - | - | - | - | - | Aroma | [60] |
| β-Ionone | C13H20O | 192 | + | - | - | - | - | - | - | - | + | - | Aroma, Antimicrobial & Insecticidal | [61] |
| Ester | ||||||||||||||
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 278 | - | - | - | - | - | - | + | - | - | - | Antioxidant & Antimicrobial | [62] |
| 1,2-Dimethylpropyl acetate | C7H14O2 | 130 | + | - | - | - | - | - | - | - | - | - | N/A | |
| 1-Ethylpentyl acetate | C9H18O2 | 158 | - | + | - | - | - | - | - | - | - | - | Antimicrobial | [63] |
| 2-(Heptyloxycarbonyl)benzoic acid | C15H20O4 | 264 | - | - | - | - | + | + | + | + | - | - | Antiobesity & Anti-hyperlipidemic | [64] |
| Triethylene glycol dimethacrylate | C14H22O6 | 286 | - | - | - | - | - | - | + | + | - | - | Cytotoxic (mammalian cells) | [65] |
| Cinnamic acid, 4-hydroxy-3-methoxy-,(5-hydroxy-2-hydroxymethyl-6-[2-(4-hydroxy-3-methoxyphenyl)ethoxy]-4-(6-methyl-3,4,5-trihydroxytetrahydropyran-2-yloxy)tetrahydropyran-3-yl) ester | C31H40O15 | 652 | - | - | + | + | + | + | + | + | - | + | Antioxidant & Antiviral | [66] |
| Dimethyl phthalate | C10H10O4 | 194 | - | - | + | - | + | + | - | - | - | + | Environmental Contaminator | [67] |
| Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | C19H34O6 | 358 | - | - | + | - | - | - | - | + | - | - | Antiviral | [68] |
| Ethyl iso-allocholate | C26H44O5 | 452 | + | + | + | + | + | + | + | + | + | + | Antiangiogenic | [69] |
| Fumaric acid, 2-isopropyl phenyl dodec-2-en-1-yl ester | C25H36O4 | 400 | - | - | - | - | - | - | - | - | + | - | Antioxidant, Immunomodulating, and Anti-inflammatory | [70] |
| Methyl salicylate | C8H8O3 | 152 | + | + | - | - | - | - | - | - | - | - | Anti-inflammatory & Analgesic agent | [71] |
| Octacosanoic acid, methyl ester | C29H58O2 | 438 | - | - | - | - | - | - | - | + | - | - | N/A | |
| Oxalic acid, bis(6-ethyloct-3-yl) ester | C22H42O4 | 370 | + | - | - | - | - | - | - | - | - | - | N/A | |
| Methoxy-Phenyl-Oxime | C8H9NO2 | 151 | + | + | - | + | + | + | + | + | + | + | Antibacterial | [72] |
| Methyl 3-hydroxybutyrate | C5H10O3 | 118 | - | + | - | - | - | - | - | - | - | - | Therapeutic effect on Alzheimer’s disease & Inhibition of apoptosis | [73] |
| ϒ-Butyrolactone | C4H6O2 | 86 | + | - | - | - | - | - | - | - | - | - | Regulate Antibiotic production in Streptomyces | [74] |
| Pantolactone | C6H10O3 | 130 | + | - | - | - | - | - | - | - | - | - | Food additive | [75] |
| Mevalonolactone | C6H10O3 | 130 | - | + | - | - | - | - | - | - | - | - | Mevalonate pathway precursor (3- Hydroxy-3-methylglutaryl Coenzyme A Reductase inhibitor) | [76] |
| 3,3,5-trimethylcyclohexyl methacrylate | C13H22O2 | 210 | - | - | + | + | - | - | - | - | - | - | N/A | |
| Organic acid | ||||||||||||||
| 10,12-Tricosadiynoic acid | C23H38O2 | 346 | - | + | - | - | - | - | - | - | - | - | ACOX1-specific inhibitor | [77] |
| Isovaleric acid | C5H10O2 | 102 | + | - | - | - | - | - | - | - | - | - | Colonic Smooth Muscle Relaxation | [78] |
| Palmitic acid | C16H32O2 | 256 | - | - | - | - | - | - | - | + | - | - | Diet Supplement for animal | [79] |
| Tetradecanoic acid | C14H28O2 | 228 | - | - | - | - | - | - | - | + | - | - | Larvicidal & mosquito repellent | [80] |
| Terpenes | ||||||||||||||
| Aristolene | C15H24 | 204 | - | + | - | - | - | - | - | - | - | - | Biopesticidal, Anti-inflammatory, Antidiabetic, Anti-urolithic, and Tyrosinase inhibitory potentials | [81,82] |
| 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene | C15H24 | 204 | + | + | - | - | - | - | - | - | - | - | Antioxidant & Antibacterial | [83] |
| Isoledene | C15H24 | 204 | + | - | - | - | - | - | - | - | - | - | Antiviral & Anti-leishmania | [84] |
| Valerena-4,7(11)-diene | C15H24 | 204 | + | - | - | - | - | - | - | - | - | - | Sedative effect | [85] |
| α-Copaene | C15H24 | 204 | + | + | - | - | - | - | - | - | - | - | Antimicrobial | [86] |
| ϒ-Cadinene | C15H24 | 204 | + | - | - | - | - | - | - | - | - | - | Antioxidant & Anti-inflammatory | [87] |
| ϒ-Muurolene | C15H24 | 204 | + | - | - | - | - | - | - | - | - | - | Antioxidant & Anti-inflammatory | [88] |
| D-Limonene | C10H16 | 136 | + | + | - | - | - | - | - | - | - | - | Anti-inflammatory & Antibiofilm | [89] |
| Rosefuran | C10H14O | 150 | - | + | - | - | - | - | - | - | - | - | Antioxidant | [90] |
| Hydrocarbon | ||||||||||||||
| 1,2,4-Trimethylcyclopentane | C8H16 | 112 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 13-Phenylpentacosane | C31H56 | 428 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 1-Heptadecene | C17H34 | 238 | + | - | - | - | - | - | - | - | - | - | N/A | |
| 2,6,10,15-Tetramethylheptadecane | C21H44 | 296 | + | - | - | - | - | - | - | - | - | - | Multiple therapeutic potentialities | [91] |
| 2-Cyclopropylidene-1,7,7-trimethylbicyclo [2.2.1]heptane | C13H20 | 176 | - | + | - | - | - | - | - | - | - | - | Antimicrobial | [92] |
| 2-Ethylhexene | C8H16 | 112 | - | + | - | - | - | - | - | - | - | - | N/A | |
| 2-Methyl-n-hexacosane | C27H56 | 380 | + | - | - | - | - | - | - | - | - | - | Anti-Carcinoma Cell Growth | [93] |
| Eicosane | C20H42 | 282 | + | - | - | - | - | - | - | - | - | - | Antifungal, Antioxidant, and Anti-inflammatory | [94,95] |
| Heneicosane | C21H44 | 296 | + | - | - | - | - | - | - | - | - | - | Antimicrobial | [96] |
| Nitrile | ||||||||||||||
| Octanenitrile | C8H15N | 125 | + | - | - | - | - | - | - | - | - | - | Aroma | [97] |
| Amine | ||||||||||||||
| Putrescine | C4H12N2 | 88 | + | - | - | - | - | - | - | - | - | - | N/A | |
| 2-Hydrazino-2-imidazoline | C3H8N4 | 100 | - | - | - | - | - | - | - | + | - | - | Antioxidant | [98] |
| Heterocyclic | ||||||||||||||
| Benzothiazole | C7H5NS | 135 | - | - | + | - | + | + | + | - | + | + | Antimicrobial, Anticonvulsant, Neuroprotective, Anti-inflammatory, and Antitumor | [99] |
| Caffeine | C8H10N4O2 | 194 | - | - | - | - | - | - | + | - | - | - | Alleviates fatigue and drowsiness | [100] |
| Amide | ||||||||||||||
| Caprolactam | C6H11NO | 113 | - | - | - | - | - | + | - | - | - | - | Caspase-1 inhibitor | [101] |
| Ether | ||||||||||||||
| β-Hydroxyethyl phenyl ether | C8H10O2 | 138 | - | - | - | - | + | - | - | - | + | - | Antibacterial & Antifungal | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros Tiburcio, P.; de Carvalho Neto, D.P.; Soccol, C.R.; Medeiros, A.B.P. Cocoa Pod Husk Valorization Through Rhizopus stolonifer Solid-State Fermentation: Enhancement in Antioxidant Activity. Microorganisms 2025, 13, 716. https://doi.org/10.3390/microorganisms13040716
Barros Tiburcio P, de Carvalho Neto DP, Soccol CR, Medeiros ABP. Cocoa Pod Husk Valorization Through Rhizopus stolonifer Solid-State Fermentation: Enhancement in Antioxidant Activity. Microorganisms. 2025; 13(4):716. https://doi.org/10.3390/microorganisms13040716
Chicago/Turabian StyleBarros Tiburcio, Patrick, Dão Pedro de Carvalho Neto, Carlos Ricardo Soccol, and Adriane Bianchi Pedroni Medeiros. 2025. "Cocoa Pod Husk Valorization Through Rhizopus stolonifer Solid-State Fermentation: Enhancement in Antioxidant Activity" Microorganisms 13, no. 4: 716. https://doi.org/10.3390/microorganisms13040716
APA StyleBarros Tiburcio, P., de Carvalho Neto, D. P., Soccol, C. R., & Medeiros, A. B. P. (2025). Cocoa Pod Husk Valorization Through Rhizopus stolonifer Solid-State Fermentation: Enhancement in Antioxidant Activity. Microorganisms, 13(4), 716. https://doi.org/10.3390/microorganisms13040716






