Impact of Zika and Chikungunya Viruses on Spontaneous Abortions: Insights from a Reference Maternity Hospital
Abstract
1. Introduction
2. Methods
2.1. Study Design and Sampling
2.2. Data Collection
2.3. Histopathology
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Clinical and Obstetric Sociodemographic Profile
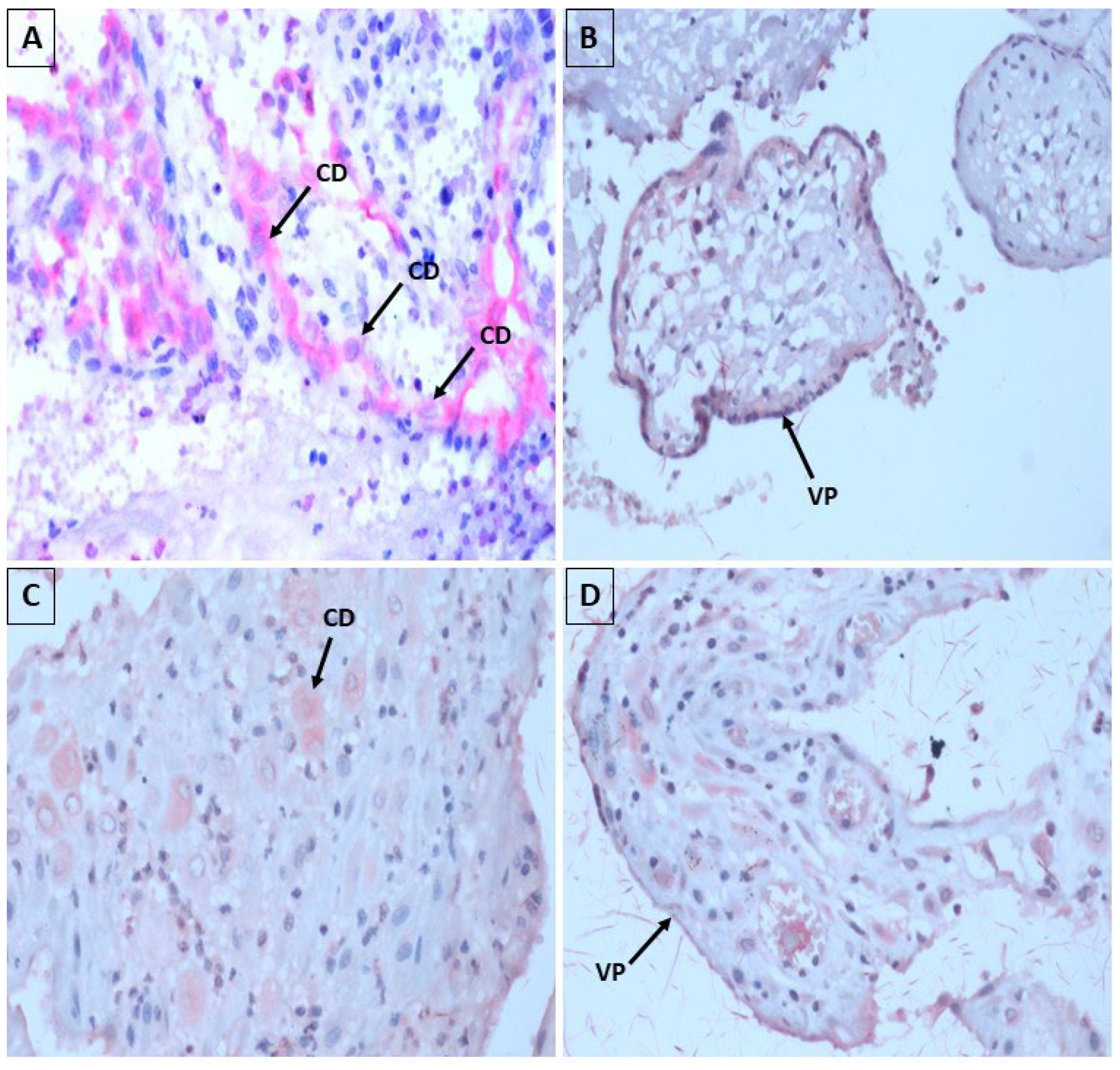
3.2. Aspects of Histopathology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveira, M.T.S.; Oliveira, C.N.T.; Marques, L.M.; Souza, C.L.; Oliveira, M.V. Fatores associados ao aborto espontâneo: Uma revisão sistemática. Rev. Bras. Saúde Matern. Infant. 2020, 20, 361–372. [Google Scholar] [CrossRef]
- Zahran, A.B.; Ali, E.A.; Siddeg, W.A.; Ali, N.I.; Bakheit, K.H. Thyrotropin and Thyroid Antibodies in Sudanese Women with Recurrent Miscarriage. Khartoum Med. J. 2016, 9, 1224–1229. [Google Scholar]
- Zhou, H.; Liu, Y.; Liu, L.; Zhang, M.; Chen, X.; Qi, Y. Maternal pre-pregnancy risk factors for miscarriage from a prevention perspective: A cohort study in China. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 206, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Craig, M.; Jacques, C.; Hall, B.; Catteau, J.; Munro, S.; Scott, G.; Camaris, C.; McIver, C.; Rawlinson, W. Correlates of placental infection with cytomegalovirus, parvovirus B19 or human herpes virus 7. J. Med. Virol. 2006, 78, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Zaki ME, S.; Goda, H. Relevance of parvovirus B19, herpes simplex virus 2, and cytomegalovirus virologic markers in maternal serum for diagnosis of unexplained recurrent abortions. Arch. Pathol. Lab. Med. 2007, 131, 956–960. [Google Scholar] [CrossRef]
- Kim, I.D.; Chang, H.S.; Hwang, K.J. Herpes simplex virus 2 infection rate and necessity of screening during pregnancy: A clinical and seroepidemiologic study. Yonsei Med. J. 2012, 53, 401–407. [Google Scholar] [CrossRef]
- Syridou, G.; Spanakis, N.; Konstantinidou, A.; Piperaki, E.; Kafetzis, D.; Patsouris, E.; Antsaklis, A.; Tsakris, A. Detection of cytomegalovirus, parvovirus B19 and herpes simplex viruses in cases of intrauterine fetal death: Association with pathological findings. J. Med. Virol. 2008, 80, 1776–1782. [Google Scholar] [CrossRef]
- Poletti, M.O.D.O.; Sousa, C.F.S.S.; Sampaio, M.G. Evidências de transmissão vertical de arbovírus. Publicação Soc. Bras. Pediatr. 2016, 6, 21–24. [Google Scholar]
- Torres, J.R.; Falleiros-Arlant, L.H.; Dueñas, L.; Pleitez-Navarrete, J.; Salgado, D.M.; Brea-Del Castillo, J. Congenital and perinatal complications of chikungunya fever: A Latin American experience. Int. J. Infect. Dis. 2016, 51, 85–88. [Google Scholar] [CrossRef]
- Machain-Williams, C.; Machain-Williams, C.; Raga, E.; Raga, E.; Baak-Baak, C.M.; Baak-Baak, C.M.; Kiem, S.; Kiem, S.; Blitvich, B.J.; Blitvich, B.J.; et al. Maternal, fetal, and neonatal outcomes in pregnant dengue patients in mexico. Biomed. Res. Int. N. Y. 2018, 1, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Diamond, M.S.; Mysorekar, I.U. Maternal-fetal transmission of zika virus: Routes and signals for infection. J. Interferon Cytokine Res. Auckl. 2017, 37, 287–294. [Google Scholar] [CrossRef]
- Lopes, N.; Nozawa, C.; Linhares, R.E.C. Características gerais e epidemiologia dos arbovírus emergentes no Brasil. Rev. Pan-Amaz. Saúde 2014, 5, 55–64. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Secretaria de Vigilância em Saúde. Monitoramento dos Casos de Dengue, Febre de Chikungunya e Febre Pelo Vírus Zika até a Semana Epidemiológica 45. 2015, 46. Available online: https://www.saude.ba.gov.br/wp-content/uploads/2024/02/Protocolo-Tecnico-INVESTIGACAOBoletimpidemiológico-OBITO-ARBO.pdf (accessed on 17 September 2023).
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Villamil-Gómez, W.; Paniz-Mondolfi, A.E. How many patients with post-chikungunya chronic inflammatory rheumatism can we expect in the new endemic areas of Latin America? Rheumatol. Int. 2015, 35, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Coronell-Rodriguez, W.; Arteta-Acosta, C.; Suárez-Fuentes, M.A.; Burgos-Rolon, M.C.; Rubio-Sotomayor, M.T.; Sarmiento-Gutierrez, M.; Corzo-Diaz, C. Zika virus infection in pregnancy, fetal and neonatal impact. Rev. Chil. Infectol. Santiago 2016, 33, 17–34. [Google Scholar]
- Rivadeneyra-Espinar, P.G.; Venegas-Esquivel, G.A.; Díaz-Espinoza, C.M.; Pérez-Robles, V.M.; González-Fernández, M.I.; Sesma-Medrano, E. Zika como causa de aborto espontáneo en zonas endémicas. Boletín Médico Hosp. Infant. Méxic 2019, 76, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Beaudoin, M.C.; Couderc, T.; Lortholary, O.; Lecuit, M. Arboviruses and pregnancy: Maternal, fetal, and neonatal effects. Lancet Child Adolesc. Health Lond. 2017, 1, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.; Nieto, A.J.; Loaiza-Osorio, S.; Barona, J.S.; Rosso, F. Pregnant women hospitalized with chikungunya virus infection, Colombia, 2015. Emerg Infect Dis. Foster City 2017, 23, 458–465. [Google Scholar] [CrossRef]
- Monteiro, L.S.; Cunha, D.B.; Sichieri, R.; Pereira, R.A. Construção e Validação de Cartilha Educativa para Prevenção das Arboviroses na Gestação; Universidade estadual do ceará, Centro de ciências da saúde, Programa de pós-graduação em saúde coletiva, Mestrado profissional em gestão em saúde: Fortaleza, Brazil, 2018. [Google Scholar]
- Carlos, A. Como Elaborar Projetos de Pesquisa, 4th ed.; Atlas: São Paulo, Brazil, 2017. [Google Scholar]
- Freire, M.C.M.; Pattussi, M.P. Tipos de estudos. In Metodologia Científica. Ciência, Ensino e Pesquisa, 3rd ed.; Estrela, C., Ed.; Artes Médicas: Porto Alegre, Brazil, 2018; pp. 109–127. [Google Scholar]
- Camargo LM, A.; Silva RP, M.; Meneguetti DU, D.O. Research methodology topics: Cohort studies or prospective and retrospective cohort studies. J. Hum. Growth Dev. 2019, 29, 433–436. [Google Scholar] [CrossRef]
- Araujo, A.G.; Aquino, C.M.D.G.D.; Souza, D.Q.S.D.; Ribeiro, L.P.; Jesus, M.A.D.A. Aspectos Morfológicos Placentários na Gestação com Suspeita de Zika Vírus; Trabalhos Conclusão de Curso Medicina; Universidade Evangélica de Goiás: Anápolis, Brazil, 2018. [Google Scholar]
- Ayres, M.; Ayres, M., Jr.; Ayres, D.L.; Santos, A.S. BioEstat 4.0: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas; Mamiraua: São Paulo, Brazil, 2015. [Google Scholar]
- Barbosa, T.; Ansaloni LV, S.; de Freitas, A.A.; de Carvalho, R.L.; Sousa, T.B.; de Oliveira, R.A. A causalidade do abortamento espontâneo: Uma revisão integrativa. Braz. J. Health Rev. Curitiba 2021, 4, 16045–16057. [Google Scholar] [CrossRef]
- Laisk, T.; Soares, A.L.G.; Ferreira, T.; Painter, J.N.; Censin, J.C.; Laber, S.; Bacelis, J.; Chen, C.-Y.; Lepamets, M.; Lin, K.; et al. The genetic architecture of sporadic and multiple consecutive miscarriage. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Curcio, H.; Monsanto Hernández, K.; Colón, J.A. Enfermedad trofoblastica gestacional diagnosticada en restos ovulares obtenidos de pacientes con abortos espontaneos. Rev. Obs. Ginecol. Venez. 2016, 76, 76–84. [Google Scholar]
- Rashid, H.; Ma, E.; Ferdous, F.; Ekström, E.C.; Wagatsuma, Y. First-trimester fetal growth restriction and the occurrence of miscarriage in rural Bangladesh: A prospective cohort study. PLoS ONE 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cecatti, J.G.; Guerra, G.V.D.Q.L.; Sousa, M.H.D.; Menezes, G.M.D.S. Aborto no Brasil: Um enfoque demografico. Rev. Bras. Ginecol. Obs. 2010, 32, 105–111. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Yang, L.; Yuan, L.; Guo, H.; Zhang, F.; Guan, Y.; Yao, W. Risk factors for early miscarriage among Chinese: A hospital-based case-control study. Fertil. Steril. 2014, 101, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Correia, L.L.; Rocha, H.A.L.; Leite, Á.J.M.; Campos, J.S.; Silva, A.C.E.; Machado, M.M.T.; Rocha, S.G.M.O.; Gomes, T.N.; da Cunha, A.J.L.A. Tendencia de abortos espontaneos e induzidos na região semiarida do Nordeste do Brasil: Uma serie transversal. Rev. Bras. Saude Mater. Infant. 2018, 18, 133–142. [Google Scholar]
- Alijotas-Reig, J.; Ferrer-Oliveras, R.; Rodrigo-Anoro Mj Farran-Codina, I.; Cabero-Roura, L.; Vilardell-Tarres, M. Anti-β2-glycoprotein-I and anti-phosphatidylserine antibodies in women with spontaneous pregnancy loss. Fertil. Steril. 2010, 93, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Durex, É.W.; Vieira Dias, F.; Braga Rodrigues, R.; Midlej Neto, T.K.; Valente Rocha, L.L. Abortos Espontâneos No Brasil E Em Suas Regiões: Estudo De Prevalência. Braz. J. Surg. Clin. Res. 2016, 14, 38–43. [Google Scholar]
- Soares, A.M.; Cançado, F.M.A.A. Perfil De Mulheres Com Perda Gestacional. Rev. Med. Minas. Gerais 2018, 28, e1930. [Google Scholar]
- Chaves, J.H.B.; Oliveira, E.M.; Bezerra, A.F.S.; Camano, L.; Sun, S.Y.; Mattar, R. O abortamento incompleto (provocado e espontâneo) em pacientes atendidas em maternidade do Sistema Único de Saúde. Rev Bras Clin Med. 2011, 9, 189–194. [Google Scholar]
- Buss, L.; Tolstrup, J.; Munk, C.; Bergholt, T.; Ottesen, B.; Grønbæk, M.; Kjaer, S. Spontaneous abortion: A prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstet. Et Gynecol. 2006, 85, 467–475. [Google Scholar] [CrossRef]
- Rosadas, C.; Brites, C.; Arakaki-Sánchez, D.; Casseb, J.; Ishak, R. Protocolo Brasileiro para Infecções Sexualmente Transmissíveis 2020: Infecção pelo vírus Zika. Epidemiol. Serv. Saúde 2021, 30, e2020605. [Google Scholar] [CrossRef]
- Kac, G.; Silveira, E.A.; Oliveira, L.C.D.; Araújo, D.M.R.; Sousa, E.B.D. Fatores associados à ocorrência de cesárea e aborto em mulheres selecionadas em um centro de saúde no município do Rio de Janeiro, Brasil. Rev. Bras. Saúde Matern. Infant. 2007, 7, 271–280. [Google Scholar] [CrossRef]
- de Araújo, C.P.; de Rezende Dornelas, A.C.V.; Sousa, A.M. Abordagem terapêutica no processo de esvaziamento uterino. Rev. Baiana Enferm. 2018, 32, e24857. [Google Scholar] [CrossRef]
- Arcanjo, F.C.N.; Ribeiro, A.S.; Teles, T.G.; Macena, R.H.M.; Carvalho, F.H.C. Uso do misoprostol em substituição à curetagem uterina em gestações interrompidas precocemente. Rev. Bras. Ginecol. Obstet. 2011, 33, 276–280. [Google Scholar]
- Secretaria de Vigilância em Saúde; Secretaria de Atenção a Saúde. ZIKA: Abordagem na Atenção Básica; UNA-SUS: Brasília, Brazil, 2013. [Google Scholar]
- Nanda, K.; Peloggia, A.; Grimes, D.; Lopez, L.; Nanda, G. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst. Rev. 2012, 3, CD003518. [Google Scholar] [CrossRef]
- Morris, J.L.; Winikoff, B.; Dabash, R.; Weeks, A.; Faundes, A.; Gemzell-Danielsson, K.; Visser, G.H.A. Recomendações atualizadas da FIGO para misoprostol utilizadas isoladamente em ginecologia e obstetrícia. Rev. Int. De. Ginecol. Obs. 2017, 138, 363–366. [Google Scholar] [CrossRef]
- Camayo, F.J.A.; Martins, L.A.B.; Cavalli, R.D.C. Perda gestacional retida: Tratamento baseado em evidência. Femina 2011, 39, 49–56. [Google Scholar]
- Bombin, M.; Mercado, J.; Zúñiga, J.; Encalada, D.; Ávila, J. Aspiración manual endouterina (AMEU): Revisión de la literatura y estudio de serie de casos. Rev. Chil. Obs. Ginecol. 2019, 84, 460–468. [Google Scholar] [CrossRef]
- Nunes, P.; Nogueira, R.; Coelho, J.; Rodrigues, F.; Salomumasobre; Joséé, C.; De Carvalho, J.; Rabelo, K.; De Azeredo, E.; Baseulio-Deoliveira, R.; et al. Investigação de múltiplos órgãos natimortos de uma infecção materna por denv-4: Caracterização histopatológica e de mediadores inflamatórios. Víruses 2019, 11, 319. [Google Scholar] [CrossRef]
- Mineiro, J.; Cao, B.; Govero, J.; Smith, A.; Fernandez, E.; Cabrera, O.; Garber, C.; Noll, M.; Klein, R.; Noguchi, K.; et al. A infecção pelo vírus Zika durante a gravidez em camundongos causa danos à placenta e morte fetal. Célula 2016, 165, 1081–1091. [Google Scholar]
- Gregory, C.J.; Oduyebo, T.; Brault, A.C.; Brooks, J.T.; Chung, K.; Hills, S.; Kuehnert, M.J.; Mead, P.; Meaney-Delman, D.; Rabe, I.; et al. Modos de transmissão do vírus Zika. J. Infect. Dis. 2017, 216 (Suppl. S10), 875–883. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Barau, G.; Michael, A.; Bintner, M.; Randrianaivo, H.; Choker, G.; Lenglet, Y.; Touret, Y.; Bouveret, A.; Grivard, P.; et al. Estudo prospectivo multidisciplinar de infecções materno-infantis pelo vírus Chikungunya na Ilha de La Ré União. PLoS Med. 2008, 5, e60. [Google Scholar]
- Touret, Y.; Randrianaivo, H.; Michael, A.; Schuffenecker, I.; Kauffmann, E.; Lenglet, Y.; Barau, G.; Fourmaintraux, A. Transmissão materno-fetal precoce do vírus Chikungunya. Press. Médica 2006, 35, 1656. [Google Scholar] [CrossRef] [PubMed]
- Grivard, P.; Le Roux, K.; Laurent, P.; Fianu, A.; Perrau, J.; Gigan, J.; Hoarau, G.; Grondin, N.; Staikowsky, F.; Favier, F.; et al. Diagnóstico molecular e sorológico da infecção pelo vírus Chikungunya. Patol. Biol. 2007, 55, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Prata-Barbosa, A.; Cleto-Yamane, T.; Robaina, J.; Guastavino, A.; De Magalhumaes-Barbosa, M.; Brindeiro, R.; Medronho, R.; Da Cunha, A. Co-infecção por vírus Zika e Chikungunya associada à morte fetal—Relato de caso. Int. J. Infectar. Des. 2018, 72, 25–27. [Google Scholar] [CrossRef]
- Imbeloni, A.A. Infecção Experimental Por Vírus Zika Em Fêmeas Prenhes De Saimiri Collinsi: Avaliação No Pré-natal E Síndrome Congênita Do Zika Vírus. 2021. Available online: https://bdtd.ibict.br/vufind/Record/IEC-2_a0927a43c5bfa6b4b44a413097d56e4b (accessed on 14 June 2023).
- van der Eijk, A.A.; van Genderen, P.J.; Verdijk, R.M.; Reusken, C.B.; Mögling, R.; van Kampen, J.J.A.; Widagdo, W.; Aron, G.I.; GeurtsvanKessel, C.H.; Pas, S.D.; et al. Miscarriage Associated with Zika Virus Infection. N. Engl. J. Med. 2022, 375, 1002–1004. [Google Scholar] [CrossRef]
- Runge-Ranzinger, S.; Morrison Ac Manrique-Saide, P.; Horstick, O. Padrões de transmissão do Zika: Uma metarevisão. Trop. Med. Int. Heal. 2019, 24, 523–529. [Google Scholar]
- Salomão, N.; Brendolin, M.; Rabelo, K.; Wakimoto, M.; De Filippis, A.; Dos Santos, F.; Moreira, M.; Basílio-De-Oliveira, C.; Avvad-Portari, E.; Paes, M.; et al. Aborto Espontâneo e Infecção por Chikungunya: Achados Patológicos. Rev. Vírus 2021, 13, 554. [Google Scholar]
- Yu, W.; Hu, X.; Cao, B. Viral Infections During Pregnancy: The Big Challenge Threatening Maternal and Fetal Health. Matern. Fetal Med. 2022, 4, 72–86. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.; Kayisli, U.A. hcg: Funções biológicas e aplicações clínicas. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.B.; Bhatnagar, J.; de Oliveira Ramos, A.M.; Davi, H.P.F.; Iglezias, A.; Kanamura, C.T.; Keating, M.K.; Hale, G.; Silva-Flannery, L.; Muehlenbachs, A.; et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet 2016, 388, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Dulay, A.T. Manual MSD. Aborto Espontâneo. 2020. Available online: https://www.msdmanuals.com/pt/casa/problemas-de-saúde-feminina/complicações-da-gravidez/trabalho-de-parto-prematuro?query=dulay (accessed on 7 November 2022).
- Mehrjardi, M.; Shobeirian, F. O papel da placenta na infecção pré-natal pelo vírus Zika. Doença Vírus. 2017, 28, 247–249. [Google Scholar]
- Azevedo, R.S.S.; Araujo, M.T.; Oliveira, C.S.; Martins Filho, A.J.; Nunes, B.T.D.; Henriques, D.F.; Silva, E.V.P.; Carvalho, V.L.; Chiang, J.O.; Martins, L.C.; et al. Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J. Clin. Med. 2018, 7, 496. [Google Scholar]
- Contopoulos-Ioannidis, D.; Newman-Linsay, S.; Chow, C.; Labeaud, A. Transmissão de mãe para filho do vírus Chikungunya; a Revisão sistemática e metanálise. PloS Negl. Trop. Dis. 2018, 12, e0006510. [Google Scholar]
- Villamil-Gómez, W.; González-Camargo, O.; Rodriguez-Ayubi, J.; Zapata-Serpa, D.; Rodriguez-Morales, A. Co-infecção de dengue, chikungunya e zika em paciente da Colômbia. J. Infectar Saúde Pública 2016, 9, 684–686. [Google Scholar]
- Mercado, M.; Acosta-Reyes, J.; Parra, E.; Pardo, L.; Rico, A.; Campo, A.; Navarro, E.; Viasus, D. Características clínicas e histopatológicas de casos fatais com coinfecção pelo vírus dengue e chikungunya na Colômbia, 2014 a 2015. Eurosurveillance 2016, 21, 30244. [Google Scholar]


| Sociodemographic Profile | n | % | p-Value 1 |
|---|---|---|---|
| Age group (in years) | |||
| 12–19 | 3 | 10.00 | 0.003 * |
| 20–29 | 16 | 53.33 | |
| 30–39 | 10 | 33.33 | |
| ≥40 | 1 | 3.33 | |
| Origin | |||
| State capital | 21 | 70.00 | <0.001 * |
| Other metropolitan areas of Belém | 5 | 16.67 | |
| Municipalities in the interior of Pará | 3 | 10.00 | |
| Not mentioned | 1 | 3.33 | |
| Race | |||
| White | 3 | 10.00 | |
| Brown | 20 | 66.67 | 0.0004 * |
| Not mentioned | 7 | 23.33 | |
| Marital status | |||
| Married | 2 | 6.67 | 0.1116 |
| Single | 11 | 36.67 | |
| Union stability | 8 | 26.67 | |
| Not mentioned | 9 | 30.00 |
| Parameters | n | % | p-Value 1 |
|---|---|---|---|
| Symptoms | |||
| Headache | 7 | 23.34 | 0.0073 |
| Fever | 6 | 20.00 | |
| Malaise | 1 | 3.33 | |
| Vomiting | 3 | 10.00 | |
| Not mentioned | 13 | 43.33 | |
| Pregnancy history | |||
| 1 | 3 | 10.00 | |
| 2 | 10 | 33.33 | |
| 3 | 8 | 26.67 | 0.2255 |
| 4 | 5 | 16.67 | |
| ≥5 | 4 | 13.33 | |
| Parity history | |||
| 0 | 6 | 20.00 | |
| 1 | 11 | 36.67 | |
| 2 | 7 | 23.33 | 0.1046 |
| 3 | 4 | 13.33 | |
| 4 | 2 | 6.67 | |
| Abortion history | |||
| 0 | 20 | 66.67 | 0.0004 * |
| 1 | 6 | 20.00 | |
| 2 | 4 | 13.33 | |
| Gestational age (weeks) | |||
| 1–13 | 18 | 60.00 | <0.0001 * |
| 14–28 | 10 | 33.33 | |
| 29–41 | 0 | 0.00 | |
| not mentioned | 2 | 6.67 | |
| Type of abortion | |||
| Incomplete abortion | 24 | 80.00 | 0.0047 * |
| Missed abortion | 6 | 20.00 | |
| Abortion procedure | |||
| Manual intrauterine aspiration | 4 | 13.33 | 0.0004 * |
| Curettage | 26 | 86.67 | |
| Year | Viral Detection | n | % | p-Value 1 |
|---|---|---|---|---|
| 2015 | ||||
| CHIKV (+) | 3 | 11.11 | ||
| CHIKV (−) | 11 | 40.74 | 0.0130 * | |
| ZIKV (+) | 2 | 7.40 | ||
| ZIKV (−) | 11 | 40.74 | ||
| 2016 | ||||
| CHIKV (+) | 0 | 0 | 1.0000 | |
| CHIKV (−) | 2 | 50 | ||
| ZIKV (+) | 0 | 0 | ||
| ZIKV (−) | 2 | 50 | ||
| 2017 | ||||
| CHIKV (+) | 7 | 25 | 0.2940 | |
| CHIKV (−) | 10 | 35.71 | ||
| ZIKV (+) | 3 | 10.71 | ||
| ZIKV (−) | 8 | 28.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, A.K.P.; Cruz, A.C.R.; Quaresma, J.A.S.; Filho, A.J.M.; Durans, D.d.B.S.; Amador Neto, O.P.; de Lima, L.d.S.O.; Assunçao, N.S.d.C.F.; Franco, E.C.S.; Cohen, P.B.; et al. Impact of Zika and Chikungunya Viruses on Spontaneous Abortions: Insights from a Reference Maternity Hospital. Microorganisms 2025, 13, 678. https://doi.org/10.3390/microorganisms13030678
de Carvalho AKP, Cruz ACR, Quaresma JAS, Filho AJM, Durans DdBS, Amador Neto OP, de Lima LdSO, Assunçao NSdCF, Franco ECS, Cohen PB, et al. Impact of Zika and Chikungunya Viruses on Spontaneous Abortions: Insights from a Reference Maternity Hospital. Microorganisms. 2025; 13(3):678. https://doi.org/10.3390/microorganisms13030678
Chicago/Turabian Stylede Carvalho, Anne Kerollen Pinheiro, Ana Cecília Ribeiro Cruz, Juarez Antônio Simões Quaresma, Arnaldo Jorge Martins Filho, Darlene de Brito Simith Durans, Orlando Pereira Amador Neto, Ligia do Socorro Oliveira de Lima, Norma Suely de Carvalho Fonseca Assunçao, Edna Cristina Santos Franco, Patrícia Brazão Cohen, and et al. 2025. "Impact of Zika and Chikungunya Viruses on Spontaneous Abortions: Insights from a Reference Maternity Hospital" Microorganisms 13, no. 3: 678. https://doi.org/10.3390/microorganisms13030678
APA Stylede Carvalho, A. K. P., Cruz, A. C. R., Quaresma, J. A. S., Filho, A. J. M., Durans, D. d. B. S., Amador Neto, O. P., de Lima, L. d. S. O., Assunçao, N. S. d. C. F., Franco, E. C. S., Cohen, P. B., & da Silva, E. V. P. (2025). Impact of Zika and Chikungunya Viruses on Spontaneous Abortions: Insights from a Reference Maternity Hospital. Microorganisms, 13(3), 678. https://doi.org/10.3390/microorganisms13030678










