Abstract
With the rapid implementation of high-pressure processing in many sectors of the food industry, considerations associated with pressure-stressed microorganisms are emerging. Nisin was utilized in this study for controlling the proliferation of Listeria monocytogenes and L. innocua inoculated on cold-smoked trout during a 4-week refrigerated shelf-life trial. Wild-type and pressure-stressed phenotypes of Listeria were compared in this study. The pressure-stressed phenotypes were prepared by treating the surrogate strain and pathogen mixture at 103.4 MPa (15K PSI) for 20 min. L. monocytogenes multiplied extensively during the 4-week refrigerated trial and counts were increased (p < 0.05) from 3.68 ± 0.1 log CFU/g on the first week to 6.03 ± 0.1 log CFU/g. Both phenotypes and the surrogate microorganisms illustrated similar (p ≥ 0.05) multiplication trends. Unlike samples subjected to water treatment, nisin was effective (p < 0.05) in keeping the microbial counts lower compared with the controls, particularly earlier during the shelf-life trial. Our study illustrates that the selected surrogate microorganism has comparable sensitivity to nisin relative to L. monocytogenes and thus could be used interchangeably in future public health microbiology challenge studies with similar scope. Additionally, we observed that pressure-stressed L. monocytogenes has proliferation and sensitivity to nisin comparable to wild-type pathogen.
1. Introduction
Listeria monocytogenes was recognized as a prominent pathogen upon its isolation during an epidemic of listeriosis in newborns in Germany in 1949 [1]. Although there are twenty-eight species in the Listeria genus, only two (monocytogenes, and ivanovii) can cause disease in humans and animals [2]. According to the U.S. Centers for Disease Control and Prevention, in a typical year, L. monocytogenes is the third leading cause of foodborne death, accounting for close to 1600 illness episodes and more than 250 annual deaths, with a mortality rate of 15.9% in the United States [3]. Of particular importance is the fact that more than 98% of Listeriosis cases in the United States are linked to contaminated food [4]. After consuming contaminated food, L. monocytogenes can travel from the human gut to various target organs through blood. The pathogen can target the liver to multiply and after proliferation in the liver, persistent cases of bacteremia and secondary invasion of other target organs like the brain and gravid uterus can occur [5,6].
One of the main sources of Listeria spp., and specifically L. monocytogenes, is ready-to-eat (RTE) food products. In a multiyear epidemiological study of RTE producers under inspection in the United States, it was observed that 71% of the facilities had at least one positive result of Listeria spp. for samples obtained from their food and/or food-contact surfaces [7]. Although it is noteworthy that there may or may not be a direct correlation between the presence of Listeria spp. and L. monocytogenes, the above-mentioned epidemiological study indicated that, overall, 4.9% of the samples were positive for Listeria spp., illustrating the ubiquitous nature of this important microorganism [7]. A Canadian outbreak in 2023 linked to recalled plant-based refrigerated beverages resulted in 20 confirmed cases, 15 hospitalizations, and three deaths, and a recent outbreak linked to deli meat initiated from the U.S. State of Virginia was linked with at least 10 deaths and 60 hospitalizations, further emphasizing the public health importance of this pathogen [8,9]. Various investigations have reported L. monocytogenes in RTE food products worldwide, with considerable variations for products and regions of the world. In Belgium, as an example, the prevalence of Listeria in RTE food products was 6.7% in deli salads with mayonnaise, 1.1% in precooked meat products, and 22% for smoked fish [10,11,12,13]. Among various commodities, a range of seafood products, such as marinated fish, cooked and frozen seafood, and smoked fish, are of particular importance and have been associated with the Listeria outbreaks. This highlights the challenge of controlling Listeria in seafood due to its ability to withstand diverse environmental stressors and multiply under refrigerated temperatures [14].
Cold-smoked rainbow trout (Oncorhynchus mykiss) is an important vehicle for this pathogen in the food chain [15,16,17]. Listeria can multiply to hazardous levels during storage in refrigerated conditions; therefore, health complications are possible even if the contamination level is less than 100 CFU/g of the product [18]. L. monocytogenes shows a higher multiplication rate in fish, compared to beef and chicken at 4 °C and under aerobic conditions [19].
The use of L. monocytogenes in commercial food processing facilities is prohibited due to its pathogenic nature since there are strict requirements for Biosafety Level II (BSL-2) facilities that conduct experiments involving L. monocytogenes because of safety concerns [20]. Thus, the ideal substitute is a surrogate bacterium that shares the common physiological traits of the pathogenic bacterium [21]. L. innocua is a nonpathogenic surrogate for L. monocytogenes, frequently found in environments similar to L. monocytogenes [22].
Although traditional preservation methods such as thermal preservation methods alter the taste, flavor, and nutritional quality of the products, they have been utilized by the food industry for decades. As a result, non-thermal methods of food preservation with minimal impact on the quality and organoleptic properties of food are gaining popularity. High-pressure processing (HPP) is a potential substitution for a thermal technique that transfers pressure instantly and uniformly throughout the sample without compromising the quality of the food sample [23,24]. HPP alters the morphology and physiology of bacteria, including membrane permeability and protein denaturation. As a result, this disruption can cause cellular damage or death, impacting vital processes and leaking cellular content. It also affects membrane proteins, protein structure, and ATP synthesis of bacteria [25]. However, limited information is available in the literature regarding the fate and multiplication of pressure-stressed microorganisms, i.e., those surviving treatment with elevated hydrostatic pressure.
A highly effective bacteriocin, nisin, produced by Lactococcus lactis, can be used to ensure the safety of ready-to-eat commodities. More than 40 countries use nisin as a preservative, as it is approved by the US Food and Drug Administration (FDA) and the European Food Safety Authority. Nisin attributes broad-spectrum antimicrobial properties, and Gram-positive bacteria are especially susceptible to its effect [26]. Nisin has demonstrated efficiency against a diverse range of Gram-positive bacteria and spoilage microorganisms, including Staphylococcus aureus, L. monocytogenes, and Clostridium spp. [27] and in a variety of foods such as dairy products and desserts, fish and seafood, fruit juices, beverages, and veterinary medicine for the treatment of bovine mastitis [28].
The current study is a 4-week shelf-life study to investigate the fate and proliferation of L. monocytogenes on cold-smoked trout during aerobic refrigerated storage. The impact of nisin during the trial was additionally investigated against wild-type and pressure-stressed phenotypes of the pathogen. The fate and proliferation of L. innocua as a surrogate for L. monocytogenes were additionally investigated in this study.
2. Materials and Methods
2.1. Preparation of Listeria Strains and Inocula
The frozen stock of five L. monocytogenes strains used in our experiment were those with ATCC® numbers 51772TM (serotype 1/2a), 51779TM (serotype 1/2c), BAA-2658TM (serotype 1/2b), 13932TM (serotype 4b), BAA-751TM (serotype 1/2b), and a non-pathogenic strain of L. innocua (ATCC® 33090TM, serotype 6a), preserved at −80 °C in glycerol. Strains that represent various lineages and ribotypes were chosen based on public health and the food industry’s significance and based on previously published work of the public health microbiology laboratory of Tennessee State University. Each strain was separately transferred into 10 mL Tryptic Soy Broth (Difco, Becton Dickinson, Franklin Lakes, NJ, USA) containing 0.6% yeast extract (TSB + YE) and incubated at 37 °C for 22–24 h. After incubation, the bacterial suspension was homogenized using a high-speed vortex (Scientific Industries, Bohemia, NY, USA, Model SI-0236), and 0.1 mL was transferred into another 10 mL TSB + YE and was then incubated again at 37 °C for 22–24 h. After incubation and homogenization, the cells were plated onto the surface of Tryptic Soy Agar (Difco, Becton Dickinson, Franklin Lakes, NJ, USA) containing 0.6% yeast extract (TSA + YE) using a sterilized inoculation loop and incubated at 37 °C for 22–24 h to obtain individual colonies. These plates were then stored in a 4 °C refrigerator for up to one month prior to the trial.
Two days before the experiment, for activation of the microorganisms, a loop-full of bacteria sourced from a single colony of the above-mentioned plates, for each strain separately, was transferred into 10 mL of TSB + YE and incubated at 37 °C for 22–24 h. After this activation and for sub-culturing, 0.1 mL (each strain separately) was pipetted aseptically into another fresh sterilized 10 mL of TSB + YE and incubated again at 37 °C for 22–24 h. After incubation, the overnight bacterial suspension of each strain was used to harvest the cells through centrifugation for 15 min at 6000 revolutions per min twice (Eppendorf North America, Hauppauge, NY, USA; Model 5424, Rotor FA-45-24-11). After the first round of centrifugation, the supernatant, containing cell components, secondary metabolite, and growth media, was discarded, and the microbial pellet was re-suspended in PBS (VWR International, Radnor, PA, USA). The process was repeated to further purify the bacterial inocula, and after the second re-suspension of the strains in PBS (VWR International, Radnor, PA, USA), the five strains of the pathogen were composited into an inoculum to ensure equal representation of all strains.
2.2. Sample Preparation and Inoculation
Farm-raised cold-smoked rainbow trout (Oncorhynchus mykiss) was obtained from a local supermarket in Nashville, Tennessee, with a sodium content of aproximately 1020 mg/100 g. The product was marketed as a “natural” commodity without any listed antimicrobials such as nitrite or nitrate. The skinless samples were then cut aseptically into 5.0 ± 0.1 g portions, and each sample was placed inside a sterile petri dish (VWR International, Radnor, PA, USA). Samples were then randomly assigned to each of the four inoculation groups of (i) a five-strain mixture of wild-type L. monocytogenes, (ii) a five-strain mixture of pressure-stressed L. monocytogenes, (iii) one strain of wild-type L. innocua, and (iv) one strain of pressure-stressed L. innocua. Each of these four was additionally divided into three treatment sections of (a) no treatment (untreated control), (b) with 0.1 mL of deionized and sterilized water added (treated control), and (c) with 5000 IU of nisin. This concentration of nisin was selected based on preliminary trials to ensure efficacy and using the antimicrobial at a common level of use in the food industry [29]. Pathogen inocula were then 10-fold serially diluted in PBS for a target inoculation level of 3 to 4 log CFU/g. Samples were stored aerobically to mimic consumers’ and food service handling of the product after opening the package and storing the commodity at refrigeration prior to consumption.
The powdered nisin (Sigma-Aldrich, St. Louis, MO, USA) was used at a concentration of 5000 IU/5.0 g of samples, with 1000 IU being equivalent to 0.025 mg of nisin [29]. The International Unit (IU) of nisin is defined as the quantity of nisin required to inhibit a single Streptococcus agalactiae cell in 1 mL of broth [29]. Before the addition of bacteriocin, nisin was transferred into Phosphate-Buffered Saline (PBS, VWR 135 International, Radnor, PA, USA), and the insoluble solids were removed by centrifuging (Eppendorf North America, Hauppauge, NY, USA; Model 5424, Rotor FA-45-24-11) at the intensity of 3000 revolutions per min for one min. After centrifuging, the supernatant was filter-sterilized and inoculated onto the surface of fish samples. The inoculated bacteriocin was then spread evenly into the surface of the product using a sterilized glass spreader prior to the inoculation of the above-mentioned four inocula.
2.3. Preparation of Pressure-Stressed Microbial Cells
A mixture of 1.5 mL of L. monocytogenes and a single strain of L. innocua was transferred to the PULSE tubes without a disk (Pressure Bioscience Inc., South Easton, MA, USA) inside PBS (VWR International, Radnor, PA, USA) at target bacterial load of 7 to 8 log CFU/mL. The PULSE tubes were then treated at 4 °C and at an elevated hydrostatic pressure of 103.4 MPa (15K PSI) for 20 min to yield the target bacterial count of 6 log CFU/mL after treatment [30]. The HUB Explorer PBI software Version 2.3.11 (Pressure Bioscience Inc., South Easton, MA, USA) was used to monitor and automatically record the temperature every 3 s. The transmission fluid for the pressure treatment was distilled water (<30 ppm total dissolved solids). Residual air from the chamber was purged using a pump on chamber closure to ensure treatments were hydrostatic in nature. The temperature was monitored using a K-type thermocouple (Omega Engineering Inc., Norwalk, CT, USA) mounted inside the chamber wall, secured with thermal paste (Model 5 AS5-3.5G, Arctic Silver, Visalia, CA, USA), and connected to HUB Explorer PBI software. The temperature was regulated by a refrigerated circulating water bath (Model 160s, VWR International, Radnor, PA, USA) mechanically connected to a stainless-steel jacket surrounding the pressure-processing chamber.
2.4. Microbiological and Physiochemical Analysis
On each day of the trial, samples were aseptically transferred into the sterile filtered stomaching bag (Whirl-Pak, Modesto, CA, USA) and 20 mL of sterile Dey/Engley neutralizing broth (D/E Broth; Difco, Becton Dickinson, Franklin Lakes, NJ, USA) was added to each bag followed by mastication for 2 min at 200 revolutions per min using a Triple Mix Paddle Blender (Boekel Scientific, Feasterville-Trevose, PA, USA) to achieve an even distribution of microorganisms within the suspension. Samples were then 10-fold serially diluted in 0.1% Maximum Recovery Diluent (MRD; Difco, Becton Dickinson, Franklin Lakes, NJ, USA) and were spread-plated onto the surface of selective and non-selective media. The selective medium, i.e., Polymyxin Acriflavin Lithium-chloride Ceftazidime Esculin Mannitol (PALCAM) Agar was supplemented with Ceftazidime (Becton, Dickinson and Company, Sparks, MD, USA) and non-selective medium of Tryptic Soy Agar (Difco, Becton Dickinson, Franklin Lakes, NJ, USA) was supplemented with 0.6% yeast extract.
After 48 h of incubation at 37 °C, the bacterial colonies were counted manually using the Quebec Colony Counter based on the U.S. Food and Drug Administration Bacteriological Analytical Method [31]. Counts of non-selective medium after incubation at 37 °C were reported as mesophilic background microbiota (mesophilic aerobic plate count). A water activity meter (Lab Swift-aw water activity meter, Novasina, Lachen, Switzerland) was also used to measure the water activity of the samples every week. The pH of the samples was similarly monitored weekly using a calibrated pH meter (S400-kit SevenExcellence, Mettler Toledo, Columbus, OH, USA).
2.5. Design and Data Analysis
This study involved two microbiologically independent replicates, serving as blocking factors within a randomized complete block design. Treatments were implemented across two distinct blocks, each comprising two replications. Each of these replications was additionally repeated twice as two microbiological repetitions and microbial analyses were conducted on selective and non-selective media. Thus, each presented value is the mean of eight independent observations (2 blocks, 2 replications, 2 microbiological repetitions). The microbial counts were log-transformed in Microsoft Excel (Microsoft Corp, Redmond, WA, USA), and the data were then imported to SAS version 9.4 software (SAS Institute Inc, Cary, NC, USA) for further inferential and descriptive statistics. Initially, the log-normality of the data was confirmed by running diagnostics using ods graphics in the General Linear Model (GLM) procedure of SAS version 9.4. After the confirmation of log normality and homogeneity of variances (Levene’s test with p ≥ 0.05), the GLM procedure was used for mean separations using Tukey-adjusted (expressed by alphabet letters in figures) and Dunnett’s adjusted (expressed by “*” in figures) analyses of variance. In the former analysis, all pair-wise comparisons were considered with the largest value receiving the letter “A” (for selective counts) and “a” (for non-selective counts), while the latter only compared each treatment with the control, thus, bars marked by “*” are statistically (p < 0.05) different than the control. For both Tukey- and Dunnett-based ANOVA, two separate analyses were conducted for selective and non-selective media.
3. Results and Discussion
The temperature of the samples remained unchanged (p ≥ 0.05) during the course of the 4-week shelf-life trial. Temperature values (°C, mean ± standard deviation) for the weeks 0 to 4 were 5.23 ± 0.1, 5.08 ± 0.1, 5.28 ± 0.2, 4.75 ± 0.4, and 5.33 ± 0.2, respectively. Similarly, the pH (mean ± standard deviation) of control and samples treated with distilled water (DW), and nisin were similar (p ≥ 0.05) and for samples of weeks 0 to 4 were 6.55 ± 0.0, 6.63 ± 0.1, 6.63 ± 0.2, 6.68 ± 0.1, and 6.72 ± 0.5, respectively. The water activity of samples without treatment (control), treated with distilled water, and nisin were also similar and remained unchanged (p ≥ 0.05) until week 3. Samples of week 4 exhibited lower (p < 0.05) water activity relative to the other weeks of the trials. The water activity (mean ± standard deviation) of samples of the weeks 0 to 4 were 0.99 ± 0.0, 0.99 ± 0.0, 0.97 ± 0.0, 0.99 ± 0.0, and 0.96 ± 0.0, respectively.
3.1. Fate of Wild-Type and Pressure-Stressed Listeria monocytogenes and Background Microbiota of Smoked Trout During 4-Week Aerobic Refrigerated Storage as Affected by Nisin
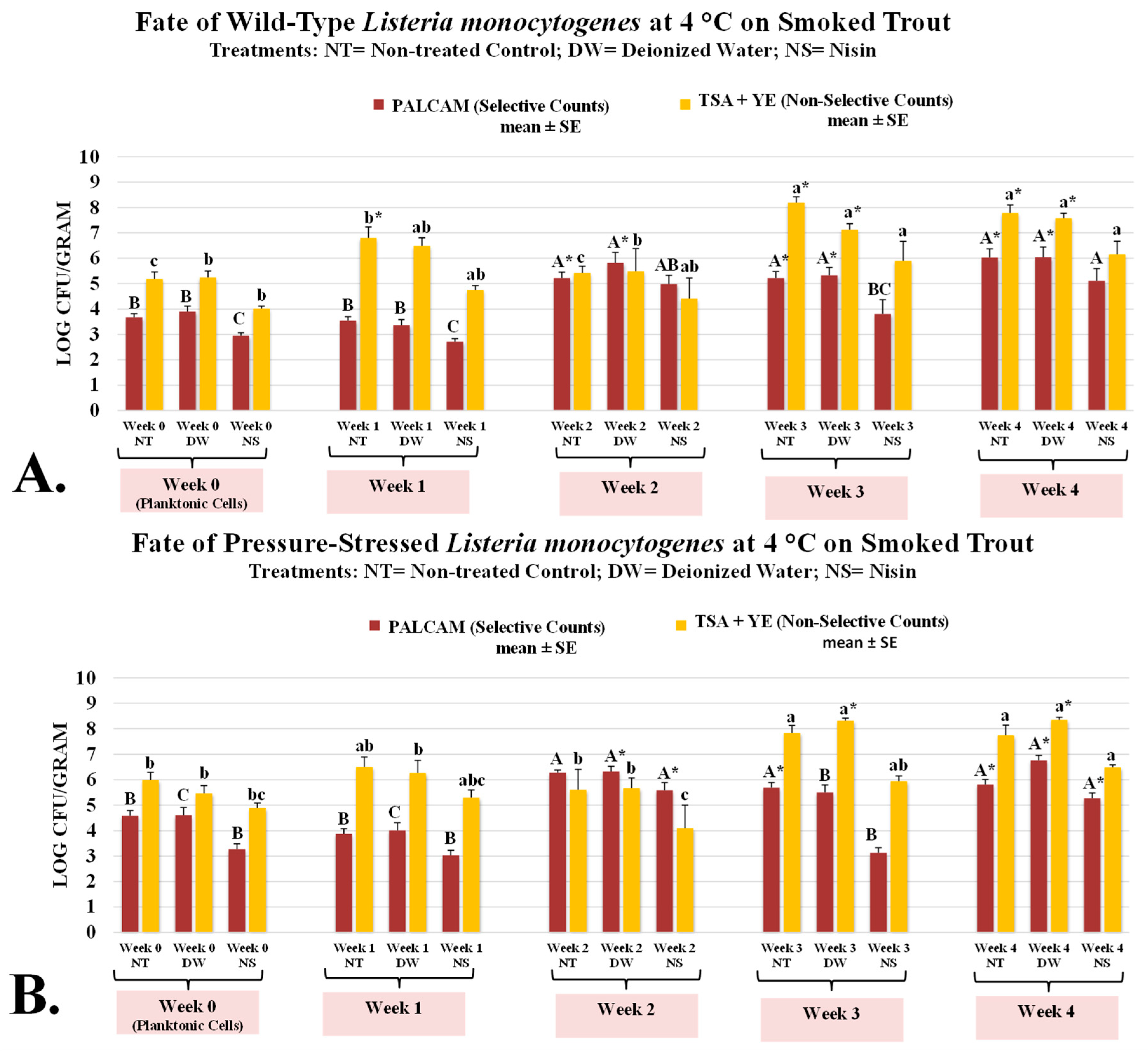
L. monocytogenes belongs to a small group of foodborne pathogens of public health concern with the capability of survival and multiplication at refrigerated temperatures [32,33]. Considering the low infective dose of the pathogen, the fate of the microorganism thus is of particular concern for ready-to-eat commodities [33,34]. Our study illustrates both selective and non-selective counts (Figure 1), representing the counts of inoculated pathogen and background microbiota (mesophilic aerobic plate count), respectively. On the day of inoculation (day 0), counts of L. monocytogenes were 3.68 ± 0.1 log CFU/g and these counts remained unchanged (p ≥ 0.05) for samples with added distilled water (DW, treated control) and were reduced (p < 0.05) for samples containing 5000 IU of nisin (Figure 1A). The corresponding counts with samples with DW (treated control) and nisin on this day were 3.90 ± 0.2 and 2.95 ± 0.1 log CFU/g, respectively (Figure 1A). Counts of background microbiota (mesophilic aerobic plate count) were similarly affected on week 0 (inoculation day). These counts for untreated control, those treated with DW (treated control), and nisin were 5.18 ± 0.3, 5.24 ± 0.3, and 4.02 ± 0.1 log CFU/g, respectively, with counts of samples containing nisin exhibiting more than one log (i.e., >90%) lower (p < 0.05) microbial counts, relative to the other two (Figure 1A). After one week of refrigerated aerobic storage counts of untreated and treated controls and those containing nisin had remained similar (p ≥ 0.05) and further showing similarity with trends observed on inoculation day (Figure 1A). However, the counts of background microbiota (mesophilic aerobic plate count) increased to a great extent during the one-week aerobic refrigerated storage. The mesophilic background microbiota counts of untreated and treated controls and nisin-containing samples were 6.80 ± 0.1, 6.48 ± 0.2, and 4.75 ± 0.1 log CFU/g, respectively (Figure 1A). Although early in the shelf-life, the addition of nisin exhibited promising potential for mitigating (p < 0.05) the counts of both pathogen and mesophilic background microbiota, this impact faded away as the shelf-life study progressed (Figure 1A). This illustrates the importance of preventive measures rather than solely relying on antimicrobials for ensuring the safety of a commodity. As an example, on the last day of the trial, the L. monocytogenes counts of untreated and treated controls and nisin-containing samples were 6.03 ± 0.3, 6.05 ± 0.4, and 5.11 ± 0.5 log CFU/g, respectively (Figure 1A). The corresponding counts for mesophilic background microbiota on the same week of the trial were higher and were 7.79 ± 0.3, 7.58 ± 0.2, and 6.15 ± 0.5 log CFU/g, respectively (Figure 1A).
Figure 1.
Bacteriostatic/bactericidal activity of nisin against wild-type and pressure-stressed Listeria monocytogenes (PALCAM counts) and mesophilic background microbiota (TSA + YE counts) during shelf-life of smoked trout. (A) Counts of wild-type L. monocytogenes. (B) Counts of pressure-stressed L. monocytogenes. PALCAM counts marked by different uppercase letters are statistically different (p < 0.05) from each other (Tukey-adjusted ANOVA). TSA + YE counts marked by different lowercase letters are statistically different (p < 0.05) from each other (Tukey-adjusted ANOVA). For each media separately, columns marked by “*” are statistically different (p < 0.05) than the control (Dunnett’s-adjusted ANOVA).
It is also of great importance to note that L. monocytogenes could extensively multiply during the 4-week aerobic refrigerated shelf-life trial. The L. monocytogenes counts without and with nisin on week 0 were 3.68 ± 0.1, and 2.95 ± 0.1 log CFU/g, respectively (Figure 1A). By weeks 2 and 4, L. monocytogenes counts of samples without nisin increased (p < 0.05) by 1.54 and 2.35 logs, respectively. The corresponding log increases for samples with nisin were 2.03 and 2.16 logs (Figure 1A). L. monocytogenes is a prevalent pathogen in the smoked fish industry, a cross-sectional study, as an example, illustrated that as high as 3.8% of raw fish and 1.3% of samples obtained from four smoked fish processing plants were contaminated with this pathogen [35]. A more recent epidemiological study in Europe, although limited in sample size and scope, showed a prevalence of L. monocytogenes as high as 18.9% in raw and smoked samples [36]. In light of these concerns, our study illustrates that relying on antimicrobial treatment alone might not be sufficient, and a more holistic approach in the context of multiple hurdle technology should be considered to ensure the safety of these high-risk products [30,37]. The hurdle technology concept proposes the application of interventions in sequence and in combination, such as the utilization of thermal and/or non-thermal processing synergized with antimicrobial treatments, to ensure the safety of the product [37,38,39]. The application of novel technologies, such as the use of elevated hydrostatic pressure, could be a very efficacious intervention for inactivation of the pathogens [33,40]. However, any application should be carefully considered to ensure the safety and effectiveness of the treatment and its impact on the sensory and quality characteristics of the product. A recent potential challenge to the use of high-pressure processing is the concerns associated with pressure-stressed and pressure-adopted strains [41]. Currently, the literature available about the fate and proliferation of pressure-stressed microorganisms, i.e., those surviving the high-pressure treatment with or without sublethal injuries, is limited. As such, in addition to the wild-type pathogen, this study investigated the fate and proliferation of pressure-stressed L. monocytogenes during the refrigerated shelf-life trials (Figure 1B). Under the conditions of our experiment, pressure-stressed L. monocytogenes was able to survive and multiply under refrigeration (Figure 1B).
This phenotype of the L. monocytogenes not only remained detectable during the trial, but as well the pressure-stressed pathogen was able to multiply significantly (p < 0.05) during the trial by > one log CFU/g (Figure 1B). These counts on week 0 to 4 were 4.59 ± 0.2, 3.88 ± 0.2, 6.28 ± 0.1, 5.69 ± 0.2, and 5.81 ± 0.2 log CFU/g, respectively (Figure 1B). Not only the pressure-stressed phenotype of the pathogen multiplied extensively under refrigeration, the rate of multiplication for pressure-stressed pathogen (Figure 1B) was comparable with the wild-type microorganism (Figure 1A). As an example, counts of untreated controls for wild-type L. monocytogenes on weeks 3 and 4 were 5.22 ± 0.3 and 6.03 ± 0.3 log CFU/g, respectively (Figure 1A). The corresponding counts for pressure-stressed L. monocytogenes on weeks 3 and 4 were 5.69 ± 0.2 and 5.81 ± 0.2 log CFU/g, respectively (Figure 1B). The pressure-stressed cells also showed similar sensitivity to nisin, relative to wild-type cells. As an example, on week 0 pressure-stressed L. monocytogenes counts of untreated and treated control samples were 4.59 ± 0.2, and 4.61 ± 0.3 log CFU/g, respectively, (Figure 1B). For the same pathogen phenotype and the same week, counts of nisin-containing samples were appreciably less (p < 0.05) and were 3.25 ± 0.2 log CFU/g (Figure 1B). This indicates the application of nisin on week 0 was able to reduce (p < 0.05) more than 90% of the pressure-stressed pathogen, compared to the controls (Figure 1B). Similar to the trends observed for the wild-type phenotype of the pathogen, bactericidal and bacteriostatic impacts of nisin faded as the days of storage increased. As an example, on the last day of the shelf-life trial counts of untreated and treated controls and nisin-containing pressure-stressed pathogen were statistically nonsignificant (p ≥ 0.05) and were 5.81 ± 0.2, 6.76 ± 0.2, and 5.28 ± 0.2 log CFU/g, respectively (Figure 1B). These findings highlight the importance of preventive measures and application of multiple hurdle technology, as discussed earlier, rather than solely relying on one antimicrobial.
3.2. Fate of Wild-Type and Pressure-Stressed Listeria innocua on Smoked Trout During 4-Week Aerobic Refrigerated Storage as Affected by Nisin
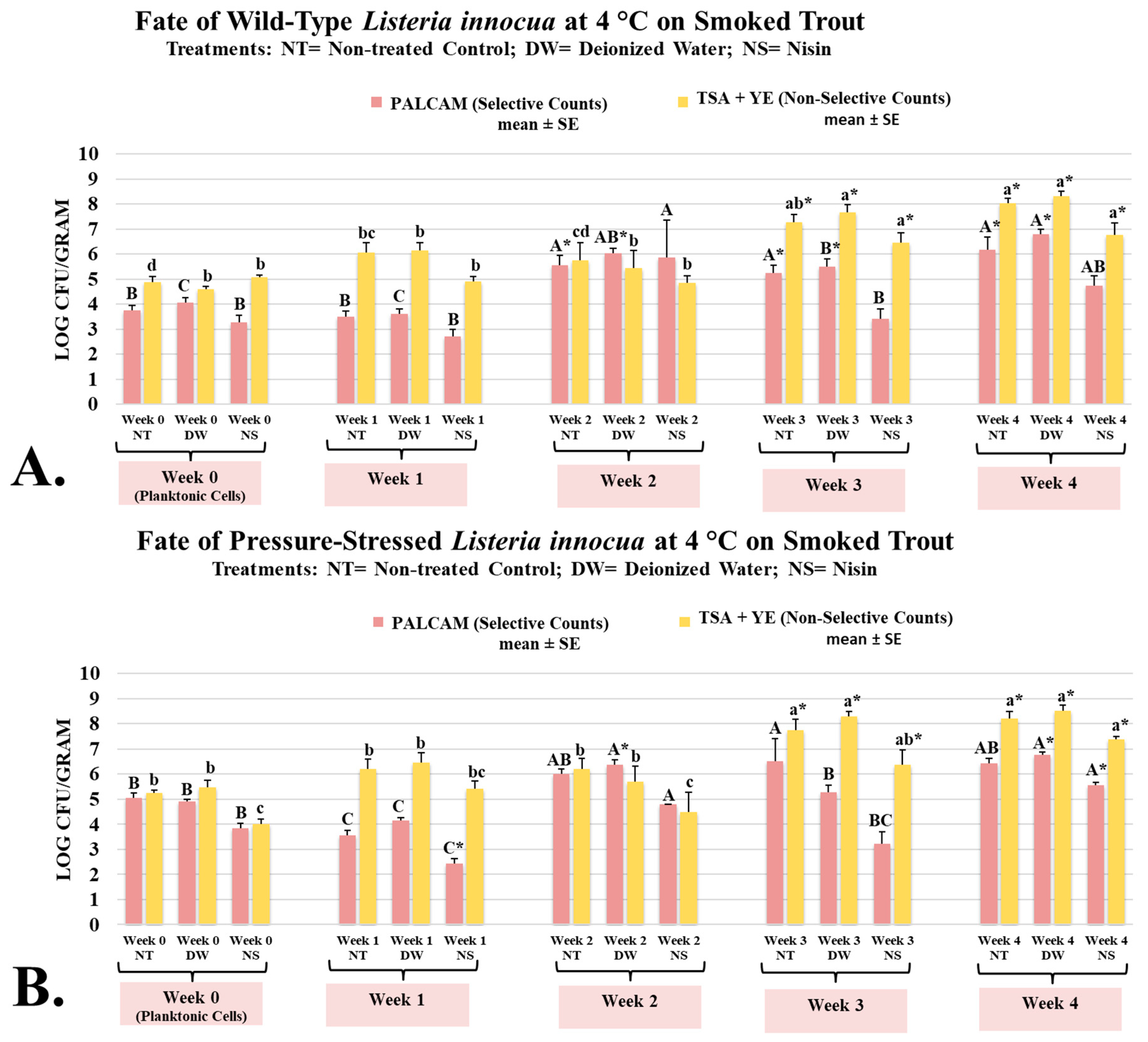
L. innocua is typically considered a microorganism that does not cause human infections, and due to genetic similarities and ecological co-habitation, this microorganism could be considered a surrogate and safe alternative for L. monocytogenes [42]. However, the use of L. innocua as a surrogate for L. monocytogenes requires conducting a validation study to ensure the two have comparable fate, proliferation, and sensitivity to treatments [43]. Thus, the current study investigated the impact of nisin on the fate and proliferation of wild-type and pressure-stressed L. innocua and L. monocytogenes. On week 0 (planktonic cells after inoculation), L. innocua wild-type counts of untreated and treated controls and nisin-containing samples were 3.75 ± 0.2, 4.05 ± 0.2, and 3.26 ± 0.3 log CFU/g, respectively (Figure 2A). The corresponding counts for L. innocua pressure-stressed phenotype were 5.05 ± 0.2, 4.91 ± 0.1, and 3.84 ± 0.2 log CFU/g, respectively (Figure 2B). Both phenotypes of L. innocua were able to multiply extensively during the aerobic refrigerated shelf-life trial. Counts of wild-type L. innocua on weeks 0 to 4 were 3.75 ± 0.2, 3.51 ± 0.2, 5.54 ± 0.4, 5.24 ± 0.3, and 6.18 ± 0.5 log CFU/g, respectively (Figure 2A). The corresponding counts of pressure-stressed L. innocua, were 5.05 ± 0.2, 3.57 ± 0.2, 6.01 ± 0.2, 6.52 ± 0.9, and 6.42 ± 0.2 log CFU/g, respectively (Figure 2B). Similar to information discussed in the previous section for L. monocytogenes, our results, thus, illustrate that pressure-stressed phenotype of L. innocua not only can tolerate cold storage after exposure to elevated hydrostatic pressure, but as well could multiply under refrigeration even in presence of background microbiota (mesophilic aerobic plate count) and under low temperatures.
Figure 2.
Bacteriostatic/bactericidal activity of nisin against wild-type and pressure-stressed Listeria innocua (PALCAM counts) and mesophilic background microbiota (TSA + YE counts) during shelf-life of smoked trout. (A) Counts of wild-type L. innocua. (B) Counts of pressure-stressed L. innocua. PALCAM counts marked by different uppercase letters are statistically different (p < 0.05) from each other (Tukey-adjusted ANOVA). TSA + YE counts marked by different lowercase letters are statistically different (p < 0.05) from each other (Tukey-adjusted ANOVA). For each media separately, columns marked by “*” are statistically different (p < 0.05) than the control (Dunnett’s-adjusted ANOVA).
Under the conditions of our experiment, we observed great similarity between fate, proliferation, and sensitivity to nisin comparing L. innocua and L. monocytogenes. This is in harmony with a previously conducted studies that supported the use of L. innocua as a surrogate for L. monocytogenes in some hurdle microbiological validation studies [44]. However, some studies, in contrast, indicate that L. innocua should not be automatically considered a surrogate for L. monocytogenes and such inference should be made only after the conduct of validation studies [43]. Thus, our results propose the use of L. innocua as a surrogate for L. monocytogenes only in conditions similar to the scope of our study.
As discussed earlier in the methods section, it is important to emphasize that the current study was designed to mimic contamination of this product after processing and when the packages are opened by consumers or food service operations where there is a chance of introduction of the pathogen to the product from the environment while samples are further stored aerobically at refrigeration temperatures. Similar to the results presented in the current study, a review of the literature illustrates that nisin has great potential for inhibiting the multiplication of Listeria spp. As an example, in a 60-day shelf-life study conducted on smoked salmon, nisin was efficacious in reducing L. monocytogenes counts at the beginning and late stages of the trial [45]. In another shelf-life trial, after 34 days, up to 1.7 log reduction of L. monocytogenes was observed in cold-smoked salmon due to the application of nisin [46], illustrating the bactericidal properties of this bacteriocin against this ubiquitous pathogen of public health concern. Others have also illustrated that while nisin is an effective bioactive compound against L. monocytogenes, the impact of this antimicrobial could vary based on the strain and lineage of the pathogen [47]. In one study conducted on cold-smoked salmon, it was concluded that serotype 1/2b of L. monocytogenes could be more susceptible to nisin relative to 1/2a and 4b serotypes [48]. A successful validation study, thus, should include a diverse selection of strains as a cocktail for inoculation, similar to the approach used in this study, rather than relying on a single-strain inoculum. This ensures external validity and generalizability of the results.
The application of nisin as an additional hurdle in the context of hurdle technology could benefit consumers and the food industry [49]. Nisin not only could provide protection for the product during the shelf-life but could also augment the decontamination efficacy of thermal and non-thermal processing due to their similarity in mechanisms of action. Similar to high-pressure processing, as an example, that primarily disrupts the function of bacterial cell membranes [50], nisin also targets the function of bacterial membranes [27], hence, could synergistically augment the efficacy of existing hurdle(s) in manufacturing of ready-to-eat commodities.
4. Conclusions
Under the conditions of our experiment, we observed L. monocytogenes could multiply extensively during the aerobic refrigerated shelf-life study. The addition of nisin was efficacious in reducing the pathogen by more than 90% early during the storage period. This impact of nisin, however, faded during the later weeks of the trial, highlighting the importance of preventive measures rather than relying solely on the application of one antimicrobial. Nisin, similarly, was able to inhibit the multiplication of mesophilic background microbiota of the product earlier in the shelf-life study. We additionally observed that pressure-stressed phenotypes of L. monocytogenes and L. innocua have similar fate, proliferation, and sensitivity to nisin relative to wild-type L. monocytogenes and L. innocua. These results, thus, indicate that the survivors of high-pressure processing should be carefully considered in hurdle validation studies and in the development of food safety and/or Hazard Analysis and Critical Control Points (HACCP) plans. Our study investigated L. innocua as a surrogate for L. monocytogenes since the former is typically considered an avirulent microorganism for humans. Under the conditions of our experiment, we observed that L. innocua has comparable fate and proliferation trends during shelf-life and sensitivity to nisin and thus could be used interchangeably with L. monocytogenes in future validation studies with similar scope.
Author Contributions
Conceptualization, A.C.F.; methodology, R.K. and A.C.F.; software, R.K. and A.C.F.; validation, A.C.F.; formal analysis, R.K. and A.C.F.; investigation, R.K. and A.C.F.; resources, A.C.F.; data curation, R.K. and A.C.F.; writing—original draft preparation, R.K. and A.C.F.; writing—review and editing, A.C.F.; visualization, R.K. and A.C.F.; supervision, A.C.F.; project administration, A.C.F.; funding acquisition, A.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded in part by the National Institute of Food and Agriculture of the United States Department of Agriculture (grant numbers 2023-70020-40768 and 2024-70020-42861), Pressure Bio Science Inc., South Easton, MA, and Public Health Microbiology FoundationSM, Nashville, TN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets of the current study can be obtained by contacting this study’s corresponding author with reasonable requests. A request could be submitted by obtaining the contact information from the Public Health Microbiology FoundationSM at https://publichealthmicrobiology.education/ (accessed on 11 February 2025). The SAS codes used for statistical analyses in the current study were derived from no-cost and publicly available sources with needed modifications and can be obtained by contacting the study’s corresponding author with reasonable requests.
Acknowledgments
Technical assistance and contributions from members of the public health microbiology program are gratefully acknowledged and appreciated by the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hof, H. History and Epidemiology of Listeriosis. FEMS Immunol. Med. Microbiol. 2003, 35, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.; Linke, K.; Wagner, M.; Stessl, B. The Saprophytic Lifestyle of Listeria monocytogenes and Entry into the Food-Processing Environment. Front. Microbiol. 2022, 13, 789801. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Get the Facts About Listeria. Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-about-listeria (accessed on 4 February 2025).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. J. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Cresence, V.M.; Rejitha, J.S.; Lekshmi, M.U.; Dharsana, K.S.; Prasad, S.P.; Vijila, H.M. Listeria—Review of Epidemiology and Pathogenesis. J. Microbiol. Immunol. Infect. 2007, 40, 4–13. [Google Scholar] [PubMed]
- Osek, J.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Uses Its Virulence Mechanisms to Infect the Hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef]
- Reinhard, R.G.; Kalinowski, R.M.; Bodnaruk, P.W.; Eifert, J.D.; Boyer, R.R.; Duncan, S.E.; Bailey, R.H. Incidence of Listeria spp. in Ready-to-Eat Food Processing Plant Environments Regulated by the US Food Safety and Inspection Service and the US Food and Drug Administration. J. Food Prot. 2018, 81, 1063–1067. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Public Health Notice: Outbreak of Listeria Infections Linked to Recalled Plant-Based Refrigerated Beverages; Public Health Agency of Canada: Ottawa, ON, Canada, 2024. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2024/outbreak-listeria-infections-recalled-refrigerated-plant-based-beverages.html (accessed on 11 January 2024).
- Centers for Disease Control and Prevention. Listeria Outbreaks; CDC: Atlanta, GA, USA, 2025. Available online: https://www.cdc.gov/listeria/outbreaks/ (accessed on 3 February 2025).
- Hamidiyan, N.; Salehi-Abargouei, A.; Rezaei, Z.; Dehghani-Tafti, R.; Akrami-Mohajeri, F. The Prevalence of Listeria spp. Food Contamination in Iran: A Systematic Review and Meta-Analysis. Food Res. Int. 2018, 107, 437–450. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria Species and L. monocytogenes in Ready-to-Eat Foods in the West Pomeranian Region of Poland: Correlations Between the Contamination Level, Serogroups, Ingredients, and Producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef]
- Castrica, M.; Andoni, E.; Intraina, I.; Curone, G.; Copelotti, E.; Massacci, F.R.; Terio, V.; Colombo, S.; Balzaretti, C.M. Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready-to-Eat Foods from Large Retailers and Canteens Over a 2-Year Period in Northern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10568. [Google Scholar] [CrossRef]
- Mpundu, P.; Mbewe, A.; Muma, J.; Mwasinga, W.; Mukumbuta, N.; Munyeme, M. A Global Perspective of Antibiotic-Resistant Listeria monocytogenes Prevalence in Assorted Ready-to-Eat Foods: A Systematic Review. Vet. World 2021, 14, 2219–2229. [Google Scholar] [CrossRef]
- Lambrechts, K.; Rip, D. Listeria monocytogenes in the Seafood Industry: Exploring Contamination Sources, Outbreaks, Antibiotic Susceptibility and Genetic Diversity. MicrobiologyOpen 2024, 13, e70003. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, H.; Eklöw, A.; Danielsson-Tham, M.L.; Loncarevic, S.; Mentzing, L.O.; Persson, I.; Unnerstad, H.; Tham, W. An Outbreak of Listeriosis Suspected to Have Been Caused by Rainbow Trout. J. Clin. Microbiol. 1997, 35, 2904–2907. [Google Scholar] [CrossRef] [PubMed]
- Tocmo, R.; Krizman, K.; Khoo, W.J.; Phua, L.K.; Kim, M.; Yuk, H. Listeria monocytogenes in Vacuum-Packed Smoked Fish Products: Occurrence, Routes of Contamination, and Potential Intervention Measures. Compr. Rev. Food Sci. Food Saf. 2014, 13, 172–189. [Google Scholar] [CrossRef]
- Halbedel, S.; Sperle, I.; Lachmann, R.; Kleta, S.; Fischer, M.A.; Wamp, S.; Holzer, A.; Lüth, S.; Murr, L.; Freitag, C.; et al. Large Multicountry Outbreak of Invasive Listeriosis by a Listeria monocytogenes ST394 Clone Linked to Smoked Rainbow Trout, 2020 to 2021. Microbiol. Spectr. 2023, 11, e03520-22. [Google Scholar] [CrossRef]
- Huang, L.; Hwang, C.A.; Sheen, S. Shelf-Life Boundaries of Listeria monocytogenes in Cold-Smoked Salmon During Refrigerated Storage and Temperature Abuse. Food Res. Int. 2023, 173, 113362. [Google Scholar] [CrossRef]
- Shineman, T.L.; Harrison, M.A. Growth of Listeria monocytogenes on Different Muscle Tissues. J. Food Prot. 1994, 57, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, E.T.; Jenkins, C.; Painset, A.; Amar, C. Listeria monocytogenes: The Silent Assassin. J. Med. Microbiol. 2024, 73, 001800. [Google Scholar] [CrossRef]
- Mohan, V.; Wibisono, R.; De Hoop, L.; Summers, G.; Fletcher, G.C. Identifying Suitable Listeria innocua Strains as Surrogates for Listeria monocytogenes for Horticultural Products. Front. Microbiol. 2019, 10, 2281. [Google Scholar] [CrossRef]
- Allerberger, F. Listeria: Growth, Phenotypic Differentiation and Molecular Microbiology. FEMS Immunol. Med. Microbiol. 2003, 35, 183–189. [Google Scholar] [CrossRef]
- Pardo, G.; Zufía, J. Life Cycle Assessment of Food-Preservation Technologies. J. Clean. Prod. 2012, 28, 198–207. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Foods 2021, 30, 19–35. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.; Grimaud, G.M.; Coakley, M.; O’Connor, P.M.; Mathur, H.; Peterson, V.L.; O’Donovan, C.M.; Lawlor, P.G.; Cotter, P.D.; Stanton, C.; et al. Modulation of the Gut Microbiome with Nisin. Sci. Rep. 2023, 13, 34586. [Google Scholar] [CrossRef] [PubMed]
- Santativongchai, P.; Tulayakul, P.; Jeon, B. Enhancement of the Antibiofilm Activity of Nisin against Listeria monocytogenes Using Food Plant Extracts. Pathogens 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; De Ullivarri, M.F.; Ross, R.P.; Hill, C. After a Century of Nisin Research—Where Are We Now? FEMS Microbiol. Rev. 2023, 47, fuad023. [Google Scholar] [CrossRef]
- Aras, S.; Kabir, M.N.; Chowdhury, S.; Fouladkhah, A.C. Augmenting the Pressure-Based Pasteurization of Listeria monocytogenes by Synergism with Nisin and Mild Heat. Int. J. Environ. Res. Public Health 2020, 17, 563. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; George, J.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. High-Pressure and Thermal-Assisted Pasteurization of Habituated, Wild-Type, and Pressure-Stressed Listeria monocytogenes, Listeria innocua, and Staphylococcus aureus. LWT. 2021, 137, 110445. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Bacteriological Analytical Manual (BAM); FDA: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam (accessed on 5 February 2025).
- Fouladkhah, A.; Geornaras, I.; Sofos, J.N. Effects of Reheating Against Listeria monocytogenes Inoculated on Cooked Chicken Breast Meat Stored Aerobically at 7 °C. Food Prot. Trends 2012, 32, 697–704. [Google Scholar]
- Kafle, R.; Fouladkhah, A.C. Effects of Thermally-Assisted and High-Pressure Processing on Background Microbiota and the Listeria monocytogenes Load of a Minimally Processed Commodity. Microorganisms 2024, 12, 1858. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Bulut, S.; Karatzas, K.A.G.; Boziaris, I.S. Inactivation of Listeria monocytogenes in Raw and Hot Smoked Trout Fillets by High Hydrostatic Pressure Processing Combined with Liquid Smoke and Freezing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102427. [Google Scholar] [CrossRef]
- Thimothe, J.; Nightingale, K.K.; Gall, K.; Scott, V.N.; Wiedmann, M. Tracking of Listeria monocytogenes in Smoked Fish Processing Plants. J. Food Prot. 2004, 67, 328–341. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Prevalence, Genetic Diversity and Antimicrobial Resistance of Listeria monocytogenes Isolated from Fresh and Smoked Fish in Poland. Food Microbiol. 2017, 64, 164–171. [Google Scholar] [CrossRef]
- Leistner, L. Basic Aspects of Food Preservation by Hurdle Technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle Technology: A Novel Approach for Enhanced Food Quality and Safety—A Review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Aras, S.; Kabir, N.; Wadood, S.; George, J.; Chowdhury, S.; Fouladkhah, A.C. Synergistic Effects of Nisin, Lysozyme, Lactic Acid, and Citricidal™ for Enhancing Pressure-Based Inactivation of Bacillus amyloliquefaciens, Geobacillus stearothermophilus, and Bacillus atrophaeus Endospores. Microorganisms 2021, 9, 653. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. Fate and Biofilm Formation of Wild-Type and Pressure-Stressed Pathogens of Public Health Concern in Surface Water and on Abiotic Surfaces. Microorganisms 2020, 8, 408. [Google Scholar] [CrossRef]
- da Silva, A.C.M.; de Oliveira Pena, P.; Júnior, S.B.P.; do Nascimento, M.D.S. Effect of Different Dry Aging Temperatures on Listeria innocua as Surrogate for Listeria monocytogenes. Meat Sci. 2019, 157, 107884. [Google Scholar] [CrossRef]
- Milillo, S.R.; Friedly, E.C.; Saldivar, J.C.; Muthaiyan, A.; O’bryan, C.; Crandall, P.G.; Johnson, M.G.; Ricke, S.C. A Review of the Ecology, Genomics, and Stress Response of Listeria innocua and Listeria monocytogenes. Crit. Rev. Food Sci. Nutr. 2012, 52, 712–725. [Google Scholar] [CrossRef]
- Silva-Angulo, A.B.; Zanini, S.F.; Rosenthal, A.; Rodrigo, D.; Klein, G.; Martínez, A. Comparative Study of the Effects of Citral on the Growth and Injury of Listeria innocua and Listeria monocytogenes Cells. PLoS ONE 2015, 10, e0114026. [Google Scholar] [CrossRef]
- Kang, J.; Stasiewicz, M.J.; Murray, D.; Boor, K.J.; Wiedmann, M.; Bergholz, T.M. Optimization of combinations of bactericidal and bacteriostatic treatments to control Listeria monocytogenes on cold-smoked salmon. Int. J. Food Microbiol. 2014, 179, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Jensen, M.R.; Aasli, A.W.; Berget, I.; Holck, A.L. Reduction and growth inhibition of Listeria monocytogenes by use of anti-listerial nisin, P100 phages and buffered dry vinegar fermentates in standard and sodium-reduced cold-smoked salmon. Foods 2023, 12, 4391. [Google Scholar] [CrossRef]
- Tang, S.; Stasiewicz, M.J.; Wiedmann, M.; Boor, K.J.; Bergholz, T.M. Efficacy of different antimicrobials on inhibition of Listeria monocytogenes growth in laboratory medium and on cold-smoked salmon. Int. J. Food Microbiol. 2013, 165, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Skeens, J.W.; Wiedmann, M.; Guariglia-Oropeza, V. The efficacy of nisin against Listeria monocytogenes on cold-smoked salmon at natural contamination levels is concentration-dependent and varies by serotype. Front. Microbiol. 2022, 13, 930400. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L.; Gorris, L.G. Food preservation by hurdle technology. Trends Food Sci Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Sibley, J.; Kafle, R.; Chowdhury, S.; Fouladkhah, A.C. Synergetic Effect of Elevated Hydrostatic Pressure, Mild Heat, and Carvacrol on Inactivation of Nontyphoidal Salmonella Serovars in Buffered Environment. Microorganisms 2025, 13, 498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).