Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Microplastic Material Preparation
2.2. Experimental Design
2.3. Sample Collection and Soil Physicochemical Property Determination Methods
2.4. High-Throughput Sequencing and Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
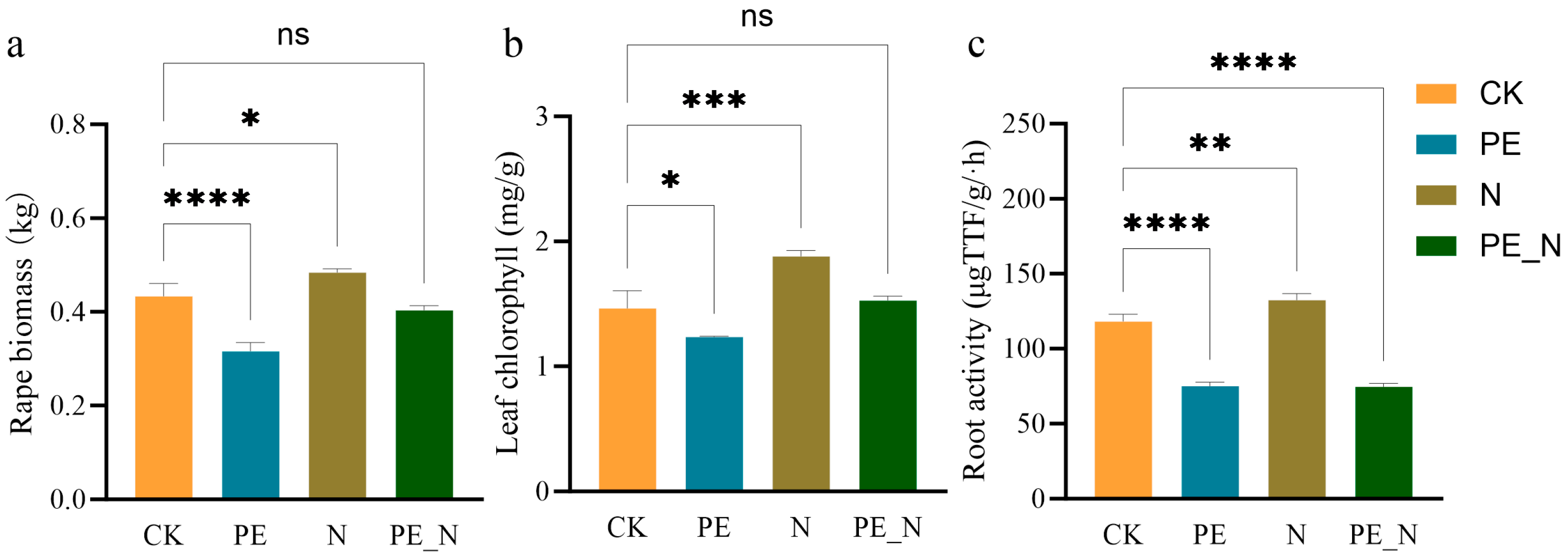
3.1. Rape Biomass and Physiological Characteristics
3.2. Changes in Soil Physicochemical Properties
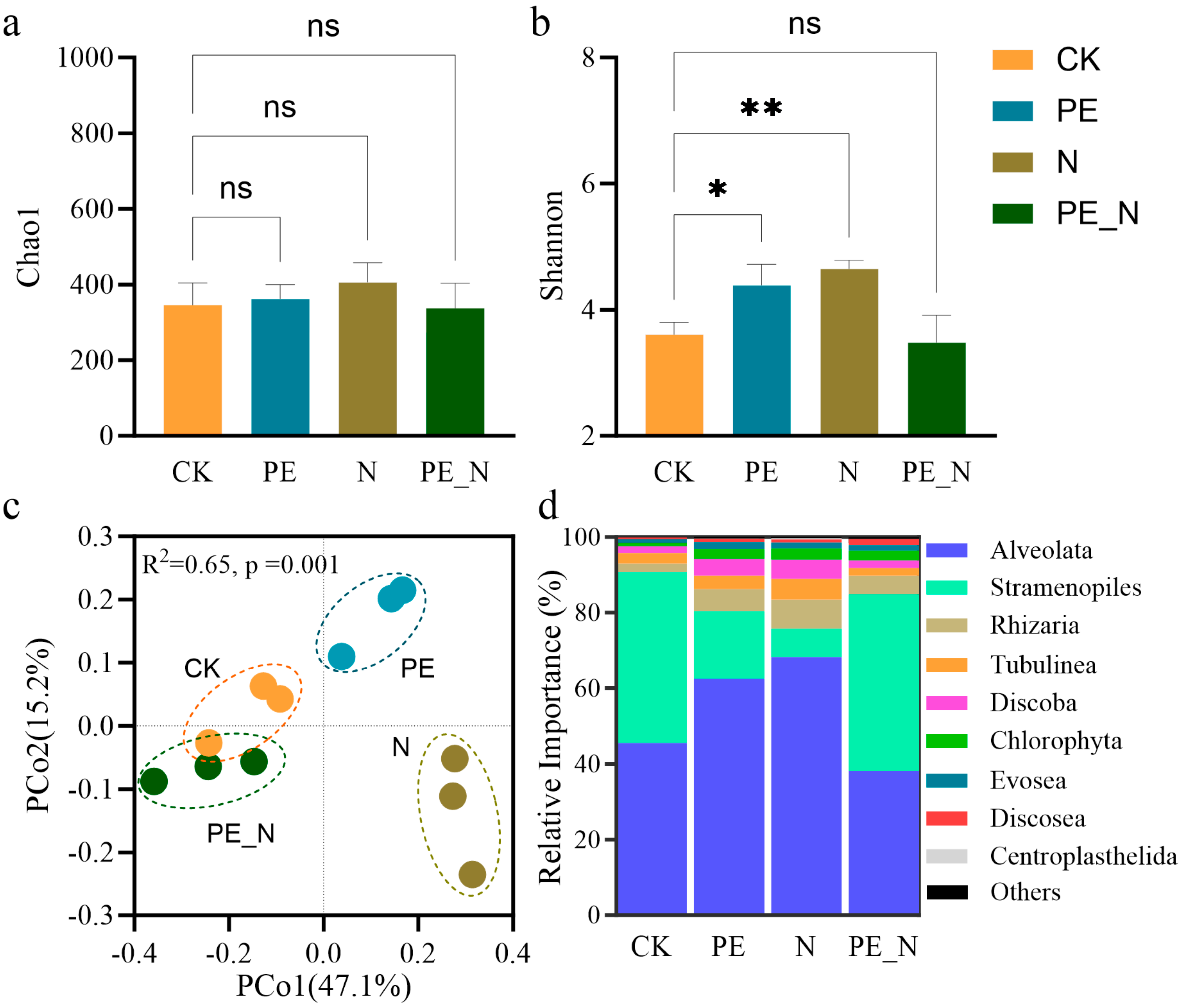
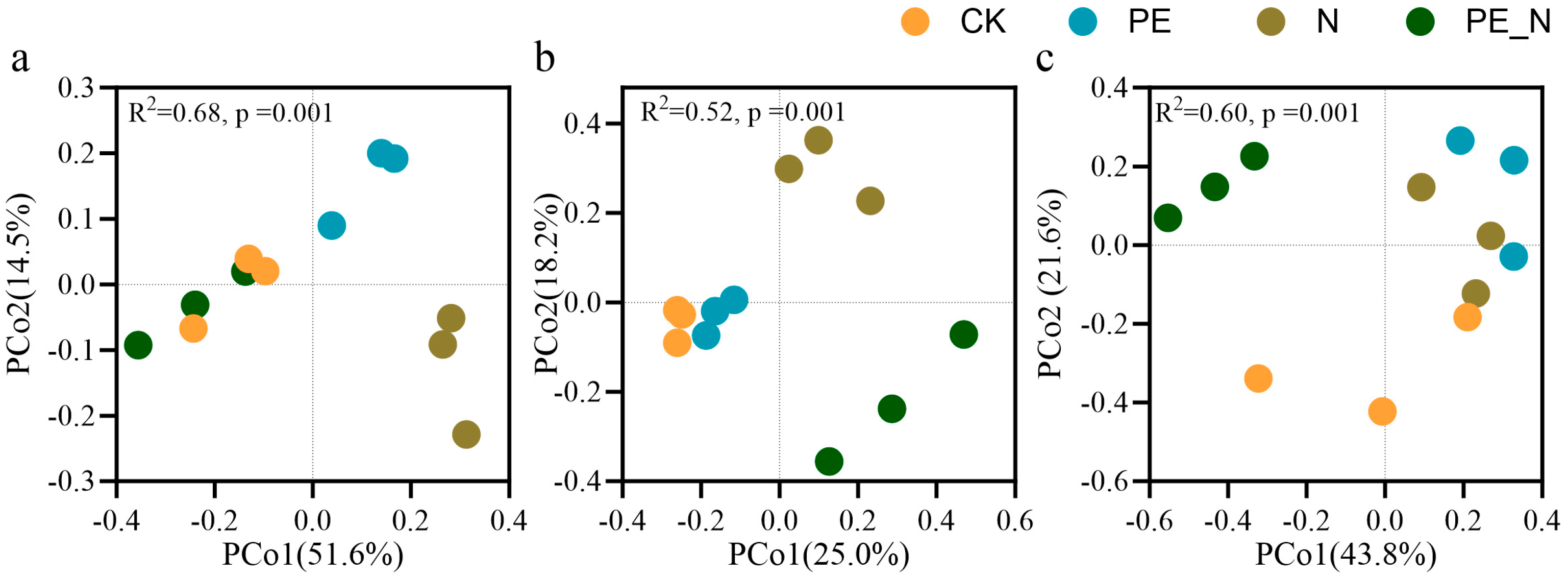
3.3. Changes in the Soil Protist Diversity and the Community Composition
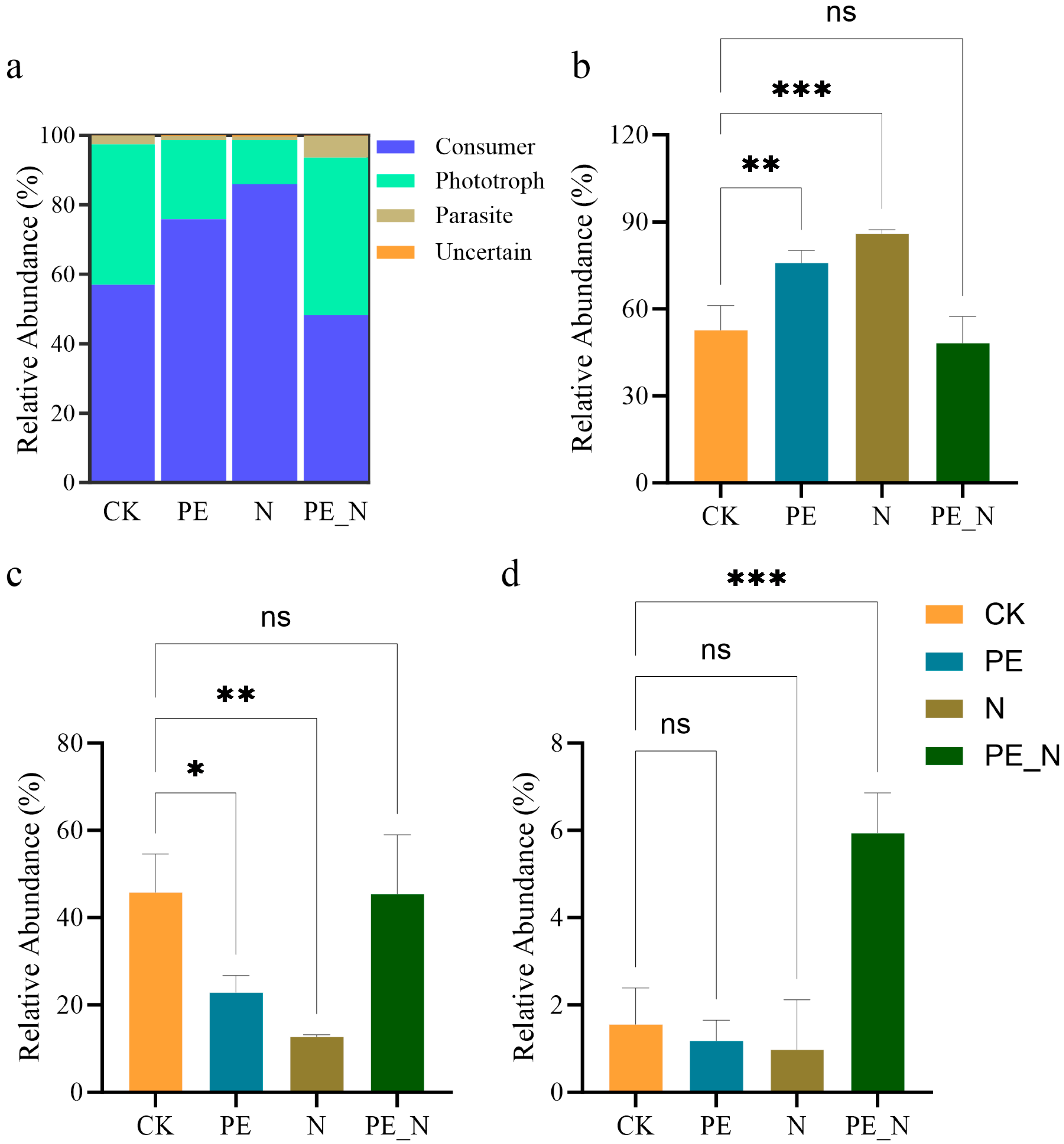
3.4. Changes in the Functional Group of the Soil Protist Community
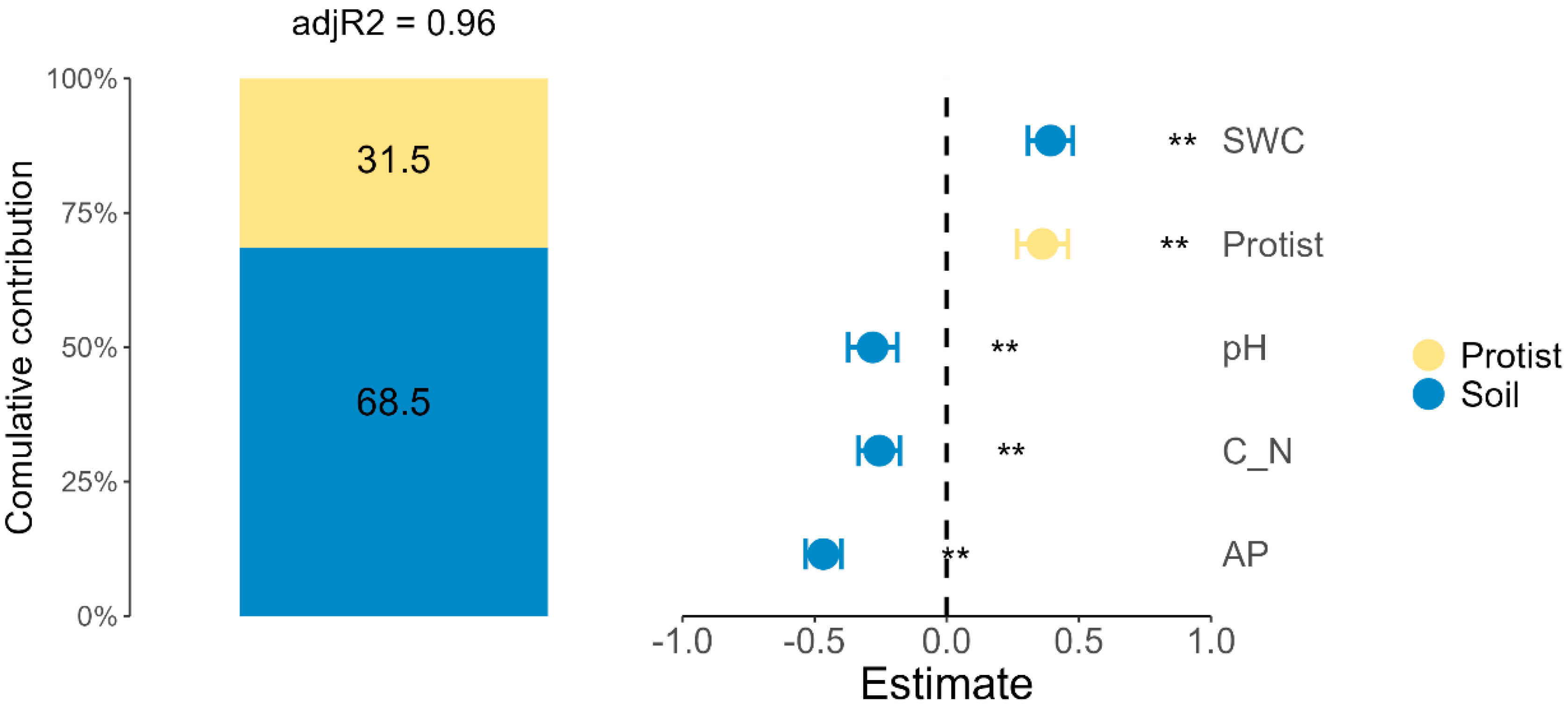
3.5. Contributions of Protist Community and Soil Characteristics in Rape Biomass
4. Discussion
4.1. Impacts of PE and Nitrogen Fertilization on Soil Nutrient Dynamics and Regulation Strategies
4.2. Impacts of PE and Nitrogen Fertilization on Soil Protist Diversity and Functional Groups
4.3. Main Drivers of Rape Growth Parameters and Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.; Yan, C.; Liu, Q.; Li, Z.; Yang, X.; Qi, R. Exploring Optimal Soil Mulching to Enhance Yield and Water Use Efficiency in Maize Cropping in China: A Meta-Analysis. Agric. Water Manag. 2019, 225, 105741. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, F.; Zhang, K.; Qin, R.; Zhang, W.; Sun, G.; Huang, J. Effects of Soil Mulching on Staple Crop Yield and Greenhouse Gas Emissions in China: A Meta-Analysis. Field Crops Res. 2022, 284, 108566. [Google Scholar] [CrossRef]
- Lv, S.; Li, J.; Yang, Z.; Yang, T.; Li, H.; Wang, X.; Peng, Y.; Zhou, C.; Wang, L.; Abdo, A.I. The Field Mulching Could Improve Sustainability of Spring Maize Production on the Loess Plateau. Agric. Water Manag. 2023, 279, 108156. [Google Scholar] [CrossRef]
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in Terrestrial Ecosystem: Sources and Migration in Soil Environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent Advances on Ecological Effects of Microplastics on Soil Environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef]
- Jannesarahmadi, S.; Aminzadeh, M.; Raga, R.; Shokri, N. Effects of Microplastics on Evaporation Dynamics in Porous Media. Chemosphere 2023, 311, 137023. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Ding, L.; Zhang, G.; Bai, B.; Han, Y.; Xiao, L.; Song, Y.; Li, Y.; Wan, S.; et al. Microplastics Reduce Nitrogen Uptake in Peanut Plants by Damaging Root Cells and Impairing Soil Nitrogen Cycling. J. Hazard. Mater. 2023, 443, 130384. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Arif, M.S.; Worthy, F.R.; Jones, D.L.; Khan, S.; Alharbi, S.A.; Filimonenko, E.; Nadir, S.; Bu, D.; et al. Do Added Microplastics, Native Soil Properties, and Prevailing Climatic Conditions Have Consequences for Carbon and Nitrogen Contents in Soil? A Global Data Synthesis of Pot and Greenhouse Studies. Environ. Sci. Technol. 2024, 58, 8464–8479. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wang, M.; Wang, K.; Meng, F.; Liu, L.; Zhao, Y.; Ma, L.; Zhu, Q.; Xu, W.; et al. Atmospheric Nitrogen Deposition: A Review of Quantification Methods and Its Spatial Pattern Derived from the Global Monitoring Networks. Ecotoxicol. Environ. Saf. 2021, 216, 112180. [Google Scholar] [CrossRef]
- Reay, M.K.; Marsden, K.A.; Powell, S.; Chadwick, D.R.; Jones, D.L.; Evershed, R.P. Combining Field and Laboratory Approaches to Quantify N Assimilation in a Soil Microbe-Plant-Animal Grazing Land System. Agric. Ecosyst. Environ. 2023, 346, 108338. [Google Scholar] [CrossRef]
- Séneca, J.; Söllinger, A.; Herbold, C.W.; Pjevac, P.; Prommer, J.; Verbruggen, E.; Sigurdsson, B.D.; Peñuelas, J.; Janssens, I.A.; Urich, T.; et al. Increased Microbial Expression of Organic Nitrogen Cycling Genes in Long-Term Warmed Grassland Soils. ISME Commun. 2021, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Lewicka-Szczebak, D.; Rohe, L. Microbial Nitrogen Transformations Tracked by Natural Abundance Isotope Studies and Microbiological Methods: A Review. Sci. Total Environ. 2024, 926, 172073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.-F.; Jin, M.-K.; Zhang, W.; Lambers, H.; Hui, D.; Liang, C.; Zhang, J.; Wu, D.; Sardans, J.; et al. Microbial Controls over Soil Priming Effects under Chronic Nitrogen and Phosphorus Additions in Subtropical Forests. ISME J. 2023, 17, 2160–2168. [Google Scholar] [CrossRef]
- Nair, G.R.; Kooverjee, B.B.; de Scally, S.; Cowan, D.A.; Makhalanyane, T.P. Changes in Nutrient Availability Substantially Alter Bacteria and Extracellular Enzymatic Activities in Antarctic Soils. FEMS Microbiol. Ecol. 2024, 100, fiae071. [Google Scholar] [CrossRef]
- Geisen, S.; Mitchell, E.A.D.; Adl, S.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernández, L.D.; Jousset, A.; Krashevska, V.; Singer, D.; et al. Soil Protists: A Fertile Frontier in Soil Biology Research. FEMS Microbiol. Rev. 2018, 42, 293–323. [Google Scholar] [CrossRef]
- Singer, D.; Seppey, C.V.W.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist Taxonomic and Functional Diversity in Soil, Freshwater and Marine Ecosystems. Environ. Int. 2021, 146, 106262. [Google Scholar] [CrossRef]
- Guo, S.; Tao, C.; Jousset, A.; Xiong, W.; Wang, Z.; Shen, Z.; Wang, B.; Xu, Z.; Gao, Z.; Liu, S.; et al. Trophic Interactions between Predatory Protists and Pathogen-Suppressive Bacteria Impact Plant Health. ISME J. 2022, 16, 1932–1943. [Google Scholar] [CrossRef]
- Gao, Z.; Karlsson, I.; Geisen, S.; Kowalchuk, G.; Jousset, A. Protists: Puppet Masters of the Rhizosphere Microbiome. Trends Plant Sci. 2019, 24, 165–176. [Google Scholar] [CrossRef]
- Murase, J.; Asiloglu, R. Protists: The Hidden Ecosystem Players in a Wetland Rice Field Soil. Biol. Fertil. Soils 2024, 60, 773–787. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Wang, X. Microplastic Presence Significantly Alters Soil Nitrogen Transformation and Decreases Nitrogen Bioavailability under Contrasting Temperatures. J. Environ. Manag. 2022, 317, 115473. [Google Scholar] [CrossRef]
- Han, L.; Chen, L.; Feng, Y.; Kuzyakov, Y.; Chen, Q.; Zhang, S.; Chao, L.; Cai, Y.; Ma, C.; Sun, K.; et al. Microplastics Alter Soil Structure and Microbial Community Composition. Environ. Int. 2024, 185, 108508. [Google Scholar] [CrossRef]
- Zi, H.; Hu, L.; Wang, C. Differentiate Responses of Soil Microbial Community and Enzyme Activities to Nitrogen and Phosphorus Addition Rates in an Alpine Meadow. Front. Plant Sci. 2022, 13, 829381. [Google Scholar] [CrossRef]
- Geisen, S.; Hu, S.; dela Cruz, T.E.E.; Veen, G.F. Protists as Catalyzers of Microbial Litter Breakdown and Carbon Cycling at Different Temperature Regimes. ISME J.—X-MOL 2021, 15, 618–621. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Dumack, K.; Trivedi, P.; Islam, Z.; Hu, H. Plant Associated Protists—Untapped Promising Candidates for Agrifood Tools. Environ. Microbiol. 2023, 25, 229–240. [Google Scholar] [CrossRef]
- Nguyen, T.B.A.; Chen, Q.; Yan, Z.; Li, C.; He, J.; Hu, H. Trophic Interrelationships of Bacteria Are Important for Shaping Soil Protist Communities. Environ. Microbiol. Rep. 2023, 15, 298–307. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal Symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef]
- Bukovská, P.; Bonkowski, M.; Konvalinková, T.; Beskid, O.; Hujslová, M.; Püschel, D.; Řezáčová, V.; Gutiérrez-Núñez, M.S.; Gryndler, M.; Jansa, J. Utilization of Organic Nitrogen by Arbuscular Mycorrhizal Fungi—Is There a Specific Role for Protists and Ammonia Oxidizers? Mycorrhiza 2018, 28, 269–283. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of Plastic Residues and Microplastics on Soil Ecosystems: A Global Meta-Analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in Terrestrial Ecosystem: Exploring the Menace to the Soil-Plant-Microbe Interactions. TrAC Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Ullah, F.; Wang, P.-Y.; Saqib, S.; Zhao, L.; Ashraf, M.; Khan, A.; Khan, W.; Khan, A.; Chen, Y.; Xiong, Y.-C. Microplastics Unveiled: Understanding Their Toxicological Impact on Terrestrial Ecosystems. iScience 2025, 28, 111879. [Google Scholar] [CrossRef]
- Rillig, M.C.; Bonkowski, M. Microplastic and Soil Protists: A Call for Research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, L.; Zhang, S.; Zhou, M.; Huang, W.; Zou, X.; He, Z.; Shu, L. Soil Protists Are More Resilient to the Combined Effect of Microplastics and Heavy Metals than Bacterial Communities. Sci. Total Environ. 2024, 906, 167645. [Google Scholar] [CrossRef]
- Ren, P.; Sun, A.; Jiao, X.; Shen, J.-P.; Yu, D.-T.; Li, F.; Wu, B.; He, J.-Z.; Hu, H.-W. Predatory Protists Play Predominant Roles in Suppressing Soil-Borne Fungal Pathogens under Organic Fertilization Regimes. Sci. Total Environ. 2023, 863, 160986. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, L.; Liu, S.; Liu, F.; Saleem, M.; Han, X.; Shu, L.; Yu, X.; Hu, R.; He, Z.; et al. Biogeography of Soil Protistan Consumer and Parasite Is Contrasting and Linked to Microbial Nutrient Mineralization in Forest Soils at a Wide-Scale. Soil Biol. Biochem. 2022, 165, 108513. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Huerta Lwanga, E.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of Plastic Mulch Film Residues on Wheat Rhizosphere and Soil Properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Qiu, X.; Sun, T.; Cao, J.; Lv, M.; Sui, Z.; Wang, Z.; Jiao, S.; Xu, Y.; et al. Discrepant Soil Microbial Community and C Cycling Function Responses to Conventional and Biodegradable Microplastics. J. Hazard. Mater. 2024, 470, 134176. [Google Scholar] [CrossRef]
- Gu, C.; Huang, W.; Li, Y.; Li, Y.; Yu, C.; Dai, J.; Hu, W.; Li, X.; Brooks, M.; Xie, L.; et al. Green Manure Amendment Can Reduce Nitrogen Fertilizer Application Rates for Oilseed Rape in Maize-Oilseed Rape Rotation. Plants 2021, 10, 2640. [Google Scholar] [CrossRef]
- Yousaf, M.; Li, X.; Zhang, Z.; Ren, T.; Cong, R.; Ata-Ul-Karim, S.T.; Fahad, S.; Shah, A.N.; Lu, J. Nitrogen Fertilizer Management for Enhancing Crop Productivity and Nitrogen Use Efficiency in a Rice-Oilseed Rape Rotation System in China. Front. Plant Sci. 2016, 7, 1496. [Google Scholar] [CrossRef]
- Li, H.; Ghafoor, A.; Karim, H.; Guo, S.; Li, Z.; Wu, Y.; Sun, Y.; Yan, F. Optimal Nitrogen Fertilization Management of Seed-Sowing Rapeseed in Yangtze River Basin of China. Pak. J. Biol. Sci. 2019, 22, 291–298. [Google Scholar] [CrossRef]
- Yang, X.X.; Zhang, L.; Huang, X.Q.; Wu, W.X.; Zhou, X.Q.; Du, L.; Li, H.Z.; Liu, Y. Difference of the microbial community structure in the rhizosphere of soybean and oilseed rape based on high-throughput pyrosequencing analysis. Ying Yong Sheng Tai Xue Bao 2019, 30, 2345–2351. [Google Scholar] [CrossRef]
- Liu, D.; Nishida, M.; Takahashi, T.; Asakawa, S. Transcription of mcrA Gene Decreases Upon Prolonged Non-Flooding Period in a Methanogenic Archaeal Community of a Paddy-Upland Rotational Field Soil. Microb. Ecol. 2018, 75, 751–760. [Google Scholar] [CrossRef]
- Bass, D.; Silberman, J.D.; Brown, M.W.; Pearce, R.A.; Tice, A.K.; Jousset, A.; Geisen, S.; Hartikainen, H. Coprophilic Amoebae and Flagellates, Including Guttulinopsis, Rosculus and Helkesimastix, Characterise a Divergent and Diverse Rhizarian Radiation and Contribute to a Large Diversity of Faecal-Associated Protists. Environ. Microbiol. 2016, 18, 1604–1619. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bj, C.; Pj, M.; Mj, R.; Aw, H.; Aj, J.; Sp, H. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Mazel, F.; Malard, L.; Niculita-Hirzel, H.; Yashiro, E.; Mod, H.K.; Mitchell, E.A.D.; Singer, D.; Buri, A.; Pinto, E.; Guex, N.; et al. Soil Protist Function Varies with Elevation in the Swiss Alps. Environ. Microbiol. 2022, 24, 1689–1702. [Google Scholar] [CrossRef]
- Fang, K.; Kou, Y.-P.; Tang, N.; Liu, J.; Zhang, X.-Y.; He, H.-L.; Xia, R.-X.; Zhao, W.-Q.; Li, D.-D.; Liu, Q. Differential Responses of Soil Bacteria, Fungi and Protists to Root Exudates and Temperature. Microbiol. Res. 2024, 286, 127829. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Kim, S.W.; Jeong, S.-W.; An, Y.-J. Microplastics Disrupt Accurate Soil Organic Carbon Measurement Based on Chemical Oxidation Method. Chemosphere 2021, 276, 130178. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, H.; Xiang, Y.; Wu, J. Effects of Microplastics Pollution on Plant and Soil Phosphorus: A Meta-Analysis. J. Hazard. Mater. 2024, 461, 132705. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic Effects on Plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of Microplastics and Nitrogen Deposition on Soil Multifunctionality, Particularly C and N Cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of Plastic Contamination on Water Evaporation and Desiccation Cracking in Soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Bodur, S.O.; Samuel, S.O.; Suzuki, K.; Harada, N.; Asiloglu, R. Nitrogen-Based Fertilizers Differentially Affect Protist Community Composition in Paddy Field Soils. Soil. Ecol. Lett. 2024, 6, 230221. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.; Jones, D.L.; Wang, J. Macro- and Microplastic Accumulation in Soil after 32 Years of Plastic Film Mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of Polystyrene on Di-Butyl Phthalate (DBP) Bioavailability and DBP-Induced Phytotoxicity in Lettuce. Environ. Pollut. 2021, 268, 115870. [Google Scholar] [CrossRef]
- Cao, X.; Liang, Y.; Jiang, J.; Mo, A.; He, D. Organic Additives in Agricultural Plastics and Their Impacts on Soil Ecosystems: Compared with Conventional and Biodegradable Plastics. TrAC Trends Anal. Chem. 2023, 166, 117212. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Shen, Y.; Lin, L.-F.; Tang, S.-L.; Liu, C.-X.; Fang, X.-H.; Guo, Z.-P.; Wang, Y.-Y.; Zhu, Y.-C. Effects of Bamboo Biochar on Soil Physicochemical Properties and Microbial Diversity in Tea Gardens. PeerJ 2024, 12, e18642. [Google Scholar] [CrossRef]
- Yang, X.; Hou, R.; Fu, Q.; Li, T.; Wang, J.; Su, Z.; Shen, W.; Zhou, W.; Wang, Y. Effect of Freeze-Thaw Cycles and Biochar Coupling on the Soil Water-Soil Environment, Nitrogen Adsorption and N2O Emissions in Seasonally Frozen Regions. Sci. Total Environ. 2023, 893, 164845. [Google Scholar] [CrossRef]
- Guo, S.; Xiong, W.; Hang, X.; Gao, Z.; Jiao, Z.; Liu, H.; Mo, Y.; Zhang, N.; Kowalchuk, G.A.; Li, R.; et al. Protists as Main Indicators and Determinants of Plant Performance. Microbiome 2021, 9, 64. [Google Scholar] [CrossRef]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic Effects on Carbon Cycling Processes in Soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef]
- Zhou, F.; Cui, J.; Zhou, J.; Yang, J.; Li, Y.; Leng, Q.; Wang, Y.; He, D.; Song, L.; Gao, M.; et al. Increasing Atmospheric Deposition Nitrogen and Ammonium Reduced Microbial Activity and Changed the Bacterial Community Composition of Red Paddy Soil. Sci. Total Environ. 2018, 633, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Guo, S.; Karlsson, I.; Jiao, Z.; Xun, W.; Kowalchuk, G.A.; Shen, Q.; Geisen, S. Microbial Amendments Alter Protist Communities within the Soil Microbiome. Soil Biol. Biochem. 2019, 135, 379–382. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, D.; Guo, W.; Wu, J.; Zhang, X.; Zhuang, X.; Kong, W. Precipitation Drives Soil Protist Diversity and Community Structure in Dry Grasslands. Microb. Ecol. 2023, 86, 2293–2304. [Google Scholar] [CrossRef]
- Hawxhurst, C.J.; Micciulla, J.L.; Bridges, C.M.; Shor, M.; Gage, D.J.; Shor, L.M. Soil Protists Can Actively Redistribute Beneficial Bacteria along Medicago Truncatula Roots. Appl. Environ. Microbiol. 2023, 89, e0181922. [Google Scholar] [CrossRef]
- Liao, H.; Hao, X.; Qin, F.; Delgado-Baquerizo, M.; Liu, Y.; Zhou, J.; Cai, P.; Chen, W.; Huang, Q. Microbial Autotrophy Explains Large-Scale Soil CO2 Fixation. Glob. Change Biol. 2023, 29, 231–242. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Song, X.; Liu, X.; Gao, H.; Liang, G.; Zhang, M.; Zheng, F.; Yang, P. Long-Term Nitrogen Fertilization Enhances Crop Yield Potential in No-Tillage Systems through Enhancing Soil Fertility. Resour. Conserv. Recycl. 2024, 206, 107622. [Google Scholar] [CrossRef]
- Fathi, A. Role of Nitrogen (N) in Plant Growth, Photosynthesis Pigments, and N Use Efficiency: A Review. Agrisost 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Han, X.; Xiao, X.; Zhang, J.; Shao, M.; Jie, Y.; Xing, H. Effects of Nitrogen Fertilizer and Planting Density on Growth, Nutrient Characteristics, and Chlorophyll Fluorescence in Silage Maize. Agronomy 2024, 14, 1352. [Google Scholar] [CrossRef]
- Gao, F.; Khan, R.; Yang, L.; Chi, Y.X.; Wang, Y.; Zhou, X.B. Uncovering the Potentials of Long-Term Straw Return and Nitrogen Supply on Subtropical Maize (Zea mays L.) Photosynthesis and Grain Yield. Field Crop. Res. 2023, 302, 109062. [Google Scholar] [CrossRef]
- Yang, H.; Dong, H.; Huang, Y.; Chen, G.; Wang, J. Interactions of Microplastics and Main Pollutants and Environmental Behavior in Soils. Sci. Total Environ. 2022, 821, 153511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-B.; He, J.-Z.; Geisen, S.; Han, L.-L.; Wang, J.-T.; Shen, J.-P.; Wei, W.-X.; Fang, Y.-T.; Li, P.-P.; Zhang, L.-M. Protist Communities Are More Sensitive to Nitrogen Fertilization than Other Microorganisms in Diverse Agricultural Soils. Microbiome 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Kang, S.; Wang, S.; Gao, T.; Wang, Z.; Luo, X.; Kang, Q.; Sajjad, W. Characteristics of Microplastics and Their Abundance Impacts on Microbial Structure and Function in Agricultural Soils of Remote Areas in West China. Environ. Pollut. 2024, 360, 124630. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Peng, Y.; Zhang, Z.; Fan, Z.; Wang, J.; Wang, X. Microbes Drive Metabolism, Community Diversity, and Interactions in Response to Microplastic-Induced Nutrient Imbalance. Sci. Total Environ. 2023, 877, 162885. [Google Scholar] [CrossRef]
- Wright, A.C.M.; Boots, B.; Ings, T.C.; Green, D.S. Impacts of Pristine, Aged and Leachate of Conventional and Biodegradable Plastics on Plant Growth and Soil Organic Carbon. Environ. Sci. Pollut. Res. Int. 2024, 31, 11766–11780. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Signarbieux, C.; Hättenschwiler, S.; Bragazza, L.; Buttler, A.; Delarue, F.; Fournier, B.; Gilbert, D.; Laggoun-Défarge, F.; Lara, E.; et al. An Unexpected Role for Mixotrophs in the Response of Peatland Carbon Cycling to Climate Warming. Sci. Rep. 2015, 5, 16931. [Google Scholar] [CrossRef]
| Treatment | SWC | pH | TC (g/kg) | TN (g/kg) | C/N | AP (mg/kg) | NH4+-N (mg/kg) | NO3+-N (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| CK | 0.23 ± 0.02 a | 6.64 ± 0.11 ab | 48.13 ± 2.7 c | 5.71 ± 0.19 a | 8.40 ± 0.83 c | 28.51 ± 3.47 a | 10.32 ± 0.45 b | 10.64 ± 0.52 c |
| PE | 0.20 ± 0.02 ab | 6.74 ± 0.20 a | 60.80 ± 3.4 ab | 5.65 ± 0.08 a | 10.53 ± 0.51 ab | 23.57 ± 0.12 b | 10.71 ± 0.44 ab | 10.56 ± 0.48 c |
| N | 0.24 ± 0.01 a | 6.30 ± 0.08 b | 52.41 ± 4.7 c | 5.77 ± 0.11 a | 9.14 ± 1.51 c | 24.65 ± 0.46 b | 10.82 ± 0.24 ab | 13.17 ± 0.64 b |
| PE_N | 0.16 ± 0.01 b | 6.45 ± 0.08 ab | 63.39 ± 4.4 a | 5.68 ± 0.05 a | 11.03 ± 2.09 a | 21.57 ± 0.18 c | 11.98 ± 0.42 a | 15.04 ± 0.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wei, M.; Sun, Q.; Shen, T.; Xie, M.; Liu, D. Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth. Microorganisms 2025, 13, 657. https://doi.org/10.3390/microorganisms13030657
Wang G, Wei M, Sun Q, Shen T, Xie M, Liu D. Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth. Microorganisms. 2025; 13(3):657. https://doi.org/10.3390/microorganisms13030657
Chicago/Turabian StyleWang, Ge, Maolu Wei, Qian Sun, Ting Shen, Miaomiao Xie, and Dongyan Liu. 2025. "Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth" Microorganisms 13, no. 3: 657. https://doi.org/10.3390/microorganisms13030657
APA StyleWang, G., Wei, M., Sun, Q., Shen, T., Xie, M., & Liu, D. (2025). Nitrogen Fertilization Alleviates Microplastic Effects on Soil Protist Communities and Rape (Brassica napus L.) Growth. Microorganisms, 13(3), 657. https://doi.org/10.3390/microorganisms13030657





