Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
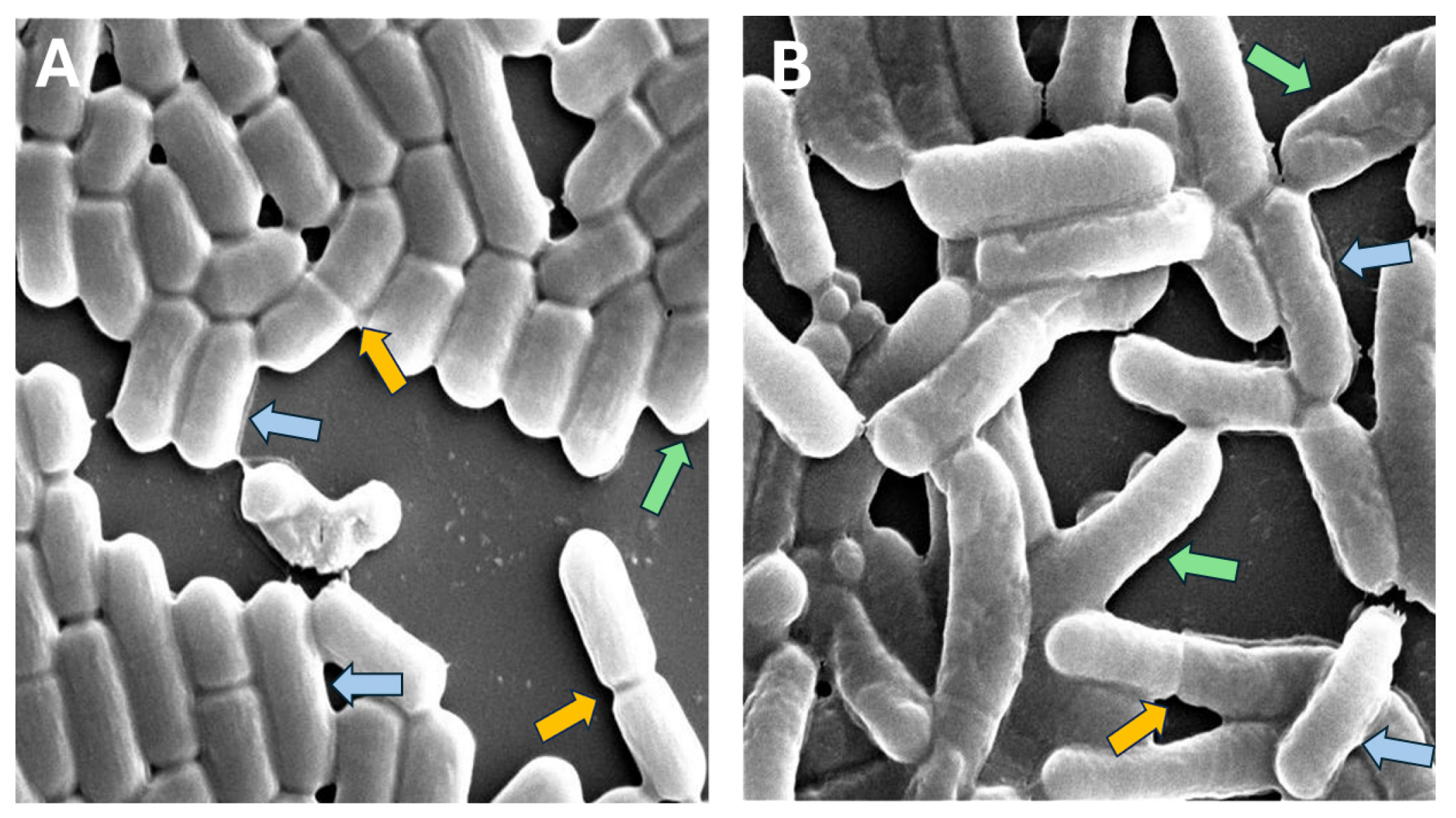
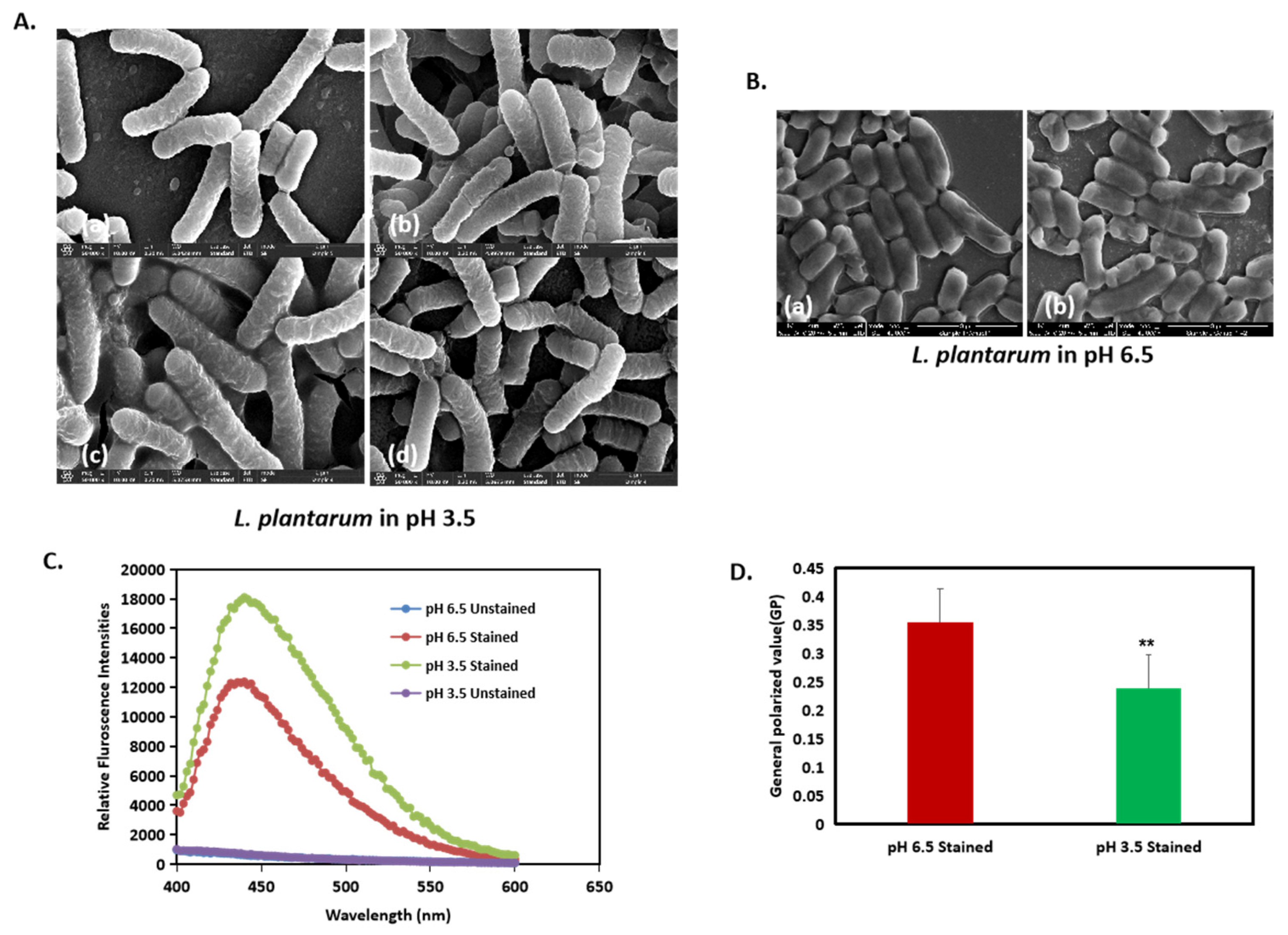
2.2. High-Resolution Scanning Electron Microscopy (HR-SEM)
2.3. Cell Dimensions Analysis from SEM Images
- Cells were shortened because of overlapping cells or partial cells near the borders of the image.
- Cells showed binary fission, e.g., they were actually two cells, not a single cell.
2.4. Image Classification: Acidic vs. Control
2.5. Measurement of Bacterial Dimensions
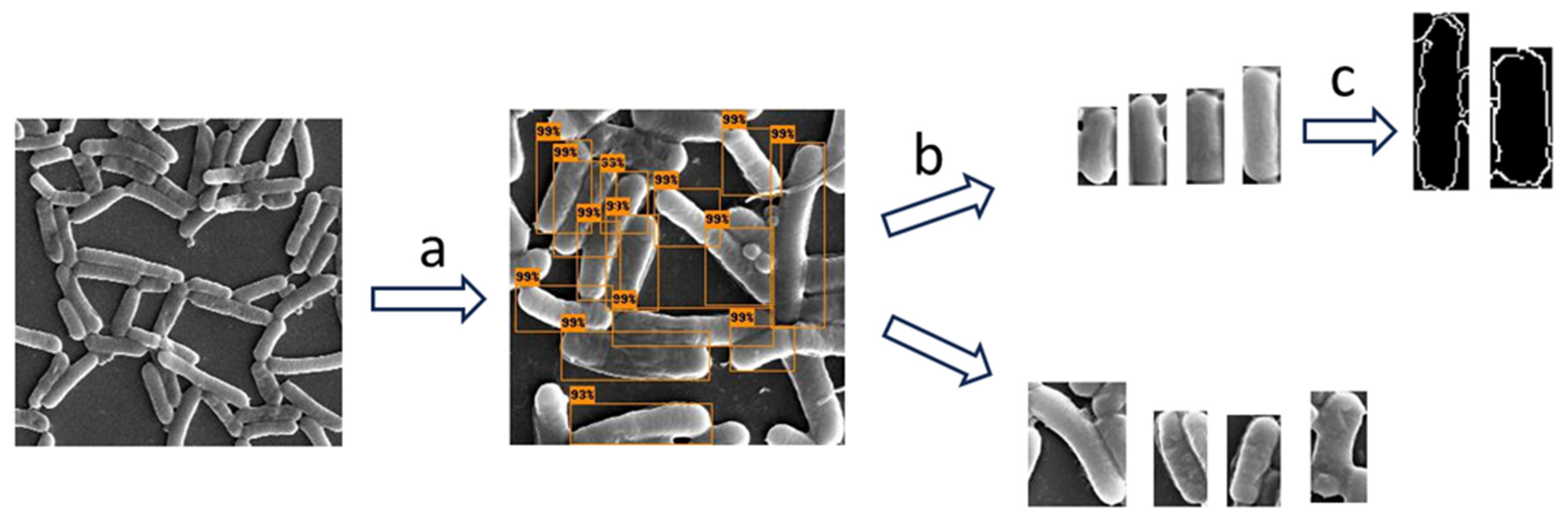
2.5.1. Step A: Object Detection
2.5.2. Step B—Image Classification
2.6. Growth Kinetic Studies
2.7. Laurdan Membrane Fluidity Assay
3. Results
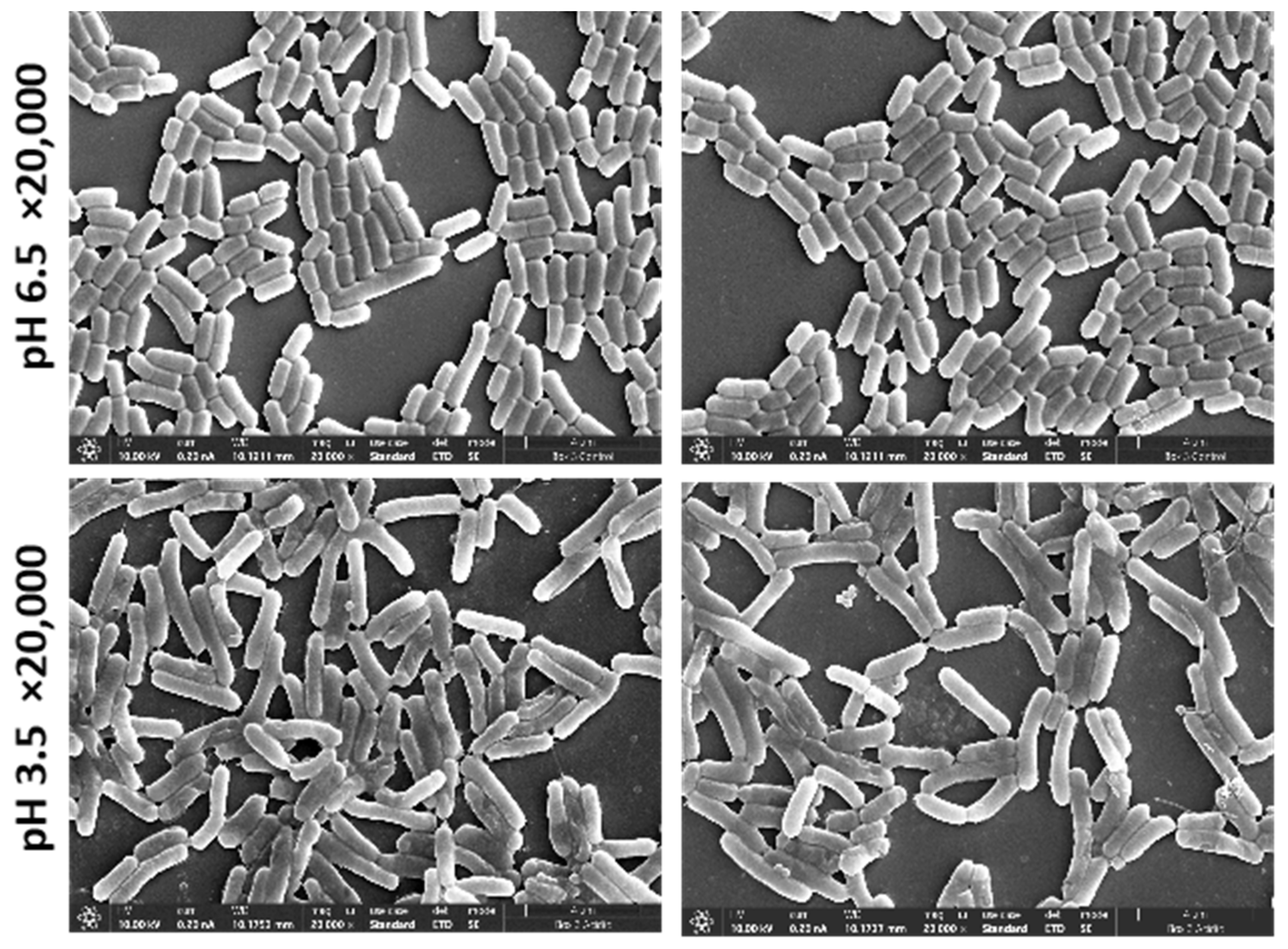
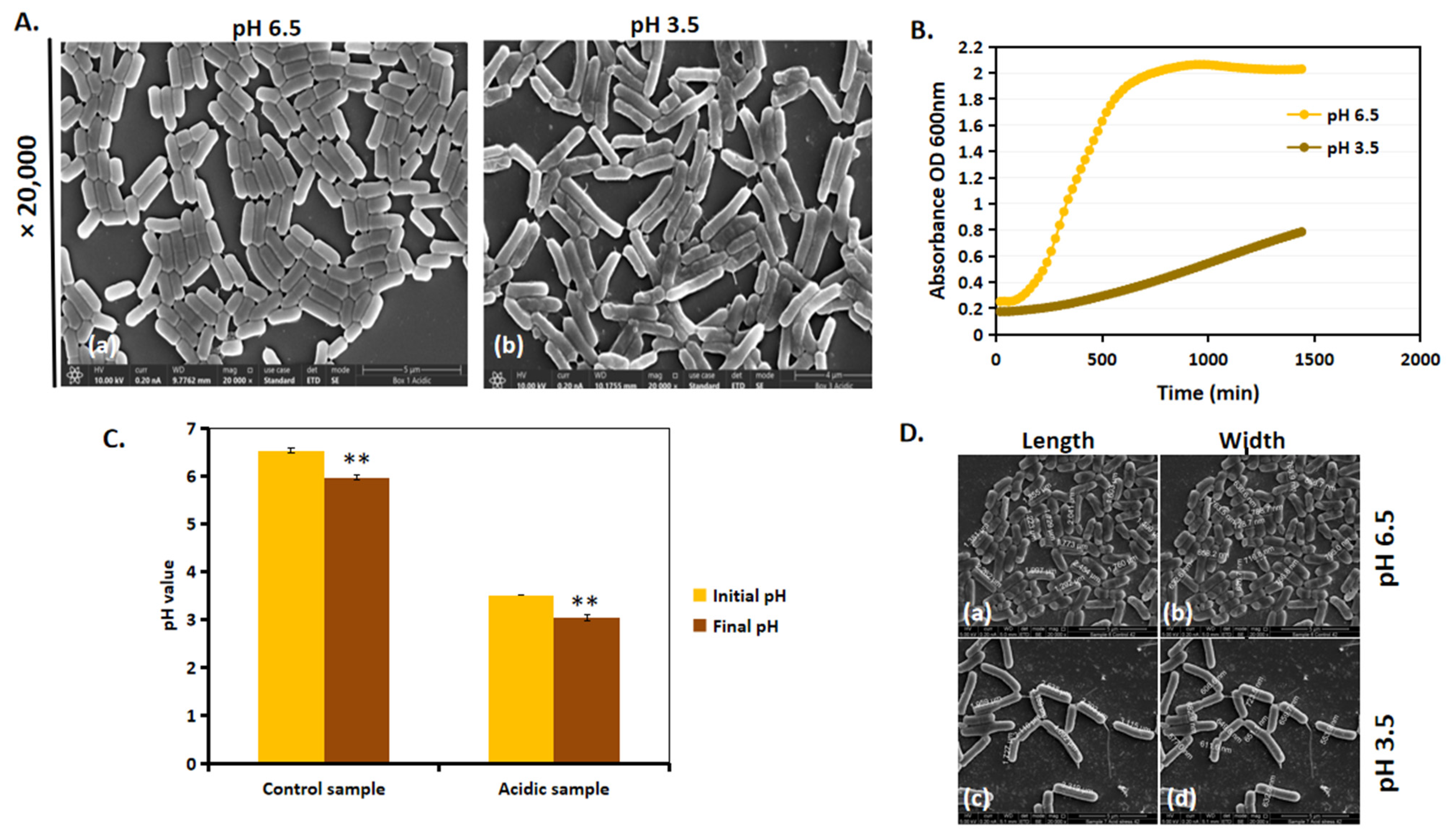
3.1. Image Classification Method Shows That L. plantarum Shows Significant Differences in Morphology When Cultured in Acid Stress
3.2. Acidic Stress Leads to Slower Growth and Increase in Length of L. plantarum
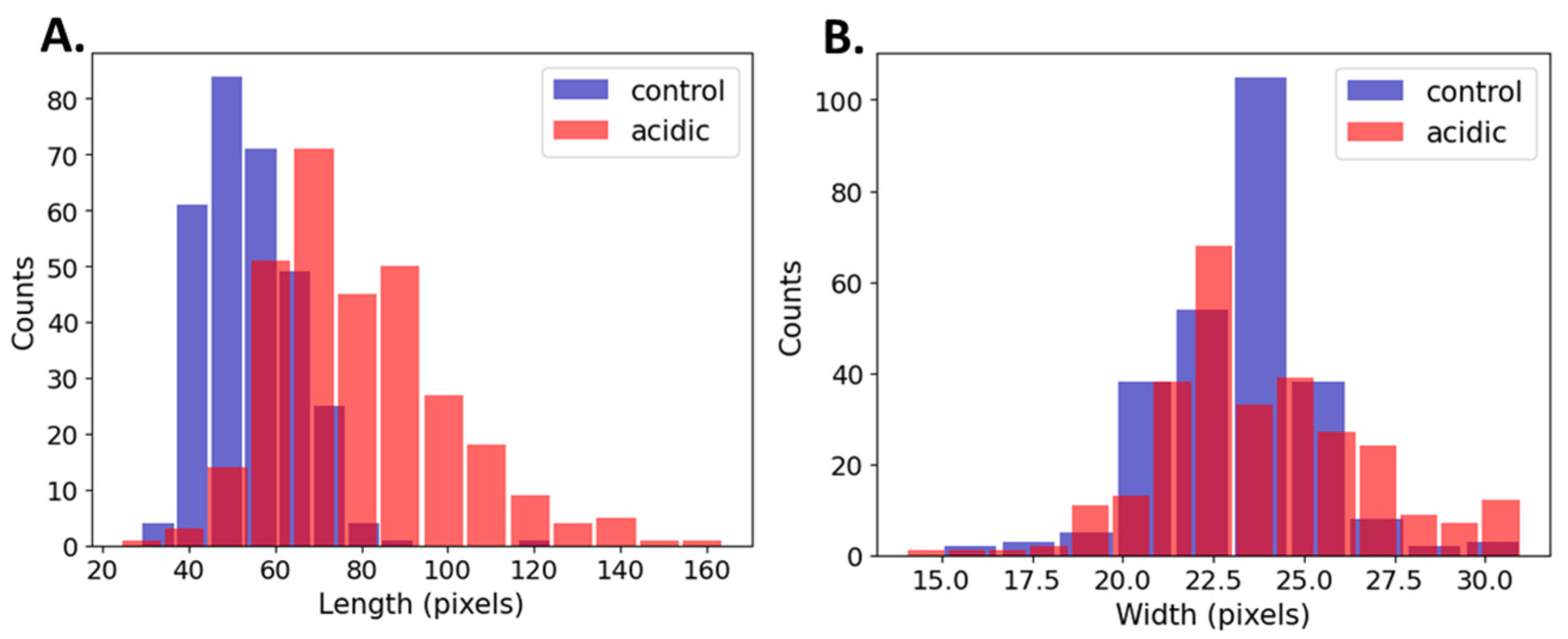
3.3. Comparison of Bacterial Dimensions in Control vs. Acidic Conditions
3.4. Membrane Fluidity in L. plantarum Is Enhanced by Acidic Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Justice, S.S.; Harrison, A.; Becknell, B.; Mason, K.M. Bacterial differentiation, development, and disease: Mechanisms for survival. FEMS Microbiol. Lett. 2014, 360, 1–8. [Google Scholar] [CrossRef]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, T.H.; Crotty, R.D.; Hubbard, G.J. Seasonal variation in cell volume of epilimnetic bacteria. Microb. Ecol. 1988, 16, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Psenner, R. Determination of size and morphology of aquatic bacteria by automated image analysis. In Handbook of Methods in Aquatic Microbial Ecology; CRC Press: Boca Raton, FL, USA, 2018; pp. 339–345. [Google Scholar]
- van Teeseling, M.C.F.; de Pedro, M.A.; Cava, F. Determinants of Bacterial Morphology: From Fundamentals to Possibilities for Antimicrobial Targeting. Front. Microbiol. 2017, 8, 1264. [Google Scholar] [CrossRef]
- Martínez, L.E.; Hardcastle, J.M.; Wang, J.; Pincus, Z.; Tsang, J.; Hoover, T.R.; Bansil, R.; Salama, N.R. Helicobacter pylori strains vary cell shape and flagellum number to maintain robust motility in viscous environments. Mol. Microbiol. 2015, 99, 88–110. [Google Scholar] [CrossRef]
- Tran, T.D.; Ali, M.A.; Lee, D.; Félix, M.-A.; Luallen, R.J. Bacterial filamentation as a mechanism for cell-to-cell spread within an animal host. Nat. Commun. 2022, 13, 693. [Google Scholar] [CrossRef]
- Steinberger, R.; Allen, A.; Hansma, H.; Holden, P. Elongation Correlates with Nutrient Deprivation in Pseudomonas aeruginosa Unsaturated Biofilms. Microb. Ecol. 2002, 43, 416–423. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, S.X. Morphology, Growth, and Size Limit of Bacterial Cells. Phys. Rev. Lett. 2010, 105, 028101. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.D.M. Lactobacillus plantarum: Characterization of the Species and Application in Food Production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Lorenzo, J.M.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Dlangalala, T.N.; Mathipa-Mdakane, M.G.; Thantsha, M.S. The Morphological and Functional Properties of Lactiplantibacillus plantarum B411 Subjected to Acid, Bile and Heat Multi-Stress Adaptation Process and Subsequent Long-Term Freezing. Microbiol. Res. 2022, 13, 909–927. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Guidone, A.; Ianniello, R.G.; Zotta, T.; Abu Sayem, S.; Varcamonti, M. Genotypic diversity of stress response in Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus. Int. J. Food Microbiol. 2012, 157, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Karasz, D.C.; Weaver, A.I.; Buckley, D.H.; Wilhelm, R.C. Conditional filamentation as an adaptive trait of bacteria and its ecological significance in soils. Environ. Microbiol. 2022, 24, 1–17. [Google Scholar] [CrossRef]
- Parlindungan, E.; May, B.K.; Jones, O.A.H. Metabolic Insights Into the Effects of Nutrient Stress on Lactobacillus plantarum B21. Front. Mol. Biosci. 2019, 6, 75. [Google Scholar] [CrossRef]
- Deghorain, M.; Fontaine, L.; David, B.; Mainardi, J.-L.; Courtin, P.; Daniel, R.; Errington, J.; Sorokin, A.; Bolotin, A.; Chapot-Chartier, M.-P.; et al. Functional and Morphological Adaptation to Peptidoglycan Precursor Alteration in Lactococcus lactis. J. Biol. Chem. 2010, 285, 24003–24013. [Google Scholar] [CrossRef]
- Ingham, C.J.; Beerthuyzen, M.; Vlieg, J.v.H. Population Heterogeneity of Lactobacillus plantarum WCFS1 Microcolonies in Response to and Recovery from Acid Stress. Appl. Environ. Microbiol. 2008, 74, 7750–7758. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Shemesh, M. Spatiotemporal bio-shielding of bacteria through consolidated geometrical structuring. npj Biofilms Microbiomes 2022, 8, 37. [Google Scholar] [CrossRef]
- Venugopal, A.; Sionov, R.V.; Kroupitski, Y.; Steinberg, D.; Shemesh, M. The V-Shaped Structuring Regulated via the LuxS-Dependent Quorum-Sensing Pathway Is Associated With Lactiplantibacillus plantarum Survivability in Acidic Environments. Food Front. 2024. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Rahaman, M.; Yao, Y.; Ma, P.; Zhang, J.; Zhao, X.; Jiang, T.; Grzegorzek, M. A comprehensive review of image analysis methods for microorganism counting: From classical image processing to deep learning approaches. Artif. Intell. Rev. 2021, 55, 2875–2944. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, C.; Rahaman, M.; Yao, Y.; Zhang, J.; Zou, S.; Zhao, X.; Grzegorzek, M. A state-of-the-art survey of object detection techniques in microorganism image analysis: From classical methods to deep learning approaches. Artif. Intell. Rev. 2022, 56, 1627–1698. [Google Scholar] [CrossRef]
- Miranda, J.C.; Gené-Mola, J.; Zude-Sasse, M.; Tsoulias, N.; Escolà, A.; Arnó, J.; Rosell-Polo, J.R.; Sanz-Cortiella, R.; Martínez-Casasnovas, J.A.; Gregorio, E. Fruit sizing using AI: A review of methods and challenges. Postharvest Biol. Technol. 2023, 206, 112587. [Google Scholar] [CrossRef]
- Kim, E.; Hong, S.-J.; Kim, S.-Y.; Lee, C.-H.; Kim, S.; Kim, H.-J.; Kim, G. CNN-based object detection and growth estimation of plum fruit (Prunus mume) using RGB and depth imaging techniques. Sci. Rep. 2022, 12, 20796. [Google Scholar] [CrossRef]
- Chotayapa, K.; Leethamchayo, T.; Chinnawong, P.; Samernate, T.; Nonejuie, P.; Achakulvisut, T. Deep Learning-Based Object Detection and Bacteria Morphological Feature Extraction for Antibiotic Mode of Action Study. In Proceedings of the 2023 15th Biomedical Engineering International Conference (BMEiCON), Tokyo, Japan, 28–31 October 2023; pp. 1–5. [Google Scholar]
- Stylianidou, S.; Brennan, C.; Nissen, S.B.; Kuwada, N.J.; Wiggins, P.A. SuperSegger: Robust image segmentation, analysis and lineage tracking of bacterial cells. Mol. Microbiol. 2016, 102, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Bai, Q.; Yang, J.; Jiang, S.; Miao, Y. Review of Image Classification Algorithms Based on Convolutional Neural Networks. Remote Sens. 2021, 13, 4712. [Google Scholar] [CrossRef]
- Gulli, A.; Pal, S. Deep Learning with Keras; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, PMLR, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar]
- Chen, L.; Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Zheng, X. {TensorFlow}: A system for {Large-Scale} machine learning. In Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation (OSDI 16), Savannah, GA, USA, 2–4 November 2016; pp. 265–283. [Google Scholar]
- Canny, J. A computational approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, PAMI-8, 679698. [Google Scholar] [CrossRef]
- Sionov, R.V.; Banerjee, S.; Bogomolov, S.; Smoum, R.; Mechoulam, R.; Steinberg, D. Targeting the Achilles’ Heel of Multidrug-Resistant Staphylococcus aureus by the Endocannabinoid Anandamide. Int. J. Mol. Sci. 2022, 23, 7798. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Chen, Q.; Wang, H.; Kong, B. Physiological, Morphological and Antioxidant Responses of Pediococcus pentosaceus R1 and Lactobacillus fermentum R6 Isolated from Harbin Dry Sausages to Oxidative Stress. Foods 2021, 10, 1203. [Google Scholar] [CrossRef]
- Rolain, T.; Bernard, E.; Courtin, P.; Bron, P.A.; Kleerebezem, M.; Chapot-Chartier, M.-P.; Hols, P. Identification of key peptidoglycan hydrolases for morphogenesis, autolysis, and peptidoglycan composition of Lactobacillus plantarum WCFS1. Microb. Cell Factories 2012, 11, 137. [Google Scholar] [CrossRef]
- Mueller, E.A.; Westfall, C.S.; Levin, P.A. pH-dependent activation of cytokinesis modulates Escherichia coli cell size. PLoS Genet. 2020, 16, e1008685. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, H.; Jammal-Touma, J.; Singh, K.; Rabin, Y.; Salman, H. Bacterial cell-size changes resulting from altering the relative expression of Min proteins. Nat. Commun. 2023, 14, 5710. [Google Scholar] [CrossRef] [PubMed]
- Cesar, S.; Huang, K.C. Thinking big: The tunability of bacterial cell size. FEMS Microbiol. Rev. 2017, 41, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.Y.; Dong, J.; Hasikin, K.; Ngui, R.; Lai, K.W.; Yeoh, P.S.Q.; Wu, X. Bacterial image analysis using multi-task deep learning approaches for clinical microscopy. PeerJ Comput. Sci. 2024, 10, e2180. [Google Scholar] [CrossRef]
- Cutler, K.J.; Stringer, C.; Lo, T.W.; Rappez, L.; Stroustrup, N.; Peterson, S.B.; Wiggins, P.A.; Mougous, J.D. Omnipose: A high-precision morphology-independent solution for bacterial cell segmentation. Nat. Methods 2022, 19, 1438–1448. [Google Scholar] [CrossRef]
- Spahn, C.; Gómez-De-Mariscal, E.; Laine, R.F.; Pereira, P.M.; von Chamier, L.; Conduit, M.; Pinho, M.G.; Jacquemet, G.; Holden, S.; Heilemann, M.; et al. DeepBacs for multi-task bacterial image analysis using open-source deep learning approaches. Commun. Biol. 2022, 5, 688. [Google Scholar] [CrossRef]
| Test Samples | Predicted Control | Predicted Acidic |
|---|---|---|
| Actual control | 2182 | 18 |
| Actual acidic | 116 | 2084 |
| Average | Median | SD | p-Value b | |
|---|---|---|---|---|
| Length Control | 52.41 | 51 | 10.87 | <<0.05 |
| Length Acidic | 75.89 | 71 | 21.17 | |
| Width Control | 23.81 | 24 | 1.97 | 0.34 |
| Width Acidic | 23.7 | 23 | 2.67 |
| Average | Median | SD | p-Value b | |
|---|---|---|---|---|
| Length Control | 54.1 | 53 | 11 | <<0.05 |
| Length Acidic | 79.42 | 75 | 21.14 | |
| Width Control | 23.01 | 23 | 2.07 | 7.0 × 10−3 |
| Width Acidic | 23.84 | 24 | 2.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopal, A.; Steinberg, D.; Moyal, O.; Yonassi, S.; Glaicher, N.; Gitelman, E.; Shemesh, M.; Amitay, M. Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress. Microorganisms 2025, 13, 647. https://doi.org/10.3390/microorganisms13030647
Venugopal A, Steinberg D, Moyal O, Yonassi S, Glaicher N, Gitelman E, Shemesh M, Amitay M. Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress. Microorganisms. 2025; 13(3):647. https://doi.org/10.3390/microorganisms13030647
Chicago/Turabian StyleVenugopal, Athira, Doron Steinberg, Ora Moyal, Shira Yonassi, Noga Glaicher, Eliraz Gitelman, Moshe Shemesh, and Moshe Amitay. 2025. "Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress" Microorganisms 13, no. 3: 647. https://doi.org/10.3390/microorganisms13030647
APA StyleVenugopal, A., Steinberg, D., Moyal, O., Yonassi, S., Glaicher, N., Gitelman, E., Shemesh, M., & Amitay, M. (2025). Computational Analysis of Morphological Changes in Lactiplantibacillus plantarum Under Acidic Stress. Microorganisms, 13(3), 647. https://doi.org/10.3390/microorganisms13030647







