Socioeconomic and Eco-Environmental Drivers Differentially Trigger and Amplify Bacterial and Viral Outbreaks of Zoonotic Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analyses
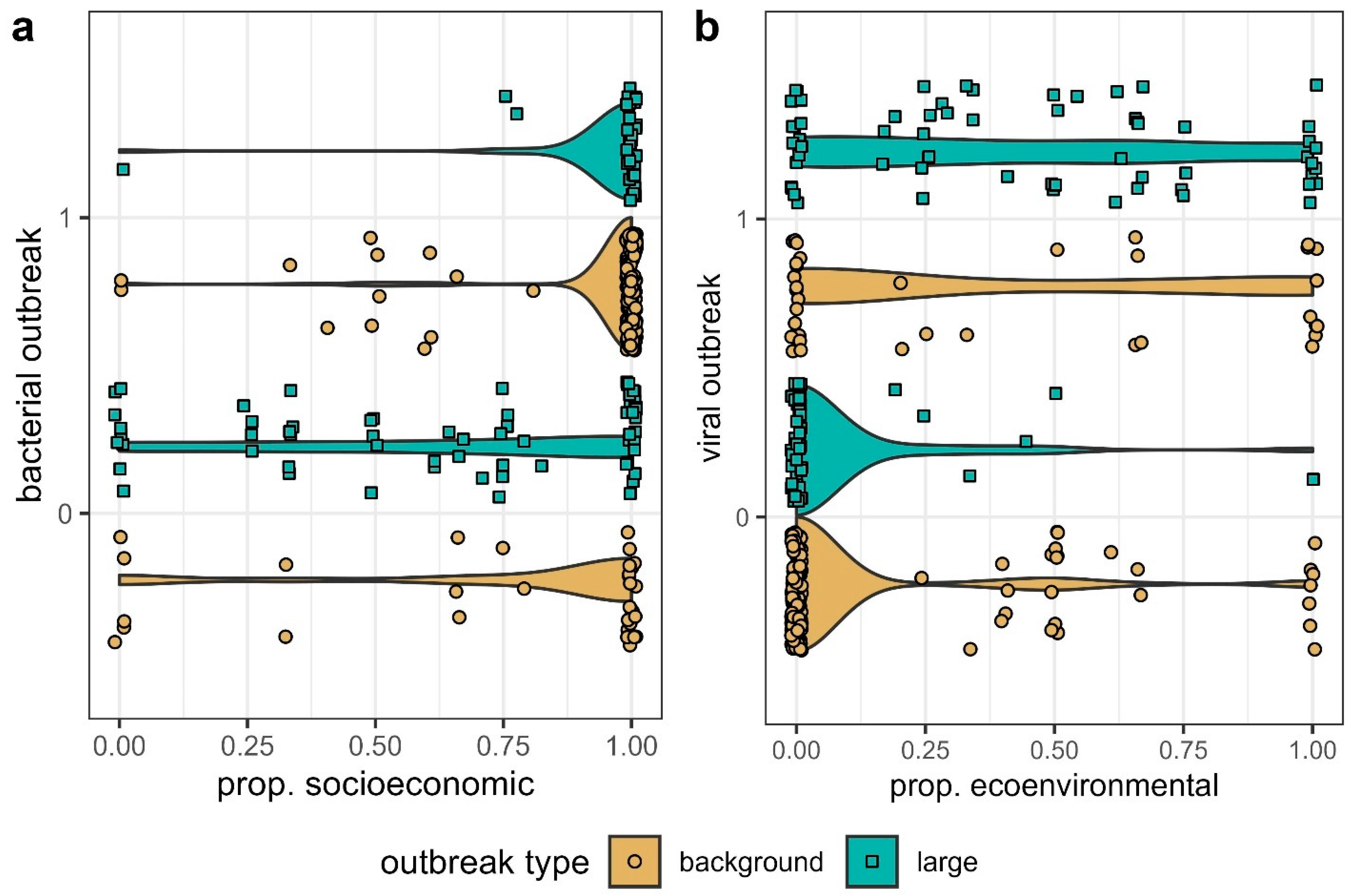
2.1.1. Triggers of Bacterial vs. Viral Outbreaks
2.1.2. Amplifiers of Bacterial and Viral Outbreaks
2.2. Sample Bias
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SE | Socioeconomic |
| EE | Ecological and environmental |
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Smith, K.F.; Goldberg, M.; Rosenthal, S.; Carlson, L.; Chen, J.; Chen, C.; Ramachandran, S. Global Rise in Human Infectious Disease Outbreaks. J. R. Soc. Interface 2014, 11, 20140950. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Goniewicz, K.; Burkle, F.M. Unleashing the Global Potential of Public Health: A Framework for Future Pandemic Response. J. Infect. Public Health 2024, 17, 82–95. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 31 January 2025).
- Disease and Urbanization, 1st ed.; Clegg, E.J., Garlick, J.P., Eds.; Routledge Library Editions: Health, Disease, and Society; Routledge: New York, NY, USA, 2022; Volume 11, ISBN 978-1-032-25318-3. [Google Scholar]
- Neiderud, C.-J. How Urbanization Affects the Epidemiology of Emerging Infectious Diseases. Infect. Ecol. Epidemiol. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis Emergence Linked to Agricultural Intensification and Environmental Change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef]
- Smith, K.F.; Sax, D.F.; Gaines, S.D.; Guernier, V.; Guégan, J.-F. Globalization of Human Infectious Disease. Ecology 2007, 88, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Sigler, T.; Mahmuda, S.; Kimpton, A.; Loginova, J.; Wohland, P.; Charles-Edwards, E.; Corcoran, J. The Socio-Spatial Determinants of COVID-19 Diffusion: The Impact of Globalisation, Settlement Characteristics and Population. Glob. Health 2021, 17, 56. [Google Scholar] [CrossRef]
- Lafferty, K.D. The Ecology of Climate Change and Infectious Diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Hamer, G.L.; Walker, E.D.; Brown, W.M.; Ruiz, M.O.; Kitron, U.D. Climatic Variability and Landscape Heterogeneity Impact Urban Mosquito Diversity and Vector Abundance and Infection. Ecosphere 2011, 2, 1–21. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T.S. Habitat Fragmentation, Biodiversity Loss and the Risk of Novel Infectious Disease Emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef]
- Deyle, E.R.; Maher, M.C.; Hernandez, R.D.; Basu, S.; Sugihara, G. Global Environmental Drivers of Influenza. Proc. Natl. Acad. Sci. USA 2016, 113, 13081–13086. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.P.; Park, A.W.; Kramer, A.M.; Han, B.A.; Alexander, L.W.; Drake, J.M. Spatiotemporal Fluctuations and Triggers of Ebola Virus Spillover. Emerg. Infect. Dis. 2017, 23, 415–422. [Google Scholar] [CrossRef]
- da Costa, A.C.C.; Codeço, C.T.; Krainski, E.T.; Gomes, M.F.d.C.; Nobre, A.A. Spatiotemporal Diffusion of Influenza A (H1N1): Starting Point and Risk Factors. PLoS ONE 2018, 13, e0202832. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.S.; Rodriguez-Barraquer, I.; Barker, C.M.; Tejedor-Garavito, N.; Harding, D.; Lorton, C.; Lukacevic, D.; Oates, G.; Espana, G.; Kraemer, M.U.G.; et al. Data Descriptor: Spatiotemporal Incidence of Zika and Associated Environmental Drivers for the 2015–2016 Epidemic in Colombia. Sci. Data 2018, 5, 180073. [Google Scholar] [CrossRef]
- Laneri, K.; Cabella, B.; Prado, P.I.; Coutinho, R.M.; Kraenkel, R.A. Climate Drivers of Malaria at Its Southern Fringe in the Americas. PLoS ONE 2019, 14, e0219249. [Google Scholar] [CrossRef]
- Wu, T. The Socioeconomic and Environmental Drivers of the COVID-19 Pandemic: A Review. Ambio 2021, 50, 822–833. [Google Scholar] [CrossRef]
- Medaglia, M.L.G.; Pereira, A.d.C.; Freitas, T.R.P.; Damaso, C.R. Swinepox Virus Outbreak, Brazil, 2011. Emerg. Infect. Dis. 2011, 17, 1976–1978. [Google Scholar] [CrossRef]
- Goldani, L.Z. Measles Outbreak in Brazil, 2018. Braz. J. Infect. Dis. 2018, 22, 359. [Google Scholar] [CrossRef]
- Kock, R.A.; Begovoeva, M.; Ansumana, R.; Suluku, R. Searching for the source of Ebola: The elusive factors driving its spillover into humans during the West African outbreak of 2013–2016. Rev. Sci. Tech. 2019, 38, 113–122. [Google Scholar] [CrossRef]
- Semenza, J.C.; Lindgren, E.; Balkanyi, L.; Espinosa, L.; Almqvist, M.S.; Penttinen, P.; Rocklöv, J. Determinants and Drivers of Infectious Disease Threat Events in Europe. Emerg. Infect. Dis. 2016, 22, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.R.; Gottdenker, N.; Schatz, A.M.; Schmidt, J.P.; Drake, J.M. Characteristics of the 100 Largest Modern Zoonotic Disease Outbreaks. Phil. Trans. R. Soc. B 2021, 376, 20200535. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.Y.; Gums, J.G. The Emergence and Evolution of Antimicrobial Resistance: Impact on a Global Scale. Bioorganic Med. Chem. 2016, 24, 6440–6445. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of Evolutionary Change in Viruses: Patterns and Determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92, e01031-17. [Google Scholar] [CrossRef]
- Shaw, L.P.; Wang, A.D.; Dylus, D.; Meier, M.; Pogacnik, G.; Dessimoz, C.; Balloux, F. The Phylogenetic Range of Bacterial and Viral Pathogens of Vertebrates. Mol. Ecol. 2020, 29, 3361–3379. [Google Scholar] [CrossRef]
- CDC Completed OHZDP Workshops. Available online: https://www.cdc.gov/one-health/php/prioritization/completed-workshops.html?CDC_AAref_Val=https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/completed-workshops.html (accessed on 1 August 2021).
- UK Public Health England List of Zoonotic Diseases. Available online: https://www.gov.uk/government/publications/list-of-zoonotic-diseases/list-of-zoonotic-diseases (accessed on 1 August 2021).
- Acha, P.N.; Szyfres, B. Zoonoses and Communicable Diseases Common to Man and Animals, 3rd ed.; Pan American Health Organization: Washington, DC, USA, 2005; Volume 1. Bacterioses and mycoses; Volume 2, p. 378. Chlamydioses, rickettsioses, and viroses; Volume 3, 408. Parasitoses, 395; ISBN 92 75 11580.
- Stephens, P.R.; Sundaram, M.; Ferreira, S.; Gottdenker, N.; Nipa, K.F.; Schatz, A.M.; Schmidt, J.P.; Drake, J.M. Drivers of African Filovirus (Ebola and Marburg) Outbreaks. Vector Borne Zoonotic Dis. 2022, 22, 478–490. [Google Scholar] [CrossRef]
- Institute of Medicine. Emerging Infections: Microbial Threats to Health in the United States, 3rd ed.; Lederberg, J., Shope, R.E., Oaks, S.C., Eds.; National Academic Press: Washington, DC, USA, 1993; ISBN 978-0-309-04741-8. [Google Scholar]
- Institute Of Medicine. Microbial Threats to Health: Emergence, Detection, and Response; Smolinski, M.S., Hamburg, M.A., Lederberg, J., Eds.; National Academic Press: Washington, DC, USA, 2003; ISBN 0-309-08864-X. [Google Scholar]
- Ceddia, M.G.; Bardsley, N.O.; Goodwin, R.; Holloway, G.J.; Nocella, G.; Stasi, A. A Complex System Perspective on the Emergence and Spread of Infectious Diseases: Integrating Economic and Ecological Aspects. Ecol. Econ. 2013, 90, 124–131. [Google Scholar] [CrossRef][Green Version]
- Gottdenker, N.L.; Streicker, D.G.; Faust, C.L.; Carroll, C.R. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. EcoHealth 2014, 11, 619–632. [Google Scholar] [CrossRef]
- Patz, J.A.; Daszak, P.; Tabor, G.M.; Aguirre, A.A.; Pearl, M.; Epstein, J.; Wolfe, N.D.; Kilpatrick, A.M.; Foufopoulos, J.; Molyneux, D.; et al. Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environ. Health Perspect. 2004, 112, 1092–1098. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Courchamp, F.; Killeen, G. Biodiversity Loss, Emerging Pathogens and Human Health Risks. Biodivers. Conserv. 2020, 29, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Anthropogenic Environmental Change and the Emergence of Infectious Diseases in Wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.; Hallaj, Z.; Keusch, G.T.; McManus, D.P.; Ngowi, H.; Cleaveland, S.; Ramos-Jimenez, P.; Gotuzzo, E.; Kar, K.; Sanchez, A.; et al. Zoonoses and Marginalised Infectious Diseases of Poverty: Where Do We Stand? Parasites Vectors 2011, 4, 106. [Google Scholar] [CrossRef]
- Grace, D.; Mutua, F.; Ochungo, P.; Kruska, R.; Jones, K.; Brierley, L.; Lapar, L.; Said, M.; Herrero, M.; Duc Phuc, P.; et al. Mapping of Poverty and Likely Zoonoses Hotspots; Department for International Development: London, UK, 2012. [Google Scholar]
- Wu, T.; Perrings, C.; Kinzig, A.; Collins, J.P.; Minteer, B.A.; Daszak, P. Economic Growth, Urbanization, Globalization, and the Risks of Emerging Infectious Diseases in China: A Review. Ambio 2017, 46, 18–29. [Google Scholar] [CrossRef]
- Madoff, L.C. ProMED-Mail: An Early Warning System for Emerging Diseases. Clin. Infect. Dis. 2004, 39, 227–232. [Google Scholar] [CrossRef]
- WHO. Ebola Haemorrhagic Fever in Sudan, 1976. Bull. World Health Organ. 1978, 56, 247–270. [Google Scholar]
- WHO. Outbreak of Ebola Haemorrhagic Fever in Yambio, South Sudan, April–June 2004. Wkly. Epidemiol. Rec. 2005, 80, 370–375. [Google Scholar]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, Dynamics, and Control of Emerging Vector-Borne Zoonotic Diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Statistics and Computing; Chambers, J., Eddy, W., Hardle, W., Sheather, S., Tierney, L., Eds.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- World Bank Open Data. Available online: https://data.worldbank.org/ (accessed on 26 February 2025).
- Kluberg, S.A.; Mekaru, S.R.; McIver, D.J.; Madoff, L.C.; Crawley, A.W.; Smolinski, M.S.; Brownstein, J.S. Global Capacity for Emerging Infectious Disease Detection, 1996–2014. Emerg. Infect. Dis. 2016, 22, e151956. [Google Scholar] [CrossRef]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef]
- Chang, H.-H.; Cohen, T.; Grad, Y.H.; Hanage, W.P.; O’Brien, T.F.; Lipsitch, M. Origin and Proliferation of Multiple-Drug Resistance in Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2015, 79, 101–116. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H. Filoviruses. A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Arch. Virol. Suppl. 2008, 20, 13–360. [Google Scholar] [PubMed]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G.; et al. Ecological Dynamics of Emerging Bat Virus Spillover. Proc. Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef]
- Eby, P.; Peel, A.J.; Hoegh, A.; Madden, W.; Giles, J.R.; Hudson, P.J.; Plowright, R.K. Pathogen Spillover Driven by Rapid Changes in Bat Ecology. Nature 2023, 613, 340–344. [Google Scholar] [CrossRef]
- Olival, K.J.; Islam, A.; Yu, M.; Anthony, S.J.; Epstein, J.H.; Khan, S.A.; Khan, S.U.; Crameri, G.; Wang, L.-F.; Lipkin, W.I.; et al. Ebola Virus Antibodies in Fruit Bats, Bangladesh. Emerg. Infect. Dis. 2013, 19, 270–273. [Google Scholar] [CrossRef]
- Filion, A.; Sundaram, M.; Stephens, P.R. Preliminary Investigation of Schmalhausen’s Law in a Directly Transmitted Pathogen Outbreak System. Viruses 2023, 15, 310. [Google Scholar] [CrossRef]
- Jameson, L.J.; Ramadani, N.; Medlock, J.M. Possible Drivers of Crimean-Congo Hemorrhagic Fever Virus Transmission in Kosova. Vector Borne Zoonotic Dis. 2012, 12, 753–757. [Google Scholar] [CrossRef]
- Lancelot, R.; Béral, M.; Rakotoharinome, V.M.; Andriamandimby, S.-F.; Héraud, J.-M.; Coste, C.; Apolloni, A.; Squarzoni-Diaw, C.; De La Rocque, S.; Formenty, P.B.H.; et al. Drivers of Rift Valley Fever Epidemics in Madagascar. Proc. Natl. Acad. Sci. USA 2017, 114, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Olivero, J. Biogeography of Diseases. In Biogeography: An Integrative Approach of the Evolution of Living; Guilbert, E., Ed.; ISTE & Wiley: London, UK, 2021; pp. 275–301. ISBN 978-1-119-88238-1. [Google Scholar]
- Sundaram, M.; Filion, A.; Akaribo, B.E.; Stephens, P.R. Footprint of War: Integrating Armed Conflicts in Disease Ecology. Trends Parasitol. 2023, 39, 238–241. [Google Scholar] [CrossRef]
- Núñez, A.; Sreeganga, S.D.; Ramaprasad, A. Access to Healthcare during COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 2980. [Google Scholar] [CrossRef]
- Morens, D.M.; Daszak, P.; Markel, H.; Taubenberger, J.K. Pandemic COVID-19 Joins History’s Pandemic Legion. mBio 2020, 11, e00812–e00820. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, L.; Pan, J.; Xu, X.; Peng, R. Correlation between Vaccine Coverage and the COVID-19 Pandemic throughout the World: Based on Real-world Data. J. Med. Virol. 2022, 94, 2181–2187. [Google Scholar] [CrossRef]
- Kauppinen, A.; Pitkänen, T.; Al-Hello, H.; Maunula, L.; Hokajärvi, A.-M.; Rimhanen-Finne, R.; Miettinen, I.T. Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. Int. J. Environ. Res. Public Health 2019, 16, 4376. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, R.V. Emerging Foodborne Diseases: An Evolving Public Health Challenge. Emerg. Infect. Dis. 1997, 3, 425–434. [Google Scholar] [CrossRef]
- CDC Ongoing Clade II Mpox Global Outbreak. Available online: https://www.cdc.gov/mpox/outbreaks/2022/index-1.html (accessed on 3 February 2025).
- WHO. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 3 February 2025).
- Eisenberg, J.N.S.; Desai, M.A.; Levy, K.; Bates, S.J.; Liang, S.; Naumoff, K.; Scott, J.C. Environmental Determinants of Infectious Disease: A Framework for Tracking Causal Links and Guiding Public Health Research. Environ. Health Perspect. 2007, 115, 1216–1223. [Google Scholar] [CrossRef]

| Driver | Type | Count |
|---|---|---|
| Food contamination | SE | 118 |
| Water contamination | SE | 82 |
| Local livestock production | SE | 54 |
| Sewage management (poor management practices, inadequate infrastructure, or structural failures) | SE | 51 |
| Weather conditions | EE | 47 |
| International travel/trade | SE | 43 |
| Change in vector abundance | EE | 33 |
| Human–animal contact (not including vectors) | B | 33 |
| Public health infrastructure (inadequate infrastructure, equipment, personnel, or monitoring) | SE | 29 |
| Antibiotics (e.g., antibiotic-resistant strains) | SE | 22 |
| Medical procedures (misdiagnosis, incorrect procedures, or failure of correct procedures) | SE | 21 |
| Industrial livestock production | SE | 19 |
| Vaccination breakdown (failure to administer) | SE | 18 |
| Human population density | SE | 17 |
| Livestock and domestic–wildlife interface | B | 16 |
| War/conflict | SE | 15 |
| Poverty (miscellaneous stressors related to poverty and/or marginalization) | SE | 14 |
| Intranational travel/trade | SE | 13 |
| Natural disasters | EE | 13 |
| Change in reservoir abundance | EE | 12 |
| Agricultural activity | SE | 11 |
| Climate change (change in long-term trends) | EE | 8 |
| Wetland cultivation | SE | 6 |
| Soil contamination | SE | 6 |
| Cultural/religious beliefs or practices (which conflict with best health and medical practices) | SE | 6 |
| Deforestation | SE | 5 |
| Socioeconomic change (e.g., broad-scale changes in governance) | SE | 5 |
| Change in vector distribution | EE | 4 |
| Encroachment | B | 4 |
| Wildlife hunting (including capture, processing, consumption; often referred to as “bushmeat”) | SE | 4 |
| Change in reservoir distribution | EE | 3 |
| Vector control (change in or reportedly deficient vector control practices) | EE | 3 |
| Immunosuppression | SE | 3 |
| Malnourishment (e.g., nutrient deficiency or lack of food in particular social groups) | SE | 3 |
| Introduced/invasive species (free living species including vectors, but not invasive pathogens) | EE | 2 |
| Mining | SE | 2 |
| Dam building | SE | 2 |
| Co-infection | SE | 2 |
| Wildlife provisioning | SE | 1 |
| Logging | SE | 1 |
| Human demographic change | SE | 1 |
| Road building | SE | 1 |
| Ineffective vaccine | SE | 1 |
| Famine (widespread nearly universal shortage of food in a region) | SE | 1 |
| Aquaculture | SE | 0 |
| Irrigation | SE | 0 |
| Reforestation | SE | 0 |
| Urbanization | SE | 0 |
| Outbreak Type | Dataset | Variable | Est. | SE | z Value | Pr(>|x|) |
|---|---|---|---|---|---|---|
| Bacterial (1) vs. viral (0) | Large | Intercept | 65.4 | 40.1 | 1.63 | 0.103 |
| Prop. SE drivers | 6.35 | 1.72 | 3.68 | <0.001 | ||
| Start year | −0.036 | 0.02 | −1.76 | 0.079 | ||
| Background | Intercept | 44.5 | 47.7 | 0.932 | 0.352 | |
| Prop. SE drivers | 2.83 | 0.720 | 3.93 | <0.001 | ||
| Start year | −0.023 | 0.024 | −0.950 | 0.342 | ||
| Viral (1) vs. bacterial (0) | Large | Intercept | −57.5 | 39.4 | −1.46 | 0.144 |
| Prop. EE drivers | 5.06 | 1.19 | 4.25 | <0.001 | ||
| Start year | 0.029 | 0.020 | 1.45 | 0.148 | ||
| Background | Intercept | −53.1 | 47.9 | −1.11 | 0.268 | |
| Prop. EE drivers | 2.45 | 0.534 | 4.59 | <0.001 | ||
| Start year | 0.025 | 0.024 | 1.07 | 0.287 |
| Outbreak Type | Dataset | Variable | Est. | SE | z Value | Pr(>|x|) |
|---|---|---|---|---|---|---|
| Bacterial | Large | Intercept | 33.4 | 33.7 | 0.992 | 0.321 |
| Prop. SE drivers | −1.11 | 1.15 | −0.966 | 0.334 | ||
| Start year | −0.011 | 0.017 | −0.673 | 0.501 | ||
| Background | Intercept | 19.4 | 26.1 | 0.743 | 0.458 | |
| Prop. SE drivers | 1.11 | 0.717 | 1.55 | 0.122 | ||
| Start year | −0.008 | 0.013 | −0.587 | 0.557 | ||
| Viral | Large | Intercept | 56.5 | 21.4 | 2.64 | 0.008 |
| Prop. SE drivers | 1.79 | 0.397 | 4.49 | <0.001 | ||
| Start year | −0.024 | 0.011 | −2.21 | 0.027 | ||
| Background | Intercept | 233 | 73.4 | 3.18 | 0.001 | |
| Prop. SE drivers | 3.44 | 0.947 | 3.63 | <0.001 | ||
| Start year | −0.115 | 0.037 | −3.12 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, P.; Nazari, N.; Dharwadkar, S.; Filion, A.; Akaribo, B.E.; Stephens, P.; Sundaram, M. Socioeconomic and Eco-Environmental Drivers Differentially Trigger and Amplify Bacterial and Viral Outbreaks of Zoonotic Pathogens. Microorganisms 2025, 13, 621. https://doi.org/10.3390/microorganisms13030621
Phillips P, Nazari N, Dharwadkar S, Filion A, Akaribo BE, Stephens P, Sundaram M. Socioeconomic and Eco-Environmental Drivers Differentially Trigger and Amplify Bacterial and Viral Outbreaks of Zoonotic Pathogens. Microorganisms. 2025; 13(3):621. https://doi.org/10.3390/microorganisms13030621
Chicago/Turabian StylePhillips, Payton, Negin Nazari, Sneha Dharwadkar, Antoine Filion, Benedicta Essuon Akaribo, Patrick Stephens, and Mekala Sundaram. 2025. "Socioeconomic and Eco-Environmental Drivers Differentially Trigger and Amplify Bacterial and Viral Outbreaks of Zoonotic Pathogens" Microorganisms 13, no. 3: 621. https://doi.org/10.3390/microorganisms13030621
APA StylePhillips, P., Nazari, N., Dharwadkar, S., Filion, A., Akaribo, B. E., Stephens, P., & Sundaram, M. (2025). Socioeconomic and Eco-Environmental Drivers Differentially Trigger and Amplify Bacterial and Viral Outbreaks of Zoonotic Pathogens. Microorganisms, 13(3), 621. https://doi.org/10.3390/microorganisms13030621





