Abstract
Oral cavity dysbiosis is associated with numerous inflammatory diseases, including diabetes, inflammatory bowel diseases, and periodontal disease. Changes in the oral microenvironment lead to bidirectional interactions between pathogens and individual host systems, which may induce systemic inflammation. There is increasing evidence linking the condition of the oral cavity with the most common causes of female infertility, such as polycystic ovary syndrome and endometriosis, as well as gestational complications, e.g., low birth weight, preterm delivery, and miscarriages. This review highlights the composition of the female oral microbiome in relation to infertility-related disorders, such as endometriosis and polycystic ovary syndrome, and provides a comprehensive overview of the current state of knowledge on the relationship between a dysbiotic oral microbiome, pregnancy, and its impact on the female reproductive tract.
1. Introduction
The human oral microbiome comprises colonies of microorganisms inhabiting the teeth, tongue, soft and hard palate, cheeks, tonsils, and gingival sulci [1], which begin to develop at birth and change throughout adolescence and adulthood [2]. This heterogeneous ecosystem, located in the initial section of the digestive tract, consists of approximately 1000 species of bacteria, viruses, fungi, protozoa, and archaea, with bacteria being the most numerous group [3]. The most common species in healthy humans are Streptococcus, Prevotella, Rothia, Neisseria, Haemophilus, Fusobacterium, Corynebacterium, Actinomyces, Capnocytophaga, Porphyromonas, and Granulicatella [4]. Moreover, individual microbial species inhabiting the oral cavity are characterized by a certain local specificity. Pioneering microorganisms first to colonize the oral cavity are Streptococcus species, among which Streptococcus mitis occurs mainly in the buccal mucosa, Streptococcus infantis colonizes the hard palate, Streptococcus sanguinis and Streptococcus gordonii colonize the teeth, and Streptococcus australis colonizes the back and lateral surface of the tongue [5,6,7,8,9]. Gemella hemolysans is the dominant species on the buccal epithelium, Granulicatella elegans is dominant on the hard palate, and the tooth surface is inhabited by Rothia dentocariosa [4]. All of these microorganisms together create a diverse and highly developed ecosystem.
The overgrowth of pathogenic species and the loss of microbial biodiversity disrupt the natural homeostasis, finally leading to a condition known as dysbiosis. Oral cavity dysbiosis may induce the development of numerous oral diseases, including dental caries and periodontal diseases [10]. Periodontal diseases are one of the most common inflammatory diseases among adults and are caused by, among other things, poor oral hygiene, diabetes, excessive sugar consumption, smoking, and obesity [11]. Several studies have shown that anaerobic Gram-negative bacteria, mainly Campylobacter rectus, Treponema denticola (T. denticola), Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans), Prevotella intermedia (P. intermedia), Porphyromonas gingivalis (P. gingivalis), and Tannerella forsythia (T. forsythia), in saliva and other internal surfaces of the oral cavity are associated with an increased risk of periodontal diseases [12,13,14,15]. Moreover, periodontal dysbiosis is also associated with the formation of pathogenic complexes consisting of anaerobic Gram-negative microbes. The orange complex is compounded with Fusobacterium nucleatum (F. nucleatum), Prevotella intermedia, Prevotella micros, and Prevotella nigrescens, shifting to the final form, the red complex, comprising P. gingivalis, T. denticola, and T. forsythia, and it appears during periodontal disease progression [16]. In turn, species such as Actinomyces gerencseriae, Bifidobacterium, Streptococcus mutans (S. mutans), Veillonella, and Lactobacillus fermentum, as well as Streptococcus salivarius (S. salivarius) and Streptococcus parasanguinis (S. parasanguins), have been associated with the development of early childhood caries [17,18].
Recently, interest in the oral microbiome has increased due to the relationship between oral dysbiosis and the development of systemic diseases. Periodontal pathogens can induce the development of systemic diseases directly, via their transmission to distant organ systems through the bloodstream; or indirectly, by promoting low-grade inflammation due to the production of proinflammatory markers, such as interleukin (IL) 1, IL-6, or tumor necrosis factor (TNF-α) [19,20]. Injuries that damage blood vessels within dental plaque may enable the transmission of pathogenic bacteria via the bloodstream from the oral cavity to other host systems. Diseases associated with oral dysbiosis include Alzheimer’s disease, diabetes and insulin resistance, cardiovascular diseases, inflammatory bowel diseases, respiratory infections and pneumonia, and even some types of cancer (Figure 1) [21,22,23,24,25,26,27,28,29,30]. Oral microbiome dysbiosis may participate in the pathogenesis of these diseases and, at the same time, lead to dental caries and periodontal diseases. Recently, a connection has also been observed between the condition of the oral cavity and reproductive functions, as systemic inflammation induced by oral dysbiosis may affect fertility by lowering the chance of implantation [31,32].
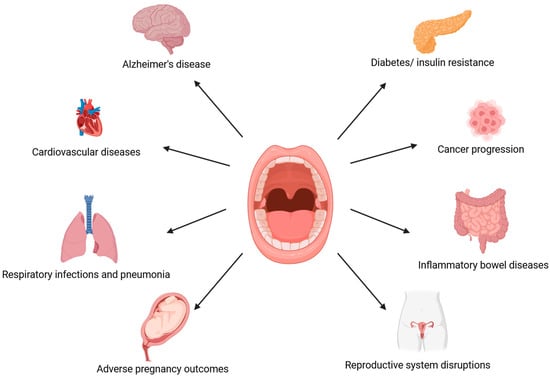
Figure 1.
A potential link between oral dysbiosis and systemic health. (The figure was created using BioRender.com). Oral cavity ecological imbalance affects individual host systems. Periodontal diseases accompanied by oral dysbiosis are associated with the development of Alzheimer’s disease, cardiovascular disease, respiratory infections and pneumonia, adverse pregnancy outcomes, diabetes and insulin resistance, carcinogenesis, inflammatory bowel diseases, and disruptions in the reproductive system [21,22,23,24,25,26,27,28,29,30,31,32].
The impact of oral health on female fertility is not yet fully understood, although some earlier reports suggest a certain relationship between the two. It is speculated that, similarly to the interaction of the oral microbiome with specific host systems, oral microbes may translocate from a dysbiotic oral cavity to the female reproductive organs, causing local inflammation. For instance, in a study by Nwhator et al. involving 70 pregnant women, the authors reported that poor oral hygiene and periodontitis were associated with an extended time to conception (>12 months) and suggested that periodontitis is positively correlated with a lower chance of conceiving [32]. However, although good oral hygiene was correlated with a shorter time to conception (<12 months) compared to poor oral hygiene, the difference was not statistically significant. Similar results were obtained in the SMILE study, which showed that among 146 women with a time to conception of >12 months, the incidence of periodontal disease was higher (34.9% vs. 25.7%) than in the group where the time to conception was shorter (<12 months) [33]. Women diagnosed with periodontal disease required an average of 7 months to conceive, whereas healthy patients conceived in an average of 5 months. In an observational and prospective study involving 256 non-pregnant women, researchers detected major periodontal pathogens in saliva and analyzed serum and saliva antibodies against these pathogens [34]. Participants underwent gynecological and clinical examinations to investigate a potential connection between periodontitis and conception. The follow-up period for becoming pregnant was 12 months. The study demonstrated that both salivary levels of P. gingivalis immunoglobulin A antibodies and the frequency of P. gingivalis in saliva samples were higher in the group of women who did not become pregnant, suggesting that P. gingivalis may be associated with conceive failure [34]. However, more research is still needed to confirm the connection between the oral and reproductive tracts and to understand this relationship.
The aim of this review was to discuss the composition of the female oral microbiota in relation to infertility-related disorders, such as endometriosis and polycystic ovary syndrome, and to describe the relationship between a dysbiotic oral microbiome, pregnancy, and its impact on the female reproductive tract. To provide a comprehensive overview of the current state of knowledge on this topic, the PubMed database was searched using the following keywords: ‘oral microbiome’ OR ‘oral microbiota’ OR ‘oral pathogens’ OR ‘oral microbes’ OR ‘oral microenvironment’ OR ‘oral health’ OR ‘mouth flora’ OR ‘periodontal health’ OR ‘periodontal disease’ OR ‘periodontitis’ OR ‘gingivitis’ AND ‘pregnancy’ OR ‘pregnancy outcomes’ OR ‘gestation’ OR ‘fertility’ OR ‘conception’ OR ‘implantation’ OR ‘reproductive health’ OR ‘reproductive tract’ OR ‘polycystic ovary syndrome’ OR ‘endometriosis’. When possible, the search was limited to the last 20 years and included only studies published in English. Both human and animal studies were included, and no restrictions were applied to the study design. Each publication was thoroughly evaluated. The authors first screened the titles and abstracts of potential articles, followed by a detailed review of the full texts to identify relevant studies. Any uncertainties or disagreements during the selection process were resolved by consensus.
2. Oral Health and Pregnancy Outcomes
Pregnancy is accompanied by very dynamic hormonal, immunological, and metabolic changes resulting from physiological alterations to establish fetal growth and development [35]. These alterations influence the mother’s microbiome of the gut, vagina, and oral cavity [36]. During pregnancy, there is an increase in the number of oral microorganisms toward dysbiosis, especially in the first trimester, which may be due to some Prevotella species, which are able to use estrogen and progesterone as substitutes for vitamin K for their growth [37,38,39,40]. Estrogen and progesterone metabolism are determined by the inflammatory component and the duration of inflammation, which influence the efficiency of enzymatic conversion within the gingiva [39,40]. Given that there are increased levels of sex steroid hormones in pregnancy, and they are able to be metabolized by localized in human gingiva-specific receptors and induce a local inflammation within, it is not surprising that oral diseases (such as gingivitis, dental caries, and periodontitis) are more prevalent in the gestational state, affecting about 40% of women worldwide [41]. Moreover, these gestational hormonal fluctuations affect the gingival tissues and cause inflammation, independent of the presence of proinflammatory cytokines such as IL-1 β or TNF-α in the gingival crevicular fluid (GCF) [42].
Although there is no established guideline for treating periodontal diseases among pregnant women, medical workers should pay more attention to oral hygiene education during pregnancy and raise awareness about dental care. According to the PERISCOPE longitudinal study among pregnant women in the first trimester, almost half of the participants (47.1%) had periodontitis, of which 66.7% had clinical signs, such as gingival bleeding [43]. Moreover, 41.3% of women declared that they had not been to the dentist at least one year before pregnancy, and 52.1% had poorer oral health, described as the presence of ≥10% dental plaque [43]. What is important is that the composition of the oral microbiome in pregnant women may change throughout pregnancy. For instance, Fujiwara et al. [38] demonstrated that in the first and second trimester, mouth flora show a predominance of P. gingivalis and A. actinomycetemcomitans compared to non-pregnant women, while Candida species were frequently abundant in the later stages of pregnancy. Moreover, it has been reported in a study by Massoni et al. [44] that there is a positive correlation between P. gingivalis and progesterone levels in the first trimester of pregnancy. During the first trimester of gestation, a higher abundance of T. forsythia in subgingival biofilm was observed, which increased susceptibility to the occurrence of gingivitis. Furthermore, Lin et al. [45] demonstrated that pregnant women are characterized by a higher abundance of Treponema, Porphyromonas, and Neisseria, as well as a reduced abundance of Veillonella and Streptococcus, compared to the non-pregnant group. The authors also demonstrated a significantly higher Shannon diversity index of the salivary microbiome in pregnant women compared to the non-pregnant group. In a study by Balan et al. [46], the authors examined bacterial communities of saliva and subgingival plaque during different stages of pregnancy and postpartum period. Using 16S rRNA sequence analysis, they aimed to determine the bacterial taxonomic differences among participants. In a group of pregnant women, the most abundant genera in saliva samples were Prevotella, Veillonella, Streptococcus, Neisseria, and Terrahaemophilus, while Prevotella, Streptococcus, Fusobacterium, Veillonella, and Terrahaemophilus predominated in subgingival plaque samples. Pregnancy was also associated with a higher abundance of pathogenic taxa at the species level, showing a predominance of Prevotella species, P. gingivalis, and F. nucleatum, which have been correlated to pregnancy gingivitis. Interestingly, the oral microbiome of pregnant women, which was characterized by an overgrowth of pathogenic bacteria, appeared to re-establish and form a healthy microbial community during the postpartum period. These findings suggest that hormonal alterations during pregnancy cause perturbations in the oral microbial environment, promoting a shift toward the overgrowth of pathogenic taxa.
Disruptions in the oral microbiome among pregnant women may not only influence maternal periodontal health but also interact with the placenta and affect the fetus. The placental microbiome is thought to be established through hematogenous microbial translocation from the maternal mouth flora, as these environments exhibit similarities to each other [47,48,49]. According to the theory of bacterial dissemination, it is suggested that periodontal pathogens may spread through the bloodstream to the amniotic fluid, leading to chorioamnionitis [50]. Given that the placental microbiome profile is similar to the supragingival plaque, it has been further suggested that oral dysbiosis during pregnancy may cause adverse pregnancy outcomes, such as premature birth, low birth weight, miscarriages, or preeclampsia [51,52,53,54,55]. Oral microorganisms found in the human placental include A. actinomycetemcomitans, P. intermedia, T. forsythia, T. denticola, Campylobacter rectus, Fusobacterium nucleatum, and Porphyromonas gingivalis, of which the most prevalent pathogens, P. gingivalis and F. nucleatum, are associated with intrauterine infections [48,52]. Periodontal pathogens such as Actinobacillus actinomycestemcomitans, F. nucleatum, P. gingivalis, P. intermedia, T. denticola, and T. forsythia were found in the placentas of pregnant women with preeclampsia [56], with increased levels of F. nucleatum and P. intermedia in the subgingival biofilm among postpartum women also associated with low birth weight and preterm delivery [57]. Cooper et al. [58] profiled subgingival and placental microbiomes of 54 women with and without preeclampsia and periodontal disease. They reported a significant predominance of oral-origin bacteria, including Veillonella, Gemella, Granulicatella, Fusobacterium, Haemophilus, Streptococcus, and Neisseria, in the placentas of pregnant women with preeclampsia compared to those without the condition (53.8% vs. 19.0%, respectively). Furthermore, the authors observed the highest prevalence of these pathogens in women with both preeclampsia and periodontal disease (58.8%). Moreover, results from recent studies have shown a connection between maternal oral health and preterm birth, suggesting that periodontitis may increase the risk of preterm delivery [59,60]. In particular, placenta colonization by oral pathogens such as P. gingivalis and F. nucleatum is suspected to contribute significantly to preterm delivery [61,62]. Periodontal infections may also influence birth weight and serve as a clinically significant factor for the risk of low birth weight. In a case–control study involving 88 postpartum women who delivered either low-birth-weight or normal-weight infants, poorer oral health, including a higher number of deep periodontal pockets, was reported among mothers of low-birth-weight babies [63]. Notably, deeper periodontal pockets increase the surface area for exchange between the bacterial biofilm and the bloodstream [64]. Additionally, both low birth weight and preterm birth have been associated with P. gingivalis, as its presence correlates with higher periodontal parameters, including plaque index, probing depth, and clinical attachment loss, and is inversely related to birth weight [65]. Interestingly, Kothwiale et al. [66] demonstrated an association between the severity of periodontal disease and lower hemoglobin levels. Both severe anemia and periodontitis, when combined, may influence fetal development, potentially leading to preterm delivery and low birth weight. In summary, pregnancy may favor the development of periodontal dysbiosis, which is accompanied by local inflammation. Consequently, oral pathogenic microbes produce proinflammatory mediators such as IL-1, IL-6, and TNF-alpha, along with bacterial endotoxins, like lipopolysaccharides (LPSs), which may disseminate systemically and affect fetus development. Selected studies investigating the relationship between periodontal health and adverse pregnancy outcomes are summarized in Table 1.

Table 1.
Summary of selected studies on the association between oral health and adverse pregnancy outcomes.
Animal studies are in line with these results, confirming the negative indirect effects of P. gingivalis on the placenta, such as fetal growth restriction caused by bacterial LPS [68]. F. nucleatum, when injected intravenously into pregnant mice, induced premature birth and stillbirths by spreading to the amniotic fluid and causing bacteriemia in the uterus, without leading to systemic dissemination [69]. These pathogenic species may induce adverse pregnancy outcomes via two possible mechanisms: directly, via hematogenous dissemination that translocates from the disrupted oral microbiome, crossing the placental barrier into the amniotic fluid and fetal circulation, consequently causing bacteremia; or indirectly, through endotoxins originating from the periodontium that enter the systemic circulation and affect the fetus (Figure 2) [67,70]. Furthermore, some oral commensal microorganisms associated with adverse pregnancy outcomes in humans may have a specific mechanism of translocation to the placenta, as there were different numbers of the microorganisms found in pregnant mice placentas than in injected saliva and subgingival plaque samples [49].
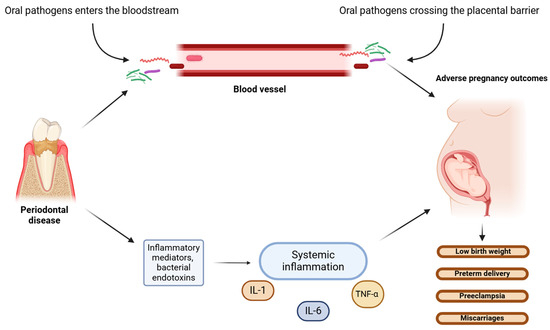
Figure 2.
Potential direct and indirect mechanisms of oral pathogens’ actions on the fetus. (The figure was created using BioRender.com). Periodontal pathogens affect the fetus through two possible mechanisms: direct action, whereby microbes from disrupted oral microbiome translocate via hematogenous dissemination to the fetal circulation and cause bacteriemia; or indirect actions via oral bacterial endotoxins and inflammatory mediators entering the systemic circulation and causing systemic inflammation [67,70]. Systemic inflammatory response is induced by bacterial lipopolysaccharides (LPSs) and mediated by proinflammatory cytokines, including interleukins (IL-1 and IL-6) and tumor necrosis factor (TNF-α). Both mechanisms cause intrauterine and placental bacteriemia, leading to adverse pregnancy outcomes.
A meta-analysis of randomized controlled trials concluded that treatment of periodontal disease during pregnancy, such as scaling or root planning, may be beneficial for both the mother and the infant. Both of these dental medicine procedures reduce the rate of low birth weight (odds ratio (OR): 0.48; 95% confidence interval (CI): 0.23–1.00) and preterm delivery (OR: 0.55; 95%CI: 0.35–0.86), probably as a result of the reduced accumulation of pathogenic bacteria in the oral cavity and, consequently, decreased microbial transmission to the amniotic fluid [71]. Additionally, periodontal disease treatment might diminish inflammatory mediator levels in the systemic circulation, minimizing their negative effects on the reproductive tract. Professional periodontal care interventions during the second trimester are safe and may improve dental health by reducing gingival inflammation [72,73], thereby decreasing the negative effects of oral pathogens on the fetus. These findings underline the influence of periodontal disease accompanied by oral dysbiosis on maternal and neonatal health.
3. Oral Dysbiosis and Fertility Disorders
According to the World Health Organization (WHO) and the International Committee for Monitoring Assisted Reproductive Technology, infertility is a disease of the human reproductive system defined by the failure to achieve a clinical pregnancy within 12 months, despite regular sexual intercourse (2–4 times a week) without using contraceptive methods [74,75]. Infertility affects approximately 17.5% of the adult worldwide population, and at least 40 to 50% of the cases are caused by a male factor [75]. Female factors associated with infertility include mainly ovulation disorders caused by excessive stress; hormonal disorders; incorrect diet and lifestyle; and endocrine/gynecological disorders, e.g., polycystic ovary syndrome (PCOS) and endometriosis [76]. The most common gynecological disorders causing infertility in women, e.g., PCOS and endometriosis, are characterized by sex steroid hormone fluctuations and low-grade systemic inflammation. Given that female sex hormones may be used by some oral bacteria species, such as P. intermedia and P. gingivalis, as a source of nutrients, these conditions may increase the risk of periodontal disease [77].
3.1. Polycystic Ovary Syndrome
PCOS affects about 5–18% of women of reproductive age [78]. According to the Rotterdam criterion, this endocrine disorder is accompanied by the presence of at least two of the following: oligo- or anovulation, hyperandrogenism (clinical and/or biochemical), and polycystic ovaries [79]. Recent studies have shown a bidirectional relationship between PCOS and periodontal diseases, as these conditions are associated with low-grade systemic inflammation and may influence each other [80,81,82]. However, while this connection has been observed, a causal relationship between PCOS and periodontal diseases has not yet been established. Nevertheless, according to recent data, PCOS increases the risk of periodontal disease by 28%; in turn, periodontal disease increases the risk of PCOS by 46% [83]. PCOS may cause repercussions in the oral microbiome via elevated levels of proinflammatory markers such as TNF-α, C-reactive protein (CRP), IL-1, or IL-6, but also through the sex steroid hormone alterations, causing gingival inflammation [84,85,86]. Women with PCOS have been shown to exhibit elevated levels of estrogen and male androgenic hormones, along with decreased progesterone levels. These alterations in circulating hormones may affect female periodontal health [87]. Estrogen receptors are highly expressed in the gingival tissues of women with PCOS, with expression levels increasing 10-fold during inflammation [88,89]. The estrogen receptor subtype, ER-beta, is present in the gingival epithelium, salivary glands, and periodontal ligament [90,91,92]. Similarly, androgen receptors are found in human gingiva, and their metabolism in gingival tissues also increases during inflammation [93,94]. Collectively, elevated levels of estrogen and androgens may modulate gingival tissue and influence the oral microbiome in women with PCOS. In turn, periodontal disease promotes insulin resistance (IR), inflammation, and oxidative stress, risk factors for PCOS development [89]. Periodontal disease is accompanied by chronic inflammation and oxidative damage, which may increase the risk of PCOS by engaging anti-inflammatory and proinflammatory pathways. In studies by Özçaka et al. [85,86], higher concentrations of IL-6 were found in GCR, saliva, and serum of women with PCOS and gingivitis compared to those with PCOS but healthy periodontium [86]. Conversely, the second study reported decreased serum concentrations of IL-17E in PCOS women with gingivitis compared to the healthy group [85]. Moreover, proinflammatory mediators, such as IL-1, IL-6, and TNF-α, which are released during periodontal disease, play an essential role in the progression of IR [95]. Notably, P. gingivalis, which is highly abundant in the dysbiotic oral microbiome associated with periodontal disease, may exacerbate IR through a macrophage-dependent immune response in the oral cavity [96]. Subsequently, IR promotes the development of PCOS via its influence on pituitary luteinizing hormone (LH) secretion, resulting in increased androgen release by the ovaries [97].
A bidirectional relationship between PCOS and periodontal disease has been previously suggested in numerous studies [98,99,100]. In a study that enrolled 196 women (98 with PCOS and 98 healthy controls), evaluating periodontal parameters among both groups, a significantly higher rate of bleeding on probing (BOP) and clinical attachment loss (CAL) were reported in PCOS women in comparison to healthy subjects [101]. The authors observed increased frequency of periodontal disease in PCOS women, suggesting a bidirectional relationship between PCOS and periodontal disease, as both conditions are related to low-grade systemic inflammation and may exacerbate each other. Another study demonstrated a similar correlation between PCOS and periodontitis, as well as an associated periodontal prooxidative state, indicating that PCOS patients have a higher susceptibility to periodontitis [102]. Moreover, the prevalence and likelihood of periodontitis are significantly higher in women with newly diagnosed PCOS [103]. These women also exhibit higher levels of periodontal inflammation and tissue breakdown compared to medically treated PCOS women and healthy controls. Additionally, the same study has also shown an association between periodontal breakdown and systemic inflammation, highlighting the role of oral health in modulating systemic inflammatory pathways. Akcali et al. [104] reported a correlation between PCOS and microbial oral dysbiosis, demonstrating that this hormonal disorder may interfere with the oral microbiome composition. Increased salivary levels of putative periodontal pathogens were observed in women with PCOS and gingivitis, such as P. gingivalis, T. forsythia, and F. nucleatum, compared to the periodontally healthy controls. A particularly strong effect was observed in the case of P. gingivalis, as PCOS increased the occurrence of this pathogen in saliva, as well as the serum antibody response, especially in the presence of gingivitis. Moreover, PCOS also selectively increased the serum antibodies to P. intermedia and S.oralis [104]. The presence of F. nucleatum, T. forsythia, P. gingivalis, and P.intermedia in the subgingival plaque is positively associated with periodontal inflammation progression [105]. Similar results among participants with PCOS and periodontitis and patients with PCOS and gingivitis were also observed in a case–control study of 40 women which demonstrated that levels of F. nucleatum and P. gingivalis in the subgingival plaque are higher in women with PCOS compared to healthy controls [80]. Interestingly, Li et al. [106] observed that PCOS patients have disrupted diurnal rhythm of the salivary microbiome and that their microbial composition may change during the day. Participants with PCOS showed differences in alpha and beta diversity at two time points compared to healthy controls with a higher salivary abundance of Fusobacterium at each time point, as well as a lower abundance of Actinobacteria [106]. Moreover, there was a reduced abundance of Actinobacteria in saliva observed in a group of periodontally healthy PCOS women [107]. The reduced abundance of Actinobacteria has been associated with periodontitis [108,109], confirming that PCOS patients have a more pathogen-favorable microenvironment, which may involve a higher susceptibility to the development of periodontal diseases affecting future gestation.
Although a connection between oral microbes and PCOS has been documented in earlier studies, there is still limited research on the influence of dental treatment on systemic health among women with PCOS. However, a randomized controlled trial by Deepti et al. [110] investigated the impact of non-surgical periodontal treatment combined with medical therapy using Myo-inositol on serological inflammatory markers, including high-sensitivity C-reactive protein (hsCRP) and insulin resistance (assessed by Homeostatic Model Assessment; HOMA-IR), among 60 women with PCOS and periodontitis who were randomly divided in two groups. Participants in both groups received Myo-inositol at a dose of 2 g per day for 6 months. In addition, women in the test group underwent 2–3 sessions of periodontal treatment, including root planning, scaling, and oral hygiene instructions. The control group received education on proper oral hygiene and information about periodontal disease but did not receive any dental therapy. Serum levels of hsCRP decreased in both groups; however, the reduction was statistically greater in the test group. The authors also reported a significant improvement in periodontal parameters in the test group, including reduced gingival inflammation, fewer sites with bleeding on probing, lower plaque levels, and decreased pocket depth. These results suggest that non-surgical periodontal treatment, such as root planning and mouth scaling, combined with medical therapy, may be beneficial in reducing low-grade systemic inflammation among patients with PCOS [110]. Moreover, it has been documented that the treatment of periodontal disease can be as effective in reducing CRP levels as pharmacological treatment or lifestyle modifications [111]. Considering that women with PCOS exhibit significantly higher levels of pro-inflammatory cytokines in saliva [112], it is plausible to suggest that dental treatments aimed at improving oral health may also help alleviate the symptoms of this hormonal disorder.
3.2. Endometriosis
Endometriosis is a chronic gynecological disease with an inflammatory component and the presence of endometrium-like epithelium outside the endometrium and myometrium [113]. Due to the often asymptomatic nature of the disease and difficulties in its diagnosis, it is impossible to determine the exact frequency of its occurrence, but, according to estimates, it may affect up to 50% of infertile women [114]. This condition is asymptomatic in about 20–25% of females, known as ‘silent endometriosis’, although typical symptoms include chronic pelvic pain, dysmenorrhea, infertility, deep dyspareunia, severe menstrual pain, etc. [115]. There is a hormonal imbalance in endometriosis, e.g., increased estrogen levels, in combination with insufficient progesterone [116].
A growing body of research demonstrates the association between the development of endometriosis and the gut microbiome. Studies have shown that the gut microbiome in endometriosis is characterized by an overgrowth of Proteobacteria, Verrucomicrobia, Fusobacteria, and Actinobacteria, with a dominance of Shigella and Escherichia, as well as significantly decreased Lactobacillaceae [117,118,119,120,121,122]. The collection of genes in the gut microbiome, which encode estrogen-metabolizing enzymes, is described as the estrobolome, and it regulates estrogen levels [123]. Given that, perturbations in the gut microenvironment may disrupt circulating estrogen levels and induce estrogen-dependent diseases [123,124]. A difference in microbial composition in subjects with endometriosis was also shown in the peritoneal fluid and female reproductive tract [118,125,126,127,128]. These alterations in the microbiome, promoting dysbiosis, play an important role in the pathogenesis of endometriosis by modifying circulating estrogen levels or inducing systemic inflammation by pathogenic bacteria [129].
There is a lack of research regarding the connection between endometriosis and oral microbiome, although a few previous studies showed an association between periodontitis and endometriosis [130,131]. Recently, patients with endometriosis and repeated implantation failure have been shown to have elevated levels of Dialister and Streptococcus in the uterine endometrium microbiome, which, along with a higher Shannon diversity index in the uterine endometrium microbiome, was suggested to be associated with the pathology of constant implantation failure in endometriosis patients [132]. Importantly, the Gram-negative Dialister species occurs in the oral cavity and has been previously associated with periodontal diseases [133]. D. pneumosintes is more frequently prevalent in the subgingival biofilm among subjects with periodontitis [133,134,135,136] and is also detected in the placenta of women with preeclampsia [136]. Jin et al. [137] proved for the first time a bidirectional causal effect between periodontitis and endometriosis, demonstrating that periodontitis has a cause–effect on endometriosis of the pelvic peritoneum. The authors also proposed a few mechanisms to explain the cause–effect of periodontitis on endometriosis of the pelvic peritoneum. Periodontal pathogens associated with dysbiosis, such as P. gingivalis, may translocate from the oral cavity to the pelvic peritoneum and induce local inflammation via activating peritoneal macrophages and interleukin-1 synthesis. A study by Young et al. [138] emphasized the crucial role of the pelvic peritoneum in the development and maintenance of endometriosis. Furthermore, recent data from an in vivo mice study by Muraoka et al. [139] demonstrated that F. nucleatum infection causes macrophage infiltration in the endometrium and promotes endometriotic lesion development. F. nucleatum was detected in the uterus of 64% of patients with endometriosis and may be related to endometriosis progression. As mentioned before, intrauterine infections may be induced by F. nucleatum due to its transmission from the oral cavity through the hematogenous system to the placenta [140]. It is hypothesized that the same transmission of pathogenic microbes from the oral cavity to the uterus occurs in patients with endometriosis who are more susceptible to oral dysbiosis and are at risk of periodontal disease. Consequently, the infiltration of F. nucleatum into endometrial tissues contributes to disease exacerbation by promoting local inflammation. These observations suggest that oral health may significantly influence the progression and course of endometriosis, as well as successful implantation (Figure 3).
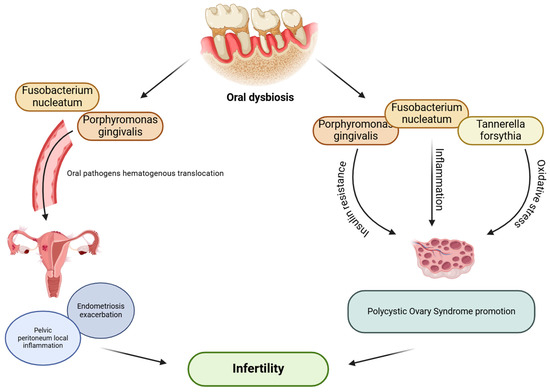
Figure 3.
Possible effect of oral dysbiosis on female infertility. (The figure was created using BioRender.com). Oral dysbiosis and periodontal pathogens associated with it, such as F. nucleatum, P. gingivalis, and T. forsythia, may translocate from disrupted oral microenvironment and affect female reproductive functions; directly, via inducing local inflammation in the pelvic peritoneum among women with endometriosis, causing exacerbation of the disease; or indirectly, via promoting inflammation, insulin resistance, or oxidative stress, which may lead to PCOS development [84,85,86,89,104,137,139].
4. Probiotics Administration in Fertility Disorders
In recent years, probiotics supplementation has emerged as a promising method to improve fertility [141]. Lactobacillus strains are of particular interest, as they might support women’s reproductive health by displacing pathogenic species and producing lactic acid within the endometrial or vaginal flora [142,143,144]. Vaginal administration of probiotics has been proposed to be more effective than oral delivery methods [145]. However, there is a lack of research examining their impact on fertility in women with periodontitis, and data regarding the influence of probiotics on periodontal disease are limited and insufficient to clearly state their benefits [146,147]. Nevertheless, probiotic administration has shown beneficial effects in PCOS patients, improving metabolic parameters crucial for fertility, including HOMA-IR, CRP and total testosterone levels, all of which are correlated with periodontitis [148,149,150]. Unfortunately, there are still limited data on the use of probiotics in women with endometriosis, although two studies have shown positive effects [151,152]. Further investigations are needed to assess the impact of the use of antibiotics or probiotics on the risk of developing both PCOS and endometriosis.
5. Conclusions and Future Perspectives
The human oral microbiome impacts systemic health, including the female reproductive tracts and reproduction. Periodontal pathogens may translocate from the oral cavity to the uterus and can also migrate to the placenta via the hematogenous system. This microbial transmission perturbs the genital microbiome, leading to local inflammation and functional impairment. Moreover, this link between oral health and reproductive functions seems to be bidirectional, as some conditions of the reproductive tract, accompanied by hormonal imbalance and/or ongoing inflammation, create a favorable environment for the development of oral dysbiosis. Disease exacerbation among women of reproductive age may consequently lead to implantation failures or even gestational complications. According to a growing body of research on the oral–reproductive connection, the oral microbial community should be considered as one of the factors influencing female fertility. The overgrowth of pathogenic bacteria in oral cavity affects the female fertility either directly, via bacterial translocation from the oral cavity to the reproductive system via the bloodstream; or indirectly, through the production of proinflammatory mediators. This relationship is particularly notable for F. nucleatum and P. gingivalis, which are associated with adverse pregnancy outcomes, as well as common causes of infertility, such as PCOS and endometriosis.
There are still insufficient data to conclusively determine whether supplementing with probiotics or improving oral health can significantly impact fertility and future conception. Some studies suggest that probiotics administration may help improve health parameters associated with periodontal disease, such as HOMA-IR, CRP, or testosterone levels in women with PCOS. Future research should focus on assessing whether increasing dental health through probiotics, antibiotics, or better oral hygiene might reduce the risk of PCOS, endometriosis, and pregnancy issues. Improving periodontal health through periodontitis treatment may reduce systemic inflammatory responses and increase the chances of successful implantation. Therefore, maintaining good oral hygiene should be a priority for every woman planning a pregnancy, particularly for those at increased risk of periodontal disease.
These insights into the relationship between the female reproductive tract and oral health emphasize the significance of periodontal health among women trying to conceive. Therefore, it is recommended that women preparing for pregnancy be surrounded by professional gynecological, as well as dental healthcare. Enhancing oral hygiene may improve reproductive health by reducing the inflammatory systemic response and alleviating disease symptoms. Future studies should explore the association between periodontal health and female fertility to inform the development of guidelines for women of reproductive age affected by infertility and during pregnancy. Dental prevention strategy may increase the chances of conception and reduce the risk of complications during gestation.
Author Contributions
Conceptualization, J.M.; methodology, J.M.; writing—original draft preparation, J.M.; writing—review and editing, M.J. and J.W.; visualization, J.M.; supervision, M.J. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Kaan, A.M.M.; Kahharova, D.; Zaura, E. Acquisition and Establishment of the Oral Microbiota. Periodontol. 2000 2021, 86, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. D efining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Houte, J.V. Bacterial adherence in oral microbial ecology. Annu. Rev. Microbiol. 1975, 29, 19–42. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Smith, C.; Martin, L.; Haffajee, J.A.; Uzel, N.G.; Goodson, J.M. Use of Checkerboard DNA–DNA Hybridization to Study Complex Microbial Ecosystems. Oral Microbiol. Immunol. 2004, 19, 352–362. [Google Scholar] [CrossRef]
- Socransky, S.S.; Manganiello, S.D. The Oral Microbiota of Man from Birth to Senility. J. Periodontol. 1971, 42, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Chen, T.; Paster, B. A Practical Guide to the Oral Microbiome and Its Relation to Health and Disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Sun, J.; Buys, N. Effects of Probiotics Consumption on Lowering Lipids and CVD Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk Factors for Periodontal Disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Slots, J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in Periodontal Disease: Introduction. Periodontol. 2000 1999, 20, 7–13. [Google Scholar] [CrossRef]
- Kinney, J.S.; Morelli, T.; Braun, T.; Ramseier, C.A.; Herr, A.E.; Sugai, J.V.; Shelburne, C.E.; Rayburn, L.A.; Singh, A.K.; Giannobile, W.V. Saliva/Pathogen Biomarker Signatures and Periodontal Disease Progression. J. Dent. Res. 2011, 90, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; Lalsiamthara, J.; Peng, Y.; Qi, L.; Deng, S.; Wang, Q. Current Research Progress on Prevotella Intermedia and Associated Diseases. Crit. Rev. Microbiol. 2024, 1–18, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Virulence Mechanisms of Tannerella Forsythia: Virulence Mechanisms of Tannerella Forsythia. Periodontol. 2000 2010, 54, 106–116. [Google Scholar] [CrossRef]
- Mohanty, R.; Asopa, S.; Joseph, M.D.; Singh, B.; Rajguru, J.; Saidath, K.; Sharma, U. Red Complex: Polymicrobial Conglomerate in Oral Flora: A Review. J. Fam. Med. Prim. Care 2019, 8, 3480. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Becker, M.R.; Paster, B.J.; Leys, E.J.; Moeschberger, M.L.; Kenyon, S.G.; Galvin, J.L.; Boches, S.K.; Dewhirst, F.E.; Griffen, A.L. Molecular Analysis of Bacterial Species Associated with Childhood Caries. J. Clin. Microbiol. 2002, 40, 1001–1009. [Google Scholar] [CrossRef]
- Pisano, M. Oral Dysbiosis and Systemic Diseases: A Two-Way Relationship? Medicina 2023, 59, 1933. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Lan, Z.; Liu, W.-J.; Cui, H.; Zou, K.-L.; Chen, H.; Zhao, Y.-Y.; Yu, G.-T. The Role of Oral Microbiota in Cancer. Front. Microbiol. 2023, 14, 1253025. [Google Scholar] [CrossRef] [PubMed]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Amador, G.; Denis, F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11157. [Google Scholar] [CrossRef] [PubMed]
- Singhrao, S.K.; Harding, A. Is Alzheimer’s Disease a Polymicrobial Host Microbiome Dysbiosis? Expert Rev. Anti Infect. Ther. 2020, 18, 275–277. [Google Scholar] [CrossRef]
- Wan, J.; Fan, H. Oral Microbiome and Alzheimer’s Disease. Microorganisms 2023, 11, 2550. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, B.; Xing, H.; Yang, S.; Xu, J.; Liu, H. Patients with Chronic Obstructive Pulmonary Disease Suffer from Worse Periodontal Health—Evidence from a Meta-Analysis. Front. Physiol. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Harish, Y.; Hiremath, S.; Puranik, M. A Cross-Sectional Survey to Study the Relationship of Periodontal Disease with Cardiovascular Disease, Respiratory Disease, and Diabetes Mellitus. J. Indian Soc. Periodontol. 2016, 20, 446. [Google Scholar] [CrossRef]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of Incident Cardiovascular Disease in People with Periodontal Disease: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, D.; Chen, R.; Zhu, F.; Gong, J.; Yan, F. The Association between Periodontitis and Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Sun, B.; Li, L.; Sun, Z.; Zhu, X.; Zhong, X.; Xu, Y. The Oral Microbiome Analysis Reveals the Similarities and Differences between Periodontitis and Crohn’s Disease-Associated Periodontitis. FEMS Microbiol. Lett. 2022, 369, fnac054. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Racicot, K.; Mor, G. The Role of Inflammation for a Successful Implantation. Am. J. Reprod. Immunol. 2014, 72, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nwhator, S.; Opeodu, O.; Ayanbadejo, P.; Umeizudike, K.; Olamijulo, J.; Alade, G.; Agbelusi, G.; Arowojolu, M.; Sorsa, T. Could Periodontitis Affect Time to Conception? Ann. Med. Health Sci. Res. 2014, 4, 817. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Doherty, D.A.; Pennell, C.E.; Newnham, I.A.; Newnham, J.P. Periodontal Disease: A Potential Modifiable Risk Factor Limiting Conception. Hum. Reprod. 2012, 27, 1332–1342. [Google Scholar] [CrossRef]
- Paju, S.; Oittinen, J.; Haapala, H.; Asikainen, S.; Paavonen, J.; Pussinen, P.J. Porphyromonas gingivalis May Interfere with Conception in Women. J. Oral Microbiol. 2017, 9, 1330644. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Magon, N. Hormones in Pregnancy. Niger. Med. J. 2012, 53, 179. [Google Scholar] [CrossRef]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Llurba Olivé, E.; García Algar, O.; Solana, M.J.; Cabero Perez, M.J.; Sainz, T.; Martinez, L.; et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef]
- Kornman, K.S.; Loesche, W.J. Effects of Estradiol and Progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect. Immun. 1982, 35, 256–263. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tsuruda, K.; Iwamoto, Y.; Kato, F.; Odaki, T.; Yamane, N.; Hori, Y.; Harashima, Y.; Sakoda, A.; Tagaya, A.; et al. Significant Increase of Oral Bacteria in the Early Pregnancy Period in Japanese Women. J. Investig. Clin. Dent. 2017, 8, e12189. [Google Scholar] [CrossRef]
- Sooriyamoorthy, M.; Gower, D.B. Hormonal Influences on Gingival Tissue: Relationship to Periodontal Disease. J. Clin. Periodontol. 1989, 16, 201–208. [Google Scholar] [CrossRef]
- Vittek, J.; Hernandez, M.R.; Wenk, E.J.; Rappaport, S.C.; Southren, A.L. Specific Estrogen Receptors in Human Gingiva. J. Clin. Endocrinol. Metab. 1982, 54, 608–612. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Wang, L.; Gu, Z.; Ma, L. Bibliometric Analysis of the Knowledge Landscape of Periodontal Disease in Pregnancy: A Noteworthy Multidisciplinary Issue. J. Multidiscip. Healthc. 2023, 16, 3941–3957. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, S.-W.; Su, W.-L.; Zhu, H.-Y.; Ouyang, S.-Y.; Cao, Y.-T.; Jiang, S.-Y. Sex Hormones Enhance Gingival Inflammation without Affecting IL-1 β and TNF- α in Periodontally Healthy Women during Pregnancy. Mediators Inflamm. 2016, 2016, 4897890. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Timofeeva, I.; Bouchoucha, E.; Canceill, T.; Champion, C.; Groussolles, M.; Arnaud, C.; Vayssière, C.; Nabet, C.; Laurencin-Dalicieux, S. Oral and Periodontal Assessment at the First Trimester of Pregnancy: The PERISCOPE Longitudinal Study. Acta Obstet. Gynecol. Scand. 2023, 102, 669–680. [Google Scholar] [CrossRef]
- Massoni, R.S.D.S.; Aranha, A.M.F.; Matos, F.Z.; Guedes, O.A.; Borges, Á.H.; Miotto, M.; Porto, A.N. Correlation of Periodontal and Microbiological Evaluations, with Serum Levels of Estradiol and Progesterone, during Different Trimesters of Gestation. Sci. Rep. 2019, 9, 11762. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Jiang, W.; Hu, X.; Gao, L.; Ai, D.; Pan, H.; Niu, C.; Yuan, K.; Zhou, X.; Xu, C.; et al. Ecological Shifts of Supragingival Microbiota in Association with Pregnancy. Front. Cell. Infect. Microbiol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Balan, P.; Chong, Y.S.; Umashankar, S.; Swarup, S.; Loke, W.M.; Lopez, V.; He, H.G.; Seneviratne, C.J. Keystone Species in Pregnancy Gingivitis: A Snapshot of Oral Microbiome During Pregnancy and Postpartum Period. Front. Microbiol. 2018, 9, 2360. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Han, Y.W.; Shen, T.; Chung, P.; Buhimschi, I.A.; Buhimschi, C.S. Uncultivated Bacteria as Etiologic Agents of Intra-Amniotic Inflammation Leading to Preterm Birth. J. Clin. Microbiol. 2009, 47, 38–47. [Google Scholar] [CrossRef]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of Diverse Oral Bacteria to Murine Placenta: Evidence for the Oral Microbiome as a Potential Source of Intrauterine Infection. Infect. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- Wen, X.; Fu, X.; Zhao, C.; Yang, L.; Huang, R. The Bidirectional Relationship between Periodontal Disease and Pregnancy via the Interaction of Oral Microorganisms, Hormone and Immune Response. Front. Microbiol. 2023, 14, 1070917. [Google Scholar] [CrossRef]
- Ye, C.; Kapila, Y. Oral Microbiome Shifts during Pregnancy and Adverse Pregnancy Outcomes: Hormonal and Immunologic Changes at Play. Periodontol. 2000 2021, 87, 276–281. [Google Scholar] [CrossRef]
- Moore, S.; Ide, M.; Coward, P.Y.; Randhawa, M.; Borkowska, E.; Baylis, R.; Wilson, R.F. A Prospective Study to Investigate the Relationship between Periodontal Disease and Adverse Pregnancy Outcome. Br. Dent. J. 2004, 197, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.; Ide, M.; Wilson, R.F. The Relationship between Maternal Periodontitis, Adverse Pregnancy Outcome and Miscarriage in Never Smokers. J. Clin. Periodontol. 2006, 33, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Konopka, T.; Zakrzewska, A. Periodontitis and Risk for Preeclampsia—A Systematic Review. Ginekol. Pol. 2020, 91, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral Microflora and Pregnancy: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef]
- Barak, S.; Oettinger-Barak, O.; Machtei, E.E.; Sprecher, H.; Ohel, G. Evidence of Periopathogenic Microorganisms in Placentas of Women with Preeclampsia. J. Periodontol. 2007, 78, 670–676. [Google Scholar] [CrossRef]
- Lima, K.M.; Alves, C.M.; Vidal, F.C.; Gomes-Filho, I.S.; Costa, J.C.; Coletta, R.D.; Rodrigues, V.P.; Lopes, F.F. Fusobacterium Nucleatum and Prevotella in Women with Periodontitis and Preterm Birth. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e450–e456. [Google Scholar] [CrossRef]
- Cooper, S.M.; Borgida, A.; Thacker, S.; Hammer, E.; Hariharan, A.; Kuo, C.; Blanck, N.; Yuan, H.; Panier, H.; Lin, Q.; et al. Oral Origin of the Placenta Microbiome in Pregnant Women with Preeclampsia. Front. Bacteriol. 2024, 2, 1322165. [Google Scholar] [CrossRef]
- Shaggag, L.M.; ALhabardi, N.; Adam, I. The Association between Maternal Periodontitis and Preterm Birth: A Case-Control Study in a Low-Resource Setting in Sudan, Africa. Medicina 2022, 58, 632. [Google Scholar] [CrossRef]
- De Oliveira, L.J.C.; Cademartori, M.G.; Schuch, H.S.; Barros, F.C.; Silveira, M.F.; Correa, M.B.; Demarco, F.F. Periodontal Disease and Preterm Birth: Findings from the 2015 Pelotas Birth Cohort Study. Oral Dis. 2021, 27, 1519–1527. [Google Scholar] [CrossRef]
- Katz, J.; Chegini, N.; Shiverick, K.T.; Lamont, R.J. Localization of P. gingivalis in Preterm Delivery Placenta. J. Dent. Res. 2009, 88, 575–578. [Google Scholar] [CrossRef]
- Ye, C.; Katagiri, S.; Miyasaka, N.; Kobayashi, H.; Khemwong, T.; Nagasawa, T.; Izumi, Y. The Periodontopathic Bacteria in Placenta, Saliva and Subgingival Plaque of Threatened Preterm Labor and Preterm Low Birth Weight Cases: A Longitudinal Study in Japanese Pregnant Women. Clin. Oral Investig. 2020, 24, 4261–4270. [Google Scholar] [CrossRef] [PubMed]
- Haerian-Ardakani, A.; Haerian, A. Relationship between Maternal Periodontal Disease and Low Birth Weight Babies. Iran J. Reprod. Med. 2013, 11, 625–630. [Google Scholar] [PubMed]
- Loos, B.G. Systemic Markers of Inflammation in Periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, L.; Kang, J.; Huang, Z.; Sha, Y.; Zhu, W.; Yan, L. Relationship between the preterm low birth weight infant and the periodontal pathogen bacteria in maternal saliva. Beijing Da Xue Xue Bao Yi Xue Ban 2012, 44, 29–33. [Google Scholar]
- Kothwiale, S.V.; Desai, B.R.; Kothwiale, V.A.; Ganhid, M.; Konin, S. Periodontal Disease as a Potential Risk Factor for Low Birth Weight and Reduced Maternal Haemomglobin Levels. Oral Health Prev. Dent. 2014, 12, 83–90. [Google Scholar] [CrossRef]
- Perunovic, N.D.J.; Rakic, M.M.; Nikolic, L.I.; Jankovic, S.M.; Aleksic, Z.M.; Plecas, D.V.; Madianos, P.N.; Cakic, S.S. The Association Between Periodontal Inflammation and Labor Triggers (Elevated Cytokine Levels) in Preterm Birth: A Cross-Sectional Study. J. Periodontol. 2016, 87, 248–256. [Google Scholar] [CrossRef]
- Kunnen, A.; Van Pampus, M.; Aarnoudse, J.; Van Der Schans, C.; Abbas, F.; Faas, M. The Effect of Porphyromonas gingivalis Lipopolysaccharide on Pregnancy in the Rat. Oral Dis. 2014, 20, 591–601. [Google Scholar] [CrossRef]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium nucleatum Induces Premature and Term Stillbirths in Pregnant Mice: Implication of Oral Bacteria in Preterm Birth. Infect. Immun. 2004, 72, 2272–2279. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Polyzos, N.P.; Polyzos, I.P.; Mauri, D.; Tzioras, S.; Tsappi, M.; Cortinovis, I.; Casazza, G. Effect of Periodontal Disease Treatment during Pregnancy on Preterm Birth Incidence: A Metaanalysis of Randomized Trials. Am. J. Obstet. Gynecol. 2009, 200, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kurien, S.; Kattimani, V.S.; Sriram, R.R.; Sriram, S.K.; Bhupathi, A.; Bodduru, R.R.; Patil, N.N. Management of Pregnant Patient in Dentistry. J. Int. Oral Health 2013, 5, 88–97. [Google Scholar] [PubMed]
- Bostanci, N. Periodontal Health and Pregnancy Outcomes: Time to Deliver. Acta Obstet. Gynecol. Scand. 2023, 102, 648–651. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; De Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary of ART Terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021, 1st ed.; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-006831-5. [Google Scholar]
- Koroma, L.; Stewart, L. Infertility: Evaluation and Initial Management. J. Midwifery Womens Health 2012, 57, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Jafri, Z.; Bhardwaj, A.; Sawai, M.; Sultan, N. Influence of Female Sex Hormones on Periodontium: A Case Series. J. Nat. Sci. Biol. Med. 2015, 6, 146. [Google Scholar] [CrossRef]
- Jakubowska-Kowal, K.M.; Skrzynska, K.J.; Gawlik-Starzyk, A.M. Prevalence and Diagnosis of Polycystic Ovary Syndrome (PCOS) in Adolescents—What’s New in 2023? Systematic Review. Ginekol. Pol. 2024, 95, 643–649. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Achu Joseph, R.; Ajitkumar, S.; Subbusamy Kanakasabapathy, B.; Santhanakrishnan, M. Evaluation of Microbial Profile in Patients with Polycystic Ovary Syndrome and Periodontal Disease: A Case-Control Study. Int. J. Fertil. Steril. 2023, 17, 248–253. [Google Scholar] [CrossRef]
- Jaglan, S.; Tewari, S.; Singhal, S.R.; Sharma, R.K. Impact of Polycystic Ovary Syndrome on Periodontal Status of Women of Adolescent and Adult Age Groups: A Cross-Sectional Study. Med. Princ. Pract. 2024, 33, 148–156. [Google Scholar] [CrossRef]
- Pavankumar, S.; Yellarthi, P.K.; Sandeep, J.N.; Boyapati, R.; Damera, T.K. Evaluation of Periodontal Status in Women with Polycystic Ovary Syndrome versus Healthy Women: A Cross-Sectional Study. J. Yeungnam Med. Sci. 2023, 40, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Escalda, C.; Proença, L.; Mendes, J.J.; Botelho, J. Is There a Bidirectional Association between Polycystic Ovarian Syndrome and Periodontitis? A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1961. [Google Scholar] [CrossRef]
- Tanguturi, S.; Nagarakanti, S. Polycystic Ovary Syndrome and Periodontal Disease: Underlying Links- A Review. Indian J. Endocrinol. Metab. 2018, 22, 267. [Google Scholar] [CrossRef] [PubMed]
- Özçaka, Ö.; Buduneli, N.; Ceyhan, B.O.; Akcali, A.; Hannah, V.; Nile, C.; Lappin, D.F. Is Interleukin-17 Involved in the Interaction Between Polycystic Ovary Syndrome and Gingival Inflammation? J. Periodontol. 2013, 84, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Özçaka, Ö.; Ceyhan, B.Ö.; Akcali, A.; Biçakci, N.; Lappin, D.F.; Buduneli, N. Is There an Interaction Between Polycystic Ovary Syndrome and Gingival Inflammation? J. Periodontol. 2012, 83, 1529–1537. [Google Scholar] [CrossRef]
- Tsilchorozidou, T.; Overton, C.; Conway, G.S. The Pathophysiology of Polycystic Ovary Syndrome. Clin. Endocrinol. 2004, 60, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Asnani, K.; Hingorani, D.; Kheur, S.; Deshmukh, V.; Romanos, G. Expression of Nuclear Receptors of Gingiva in Polycystic Ovarian Syndrome: A Preliminary Case Study. Aust. Dent. J. 2014, 59, 252–257. [Google Scholar] [CrossRef]
- Dou, Y.; Xin, J.; Zhou, P.; Tang, J.; Xie, H.; Fan, W.; Zhang, Z.; Wu, D. Bidirectional Association between Polycystic Ovary Syndrome and Periodontal Diseases. Front. Endocrinol. 2023, 14, 1008675. [Google Scholar] [CrossRef]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.-Å.; Laine, M. Estrogen Receptor- Is the Predominant Estrogen Receptor Subtype in Human Oral Epithelium and Salivary Glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef]
- Nebel, D.; Bratthall, G.; Ekblad, E.; Norderyd, O.; Nilsson, B.-O. Estrogen Regulates DNA Synthesis in Human Gingival Epithelial Cells Displaying Strong Estrogen Receptor β Immunoreactivity: Estrogen Receptors in Human Gingiva. J. Periodontal Res. 2011, 46, 622–628. [Google Scholar] [CrossRef]
- Palanisamy, S. The Impact of Estrogen on Periodontal Tissue Integrity and Inflammation—A Mini Review. Front. Dent. Med. 2025, 6, 1455755. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A. Sex Steroid Hormones and Cell Dynamics in the Periodontium. Crit. Rev. Oral Biol. Med. 1994, 5, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Ojanotko-Harri, A.; Forssell, H.; Laine, M.; Hurttia, H.; Bläuer, M.; Tuohimaa, P. Immunohistochemical Detection of Androgen Receptors in Human Oral Mucosa. Arch. Oral Biol. 1992, 37, 511–514. [Google Scholar] [CrossRef]
- Gurav, A.N. Periodontitis and Insulin Resistance: Casual or Causal Relationship? Diabetes Metab. J. 2012, 36, 404. [Google Scholar] [CrossRef]
- Minty, M.; Canceil, T.; Serino, M.; Burcelin, R.; Tercé, F.; Blasco-Baque, V. Oral Microbiota-Induced Periodontitis: A New Risk Factor of Metabolic Diseases. Rev. Endocr. Metab. Disord. 2019, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic Ovary Syndrome: Insight into Pathogenesis and a Common Association with Insulin Resistance. Clin. Med. 2016, 16, 262–266. [Google Scholar] [CrossRef]
- Rathi, N.; Reche, A. Risk of Periodontal Diseases in Women with Polycystic Ovary Syndrome: An Overview. Cureus 2023, 15, e47169. [Google Scholar] [CrossRef]
- Farook, F.F.; Ng, K.T.; Mnm, N.; Koh, W.J.; Teoh, W.Y. Association of Periodontal Disease and Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Open Dent. J. 2019, 13, 478–487. [Google Scholar] [CrossRef]
- Márquez-Arrico, C.F.; Silvestre-Rangil, J.; Gutiérrez-Castillo, L.; Martinez-Herrera, M.; Silvestre, F.J.; Rocha, M. Association between Periodontal Diseases and Polycystic Ovary Syndrome: A Systematic Review. J. Clin. Med. 2020, 9, 1586. [Google Scholar] [CrossRef]
- Rahiminejad, M.; Moaddab, A.; Zaryoun, H.; Rabiee, S.; Moaddab, A.; Khodadoustan, A. Comparison of Prevalence of Periodontal Disease in Women with Polycystic Ovary Syndrome and Healthy Controls. Dent. Res. J. 2015, 12, 507. [Google Scholar] [CrossRef]
- Dursun, E.; Akalın, F.A.; Güncü, G.N.; Çınar, N.; Aksoy, D.Y.; Tözüm, T.F.; Kılınc, K.; Yıldız, B.O. Periodontal Disease in Polycystic Ovary Syndrome. Fertil. Steril. 2011, 95, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Porwal, S.; Tewari, S.; Sharma, R.K.; Singhal, S.R.; Narula, S.C. Periodontal Status and High-Sensitivity C-Reactive Protein Levels in Polycystic Ovary Syndrome with and Without Medical Treatment. J. Periodontol. 2014, 85, 1380–1389. [Google Scholar] [CrossRef]
- Akcalı, A.; Bostanci, N.; Özçaka, Ö.; Öztürk-Ceyhan, B.; Gümüş, P.; Buduneli, N.; Belibasakis, G.N. Association between Polycystic Ovary Syndrome, Oral Microbiota and Systemic Antibody Responses. PLoS ONE 2014, 9, e108074. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Q.; Li, J.; Yang, S.; Shi, Q. Progression of Periodontal Inflammation in Adolescents Is Associated with Increased Number of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Fusobacterium nucleatum. Int. J. Paediatr. Dent. 2014, 24, 226–233. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Qian, C.; Liu, Q.; Cao, W.; Ma, M.; He, R.; Chen, R.; Geng, R.; Liu, Y. Dysbiosis of the Saliva Microbiome in Patients with Polycystic Ovary Syndrome. Front. Cell. Infect. Microbiol. 2021, 10, 624504. [Google Scholar] [CrossRef] [PubMed]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Pieber, T.R.; Gorkiewicz, G.; Obermayer-Pietsch, B. The Salivary Microbiome in Polycystic Ovary Syndrome (PCOS) and Its Association with Disease-Related Parameters: A Pilot Study. Front. Microbiol. 2016, 7, 1270. [Google Scholar] [CrossRef]
- Liu, B.; Faller, L.L.; Klitgord, N.; Mazumdar, V.; Ghodsi, M.; Sommer, D.D.; Gibbons, T.R.; Treangen, T.J.; Chang, Y.-C.; Li, S.; et al. Deep Sequencing of the Oral Microbiome Reveals Signatures of Periodontal Disease. PLoS ONE 2012, 7, e37919. [Google Scholar] [CrossRef]
- Wang, J.; Qi, J.; Zhao, H.; He, S.; Zhang, Y.; Wei, S.; Zhao, F. Metagenomic Sequencing Reveals Microbiota and Its Functional Potential Associated with Periodontal Disease. Sci. Rep. 2013, 3, 1843. [Google Scholar] [CrossRef] [PubMed]
- Deepti; Tewari, S.; Narula, S.C.; Singhal, S.R.; Sharma, R.K. Effect of Non-Surgical Periodontal Therapy Along with Myo-Inositol on High-Sensitivity C-Reactive Protein and Insulin Resistance in Women with Polycystic Ovary Syndrome and Chronic Periodontitis: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 999–1011. [Google Scholar] [CrossRef]
- Luthra, S.; Orlandi, M.; Hussain, S.B.; Leira, Y.; Botelho, J.; Machado, V.; Mendes, J.J.; Marletta, D.; Harden, S.; D’Aiuto, F. Treatment of Periodontitis and C -reactive Protein: A Systematic Review and Meta-analysis of Randomized Clinical Trials. J. Clin. Periodontol. 2023, 50, 45–60. [Google Scholar] [CrossRef]
- Wendland, N.; Opydo-Szymaczek, J.; Formanowicz, D.; Blacha, A.; Jarząbek-Bielecka, G.; Mizgier, M. Association between Metabolic and Hormonal Profile, Proinflammatory Cytokines in Saliva and Gingival Health in Adolescent Females with Polycystic Ovary Syndrome. BMC Oral Health 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High Prevalence of Endometriosis in Infertile Women with Normal Ovulation and Normospermic Partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and Infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef]
- Shan, J.; Ni, Z.; Cheng, W.; Zhou, L.; Zhai, D.; Sun, S.; Yu, C. Gut Microbiota Imbalance and Its Correlations with Hormone and Inflammatory Factors in Patients with Stage 3/4 Endometriosis. Arch. Gynecol. Obstet. 2021, 304, 1363–1373. [Google Scholar] [CrossRef]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef] [PubMed]
- Talwar, C.; Singh, V.; Kommagani, R. The Gut Microbiota: A Double-Edged Sword in Endometriosis. Biol. Reprod. 2022, 107, ioac147. [Google Scholar] [CrossRef]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis Induces Gut Microbiota Alterations in Mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef]
- Leonardi, M.; Hicks, C.; El-Assaad, F.; El-Omar, E.; Condous, G. Endometriosis and the Microbiome: A Systematic Review. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 239–249. [Google Scholar] [CrossRef]
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular Detection of Microbial Colonization in Cervical Mucus of Women with and without Endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147. [Google Scholar] [CrossRef] [PubMed]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The Role of Gut and Genital Microbiota and the Estrobolome in Endometriosis, Infertility and Chronic Pelvic Pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wu, Y.; Chai, X.; Wu, X. The Colonized Microbiota Composition in the Peritoneal Fluid in Women with Endometriosis. Arch. Gynecol. Obstet. 2022, 305, 1573–1580. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota Composition and Distribution along the Female Reproductive Tract of Women with Endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15. [Google Scholar] [CrossRef]
- Lee, S.-R.; Lee, J.-C.; Kim, S.-H.; Oh, Y.-S.; Chae, H.-D.; Seo, H.; Kang, C.-S.; Shin, T.-S. Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid. Int. J. Mol. Sci. 2021, 22, 4608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The Gut Microbiota and Endometriosis: From Pathogenesis to Diagnosis and Treatment. Front. Cell. Infect. Microbiol. 2022, 12, 1069557. [Google Scholar] [CrossRef]
- Kavoussi, S.K.; West, B.T.; Taylor, G.W.; Lebovic, D.I. Periodontal Disease and Endometriosis: Analysis of the National Health and Nutrition Examination Survey. Fertil. Steril. 2009, 91, 335–342. [Google Scholar] [CrossRef]
- Thomas, V.; Uppoor, A.; Pralhad, S.; Naik, D.; Kushtagi, P. Towards a Common Etiopathogenesis: Periodontal Disease and Endometriosis. J. Hum. Reprod. Sci. 2018, 11, 269. [Google Scholar] [CrossRef]
- Ono, Y.; Kobayashi, Y.; Shimada, S.; Fukushi, Y.; Yoshino, O.; Wada, S.; Yamada, H. Uterine Endometrium Microbiome in Women with Repeated Implantation Failure Complicated by Endometriosis. J. Clin. Med. 2024, 13, 4605. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, C.T.L.; Gornic, C.; Barbosa, A.S.; Peixoto, R.J.M.; Colombo, A.P.V. Detection of Dialister Pneumosintes in the Subgingival Biofilm of Subjects with Periodontal Disease. Anaerobe 2007, 13, 244–248. [Google Scholar] [CrossRef]
- Doan, N.; Contreras, A.; Flynn, J.; Slots, J.; Chen, C. Molecular Identification of Dialister Pneumosintes in Subgingival Plaque of Humans. J. Clin. Microbiol. 2000, 38, 3043–3047. [Google Scholar] [CrossRef]
- Shaikh, H.F.M.; Oswal, P.U.; Kugaji, M.S.; Katti, S.S.; Bhat, K.G.; Kandaswamy, E.; Joshi, V.M. Association of F. Alocis and D. Pneumosintes with Periodontitis Disease Severity and Red Complex Bacteria. Dent. J. 2024, 12, 105. [Google Scholar] [CrossRef]
- Gare, J.; Kanoute, A.; Meda, N.; Viennot, S.; Bourgeois, D.; Carrouel, F. Periodontal Conditions and Pathogens Associated with Pre-Eclampsia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 7194. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, P.; Liu, P.; Wang, Y.; Guo, Y.; Wang, C.; Jia, Y.; Zou, R.; Dong, S.; Niu, L. Association between Periodontitis and Endometriosis: A Bidirectional Mendelian Randomization Study. Front. Endocrinol. 2024, 15, 1271351. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Saunders, P.T.K.; Horne, A.W. The Role of the Peritoneum in the Pathogenesis of Endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium Infection Facilitates the Development of Endometriosis through the Phenotypic Transition of Endometrial Fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef] [PubMed]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium Nucleatum and Adverse Pregnancy Outcomes: Epidemiological and Mechanistic Evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef]
- Blancafort, C.; Llácer, J. Can Probiotics Enhance Fertility Outcome? Capacity of Probiotics as a Single Intervention to Improve the Feminine Genital Tract Microbiota in Non-Symptomatic Reproductive-Aged Women. Front. Endocrinol. 2023, 13, 1081830. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The Role of Lactic Acid Production by Probiotic Lactobacillus Species in Vaginal Health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Tomusiak, A.; Strus, M.; Heczko, P.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and Safety of a Vaginal Medicinal Product Containing Three Strains of Probiotic Bacteria: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Drug Des. Dev. Ther. 2015, 9, 5345–5354. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Chatterjee, A.; Raghunathan, V. Probiotics in the Treatment of Periodontal Disease: A Systematic Review. J. Indian Soc. Periodontol. 2016, 20, 488. [Google Scholar] [CrossRef]
- Baddouri, L.; Hannig, M. Probiotics as an Adjunctive Therapy in Periodontitis Treatment—Reality or Illusion—A Clinical Perspective. Npj Biofilms Microbiomes 2024, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Probiot. Antimicrob. Proteins 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Calcaterra, V.; Rossi, V.; Massini, G.; Casini, F.; Zuccotti, G.; Fabiano, V. Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity. Nutrients 2023, 15, 3144. [Google Scholar] [CrossRef]
- Miao, C.; Guo, Q.; Fang, X.; Chen, Y.; Zhao, Y.; Zhang, Q. Effects of Probiotic and Synbiotic Supplementation on Insulin Resistance in Women with Polycystic Ovary Syndrome: A Meta-Analysis. J. Int. Med. Res. 2021, 49, 03000605211031758. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the Induced Endometriosis in Rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).