Effects of NatureKnit™, a Blend of Fruit and Vegetable Fibers Rich in Naturally Occurring Bound Polyphenols, on the Metabolic Activity and Community Composition of the Human Gut Microbiome Using the M-SHIME® Gastrointestinal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Products
2.2. Short-Term Colonic Simulation
2.3. Fermentation Profiling and Microbial Metabolic Activity
2.4. DNA Extraction and 16S rRNA Sequencing
2.5. Quantification of Total Bacterial Cells in the Lumen Samples
2.6. Statistical Analysis
3. Results
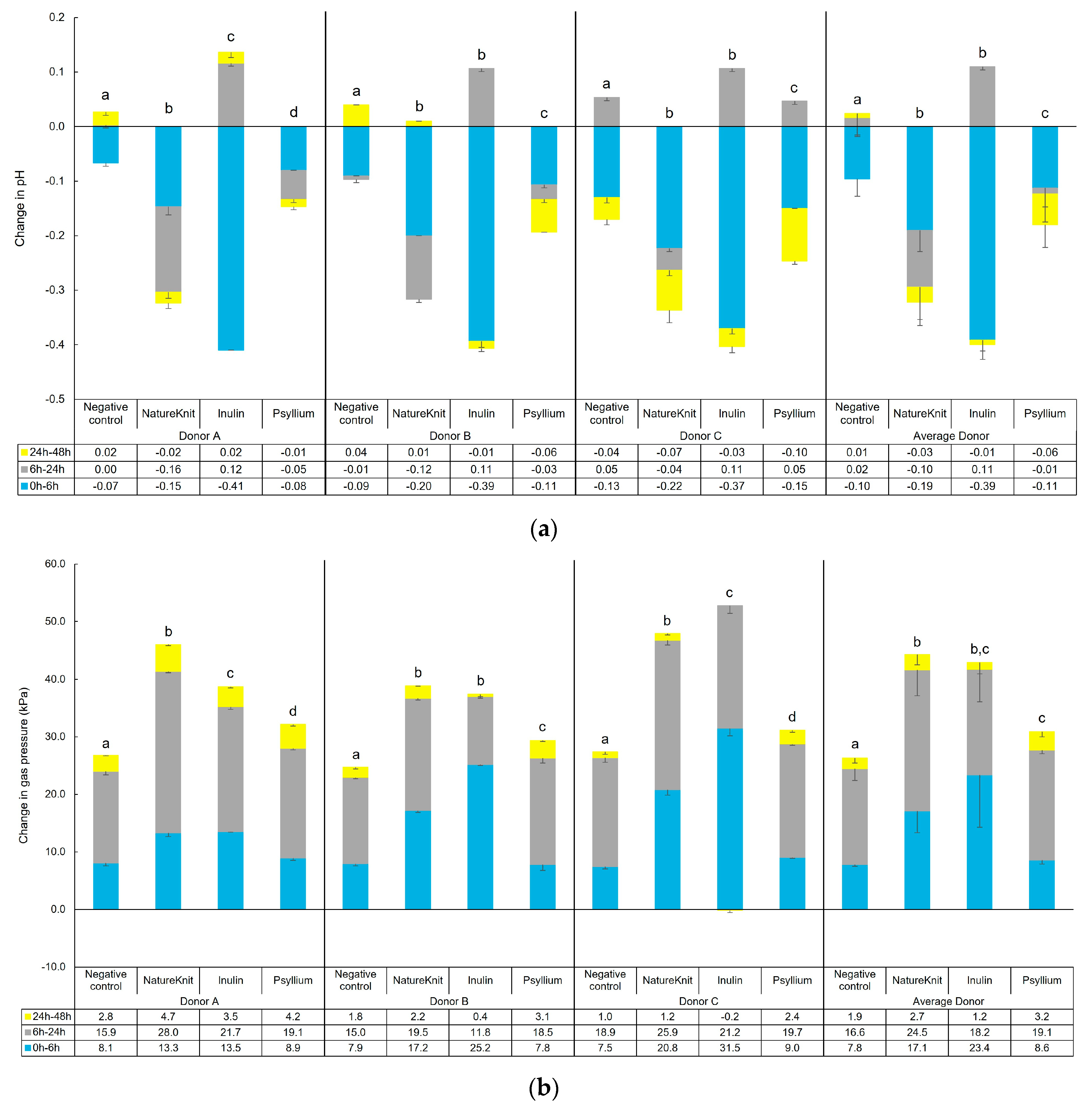
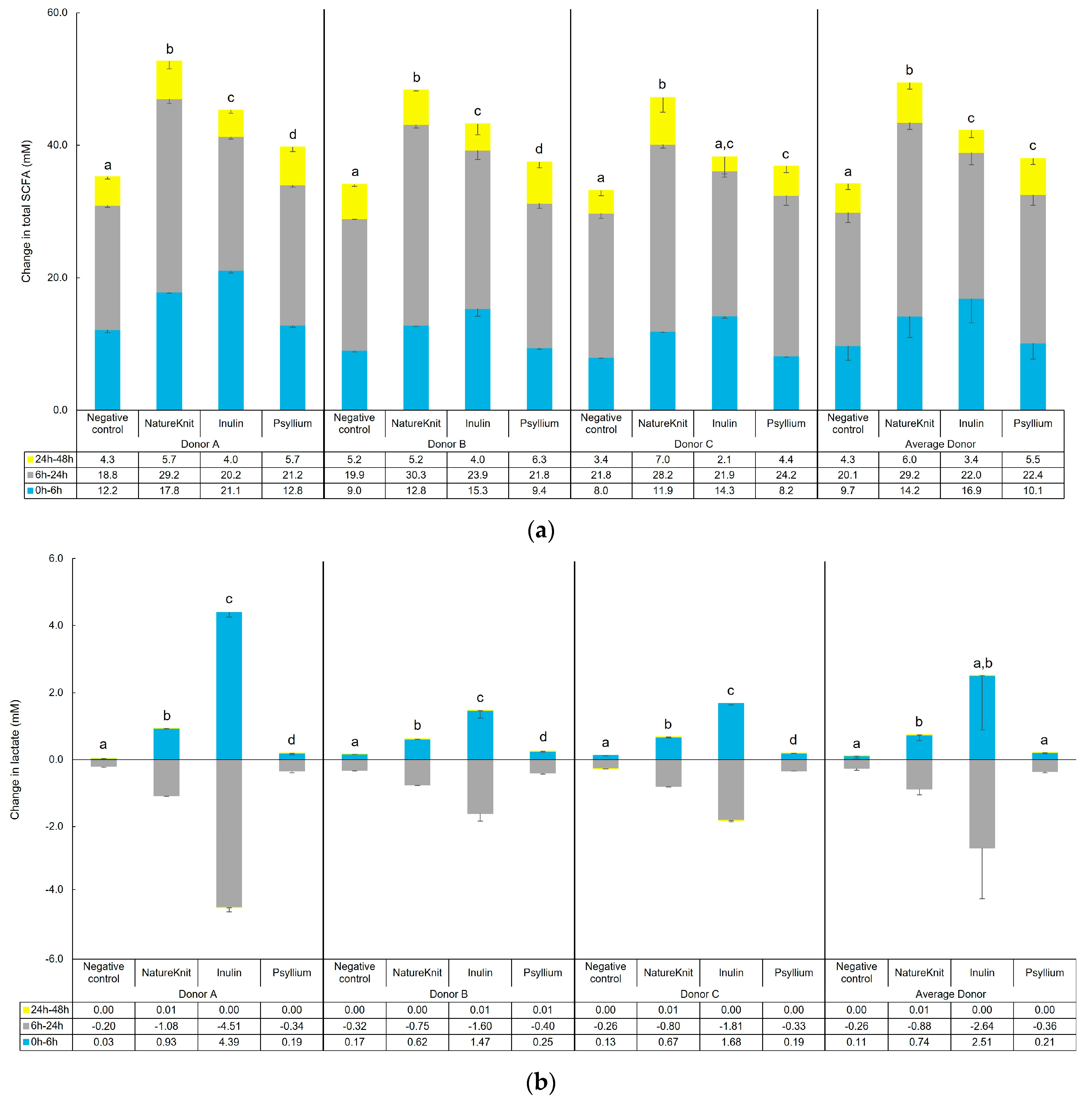
3.1. Fermentation Profile
3.2. Microbial Metabolic Activity
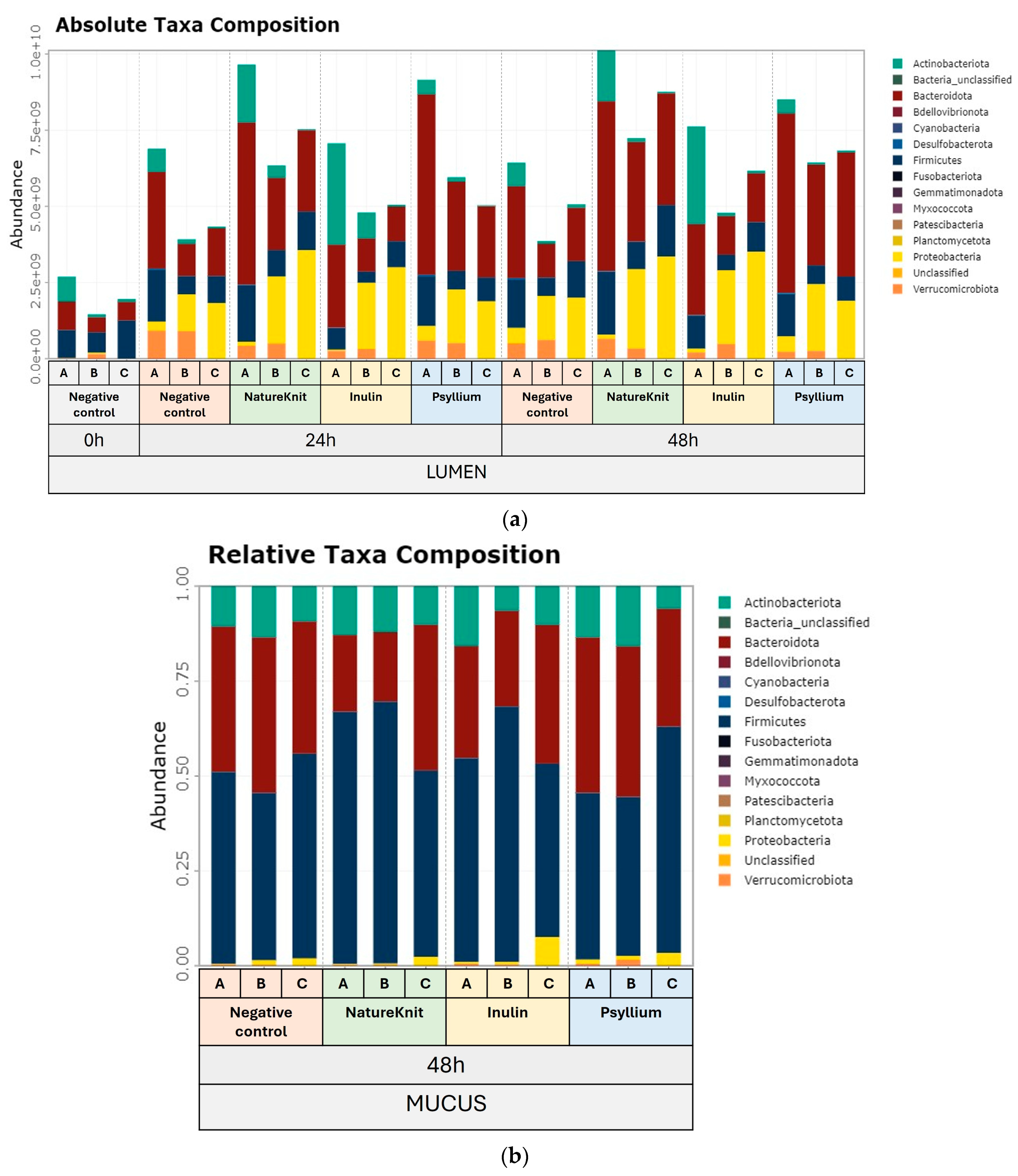
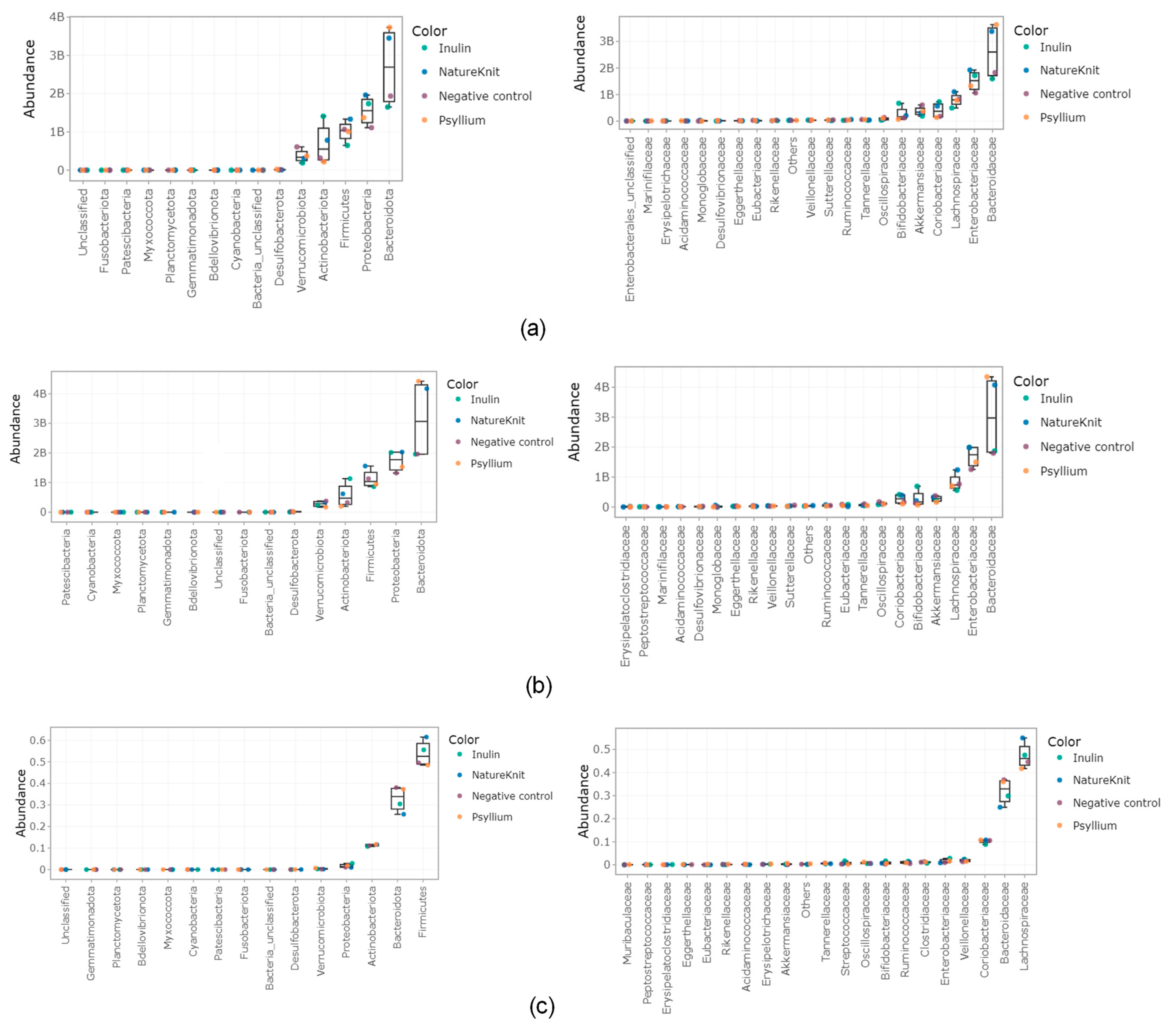
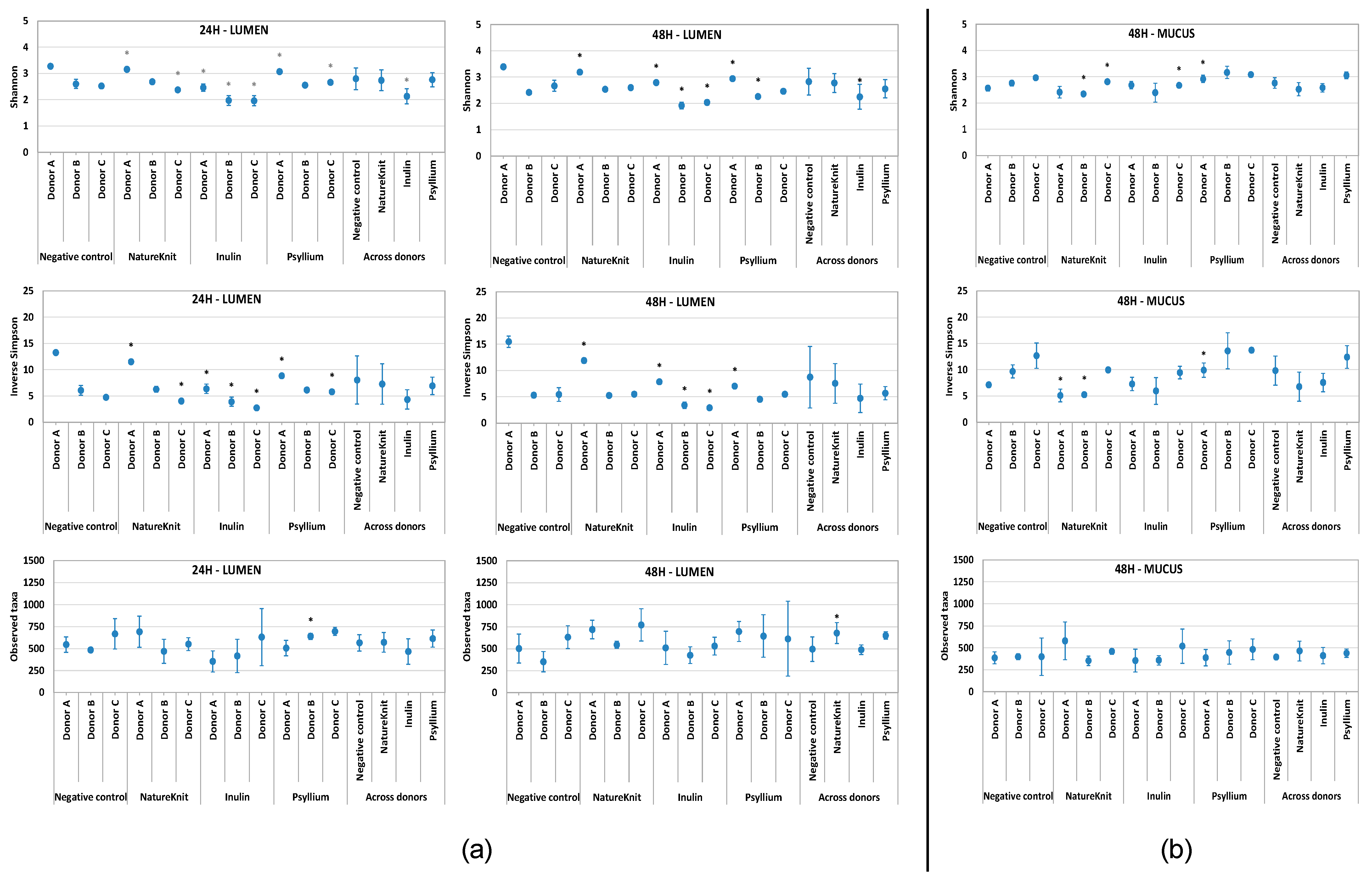
3.3. Microbial Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| M-SHIME® | Mucosal Simulator of the Human Intestinal Microbial Ecosystem |
| SCFA | Short-chain fatty acids |
| GIT | Gastrointestinal tract |
| DNA | Deoxyribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| out | Operational Taxonomic Units |
References
- Dietary Guidelines for Americans 2020–2025. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 30 August 2024).
- Brauchla, M.; Fulgoni, V.L. Cost-effective options for increasing consumption of under-consumed food groups and nutrients in the USA. Public Health Nutr. 2022, 25, 710–716. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.; Takemoto, M.L.S.; Guedes, L.S. Patterns of fiber Intake among Brazilian adults: Perceptions from an online nationwide survey. Arq. Gastroenterol. 2020, 57, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Guzman, C.; Espinoza, J.; Fuentealba, F. Pilot study to estimate dietary fiber intake in adults residing in Chile. Nutrients 2023, 15, 900. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Su, C.; Zhang, J.; Zhai, F.Y.; Zhang, B. Trends in dietary fiber intake in Chinese aged 45 years and above, 1991–2011. Eur. J. Clin. Nutr. 2014, 68, 619–622. [Google Scholar] [CrossRef]
- The National Health and Nutrition Survey (NHNS) Japan. 2019. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2019.pdf (accessed on 30 August 2024).
- Sekgala, M.D.; McHiza, Z.J.; Parker, W.A.; Monyeki, K.D. Dietary fiber intake and metabolic syndrome risk factors among young South African adults. Nutrients 2018, 10, 504. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary fibre intake in Australia. Paper I: Associations with eemographic, socio-economic, and anthropometric factors. Nutrients 2018, 10, 599. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Saura-Calixto, F. The story of the introduction of non-extractable polyphenols into polyphenol research: Origin, development and perspectives. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Perez-Jimenez, J., Eds.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Macagnan, F.T.; Bender, A.B.B.; Speroni, C.S. CHAPTER 2: Dietary Fiber and Non-extractable Polyphenols. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Perez-Jimenez, J., Eds.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gokmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Marostica, M.R., Jr.; Rodriques, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Comp. Analysis 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.T.; Dubert-Ferrandon, A.; Gareau, M.G.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 22755. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Health benefits of non-digestible oligosaccharides. Adv. Exp. Med. Biol. 1997, 427, 211–219. [Google Scholar]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The prebiotic potential of inulin-type fructans: A systematic review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Villa, M.M.; Durand, H.K.; Jiang, S.; Dallow, E.P.; Petrone, B.L.; Silverman, J.D.; Lin, P.H.; David, L.A. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome 2022, 10, 114. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo, C.; Marino, M.; Merono, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of dietary fibers on short-chain fatty acids and gut microbiota composition in healthy adults: A systematic review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Noack, J.; Timm, D.; Hospattankar, A.; Slavin, J. Fermentation profiles of wheat dextrin, inulin and partially hydrolyzed guar gum using an in vitro digestion pretreatment and in vitro batch fermentation system model. Nutrients 2013, 5, 1500–1510. [Google Scholar] [CrossRef]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J. Food Sci. 2011, 76, H137–H142. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef]
- Biagini, F.; Daddi, C.; Calvigioni, M.; De Maria, C.; Zhang, Y.S.; Ghelardi, E.; Vozzi, G. Designs and methodologies to recreate in vitro human gut microbiota models. Bio-Des. Manuf. 2023, 6, 298–318. [Google Scholar] [CrossRef]
- Cordonnier, C.; Thevenot, J.; Etienne-Mesmin, L.; Denis, S.; Alric, M.; Livrelli, V.; Blanquet-Diot, S. Dynamic in vitro models of the human gastrointestinal tract as relevant tools to assess the survival of probiotic strains and their interactions with gut microbiota. Microorganisms 2015, 3, 725–745. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Van den Abbeele, P.; Verstraete, W.; El Aidy, S.; Geirnaert, A.; Van de Wiele, T. Prebiotics, faecal transplants and microbial network units to stimulate biodiversity of the human gut microbiome. Microb. Biotechnol. 2013, 6, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb. Biotechnol. 2012, 5, 106–115. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gerard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006, 136, 1862–1867. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME((R))). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 305–317. [Google Scholar]
- Suligoj, T.; Vigsnaes, L.K.; Abbeele, P.V.D.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of human milk oligosaccharides on the adult gut microbiota and barrier function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Arroyo, M.C.; Laurie, I.; Rotsaert, C.; Marzorati, M.; Risso, D.; Karnik, K. Age-dependent prebiotic effects of soluble corn fiber in M-SHIME((R)) gut microbial ecosystems. Plant Foods Hum. Nutr. 2023, 78, 213–220. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Hao, L.; Du, P.; Li, C.; Han, H.; Xu, H.; Liu, L. The bioavailability of soybean polysaccharides and their metabolites on gut microbiota in the simulator of the human intestinal microbial ecosystem (SHIME). Food Chem. 2021, 362, 130233. [Google Scholar] [CrossRef] [PubMed]
- Joly Condette, C.; Djekkoun, N.; Reygner, J.; Depeint, F.; Delanaud, S.; Rhazi, L.; Bach, V.; Khorsi-Cauet, H. Effect of daily co-exposure to inulin and chlorpyrifos on selected microbiota endpoints in the SHIME(R) model. Environ. Pollut. 2022, 302, 118961. [Google Scholar] [CrossRef]
- Salgaco, M.K.; Perina, N.P.; Tome, T.M.; Mosquera, E.M.B.; Lazarini, T.; Sartoratto, A.; Sivieri, K. Probiotic infant cereal improves children’s gut microbiota: Insights using the Simulator of Human Intestinal Microbial Ecosystem (SHIME(R)). Food Res. Int. 2021, 143, 110292. [Google Scholar] [CrossRef] [PubMed]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.; Boschker, H.T.; Verstraete, W.; Van de Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef]
- Tzollas, N.M.; Zachariadis, G.A.; Anthemidis, A.N.; Statis, J.A. A new approach to indophenol blue method for determination of ammonium in geothermal waters with high mineral content. Int. J. Environ. Anal. Chem. 2010, 90, 115–126. [Google Scholar] [CrossRef]
- Duysburgh, C.; Van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. X 2019, 1, 100021. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 2011, 77, 3219–3226. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Vandeputte, D.; Kathagen, G.; D’Hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; DeMeo, M.T.; Keshavarzian, A.; Hamaker, B.R. Influence of dietary fiber on inflammatory bowel disease and colon cancer: Importance of fermentation pattern. Nutr. Rev. 2007, 65, 51–62. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Gibson, G.R.; Cummings, J.H. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 1992, 72, 57–64. [Google Scholar]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet(-)microbe(-)host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2018, 83, 235–247. [Google Scholar] [CrossRef]
- Whitman, J.A.; Doherty, L.A.; Pantoja-Feliciano de Goodfellow, I.G.; Racicot, K.; Anderson, D.J.; Kensil, K.; Karl, J.P.; Gibson, G.R.; Soares, J.W. In vitro fermentation shows polyphenol and fiber blends have and additive beneficial effect on gut microbiota states. Nutrients 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef]
- Devika, N.T.; Raman, K. Deciphering the metabolic capabilities of Bifidobacteria using genome-scale metabolic models. Sci. Rep. 2019, 9, 18222. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Cai, Y.; Ren, L.; Sun, L.; Gang, X.; Wang, G. Bacteroidaceae, Bacteroides, and Veillonella: Emerging protectors against Graves’ disease. Front. Cell Infect. Microbiol. 2024, 14, 1288222. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and side effects of short-chain fatty acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Heimann, E.; Nyman, M.; Palbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Hughes, R.; Kurth, M.J.; McGilligan, V.; McGlynn, H.; Rowland, I. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr. Cancer 2008, 60, 259–266. [Google Scholar] [CrossRef]
- Armstrong, H.; Alipour, M.; Valcheva, R.; Bording-Jorgensen, M.; Jovel, J.; Zaidi, D.; Shah, P.; Lou, Y.; Ebeling, C.; Mason, A.L.; et al. Host immunoglobulin G selectively identifies pathobionts in pediatric inflammatory bowel diseases. Microbiome 2019, 7, 1. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govaert, M.; Duysburgh, C.; Kesler, B.; Marzorati, M. Effects of NatureKnit™, a Blend of Fruit and Vegetable Fibers Rich in Naturally Occurring Bound Polyphenols, on the Metabolic Activity and Community Composition of the Human Gut Microbiome Using the M-SHIME® Gastrointestinal Model. Microorganisms 2025, 13, 613. https://doi.org/10.3390/microorganisms13030613
Govaert M, Duysburgh C, Kesler B, Marzorati M. Effects of NatureKnit™, a Blend of Fruit and Vegetable Fibers Rich in Naturally Occurring Bound Polyphenols, on the Metabolic Activity and Community Composition of the Human Gut Microbiome Using the M-SHIME® Gastrointestinal Model. Microorganisms. 2025; 13(3):613. https://doi.org/10.3390/microorganisms13030613
Chicago/Turabian StyleGovaert, Marlies, Cindy Duysburgh, Brendan Kesler, and Massimo Marzorati. 2025. "Effects of NatureKnit™, a Blend of Fruit and Vegetable Fibers Rich in Naturally Occurring Bound Polyphenols, on the Metabolic Activity and Community Composition of the Human Gut Microbiome Using the M-SHIME® Gastrointestinal Model" Microorganisms 13, no. 3: 613. https://doi.org/10.3390/microorganisms13030613
APA StyleGovaert, M., Duysburgh, C., Kesler, B., & Marzorati, M. (2025). Effects of NatureKnit™, a Blend of Fruit and Vegetable Fibers Rich in Naturally Occurring Bound Polyphenols, on the Metabolic Activity and Community Composition of the Human Gut Microbiome Using the M-SHIME® Gastrointestinal Model. Microorganisms, 13(3), 613. https://doi.org/10.3390/microorganisms13030613





