Ghostbuster—A Western Blot-Based Panel Method to Resolve False-Positive Brucellosis Serology Test Results
Abstract
1. Introduction
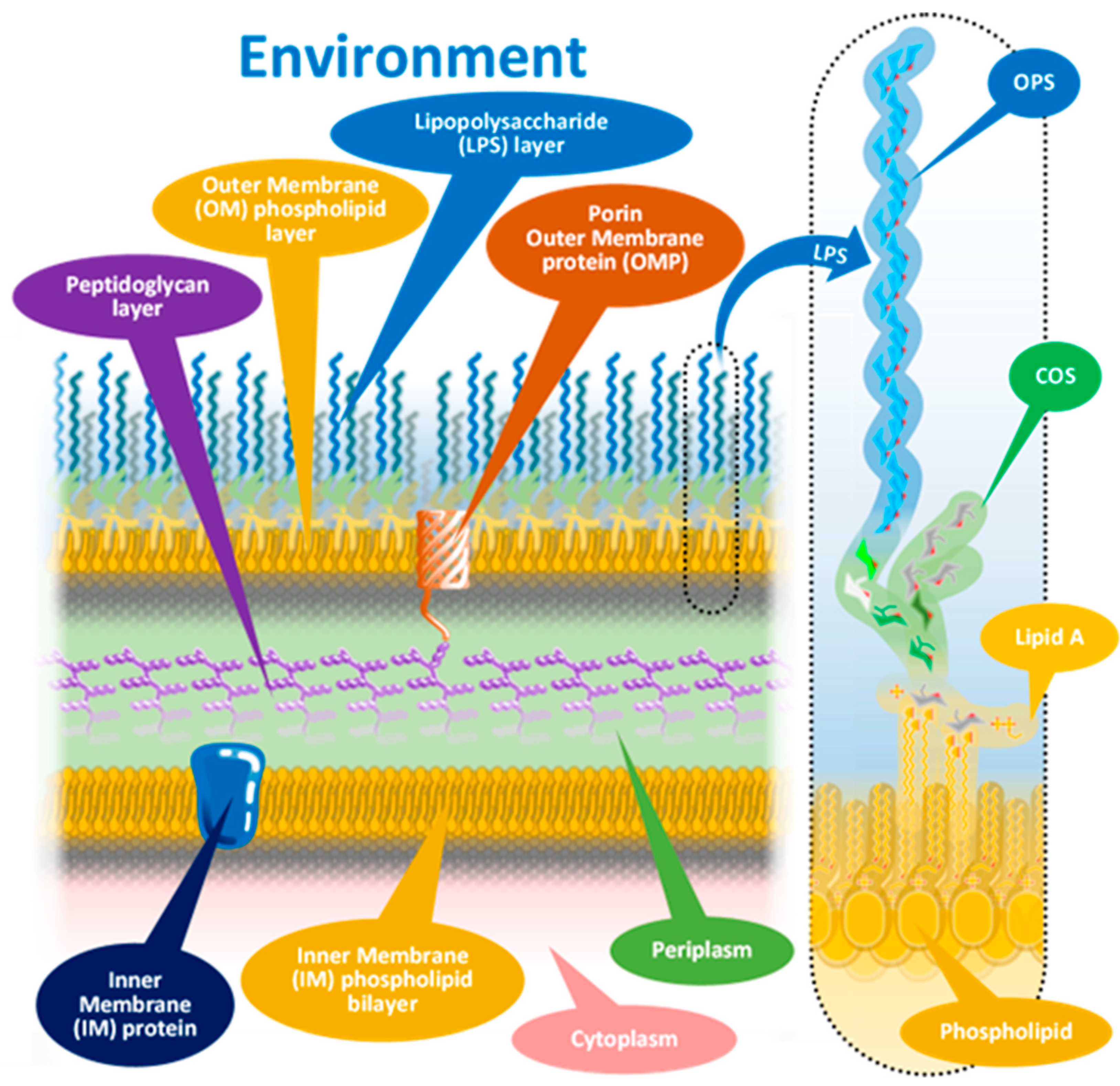
1.1. The Molecular Background of FP Serologic Test Results
1.2. Possible Solutions
2. Materials and Methods
2.1. Samples—Case Study
2.2. Samples—WB Panel Study
2.3. Serologic Laboratory Tests
2.4. Confirmatory Examinations Other than Serology
2.5. WB Panel Test—Antigen Panel Isolation—Bacterial Culture
2.6. WB Panel Test—Antigen Panel Isolation—Protein Isolation
2.7. WB Panel Test—Polyacrylamide Gel Electrophoresis (PAGE)
2.8. WB Panel Test—Western Blotting—Protein Transfer
2.9. WB Panel Test—Western Blotting—The Development of the Blots
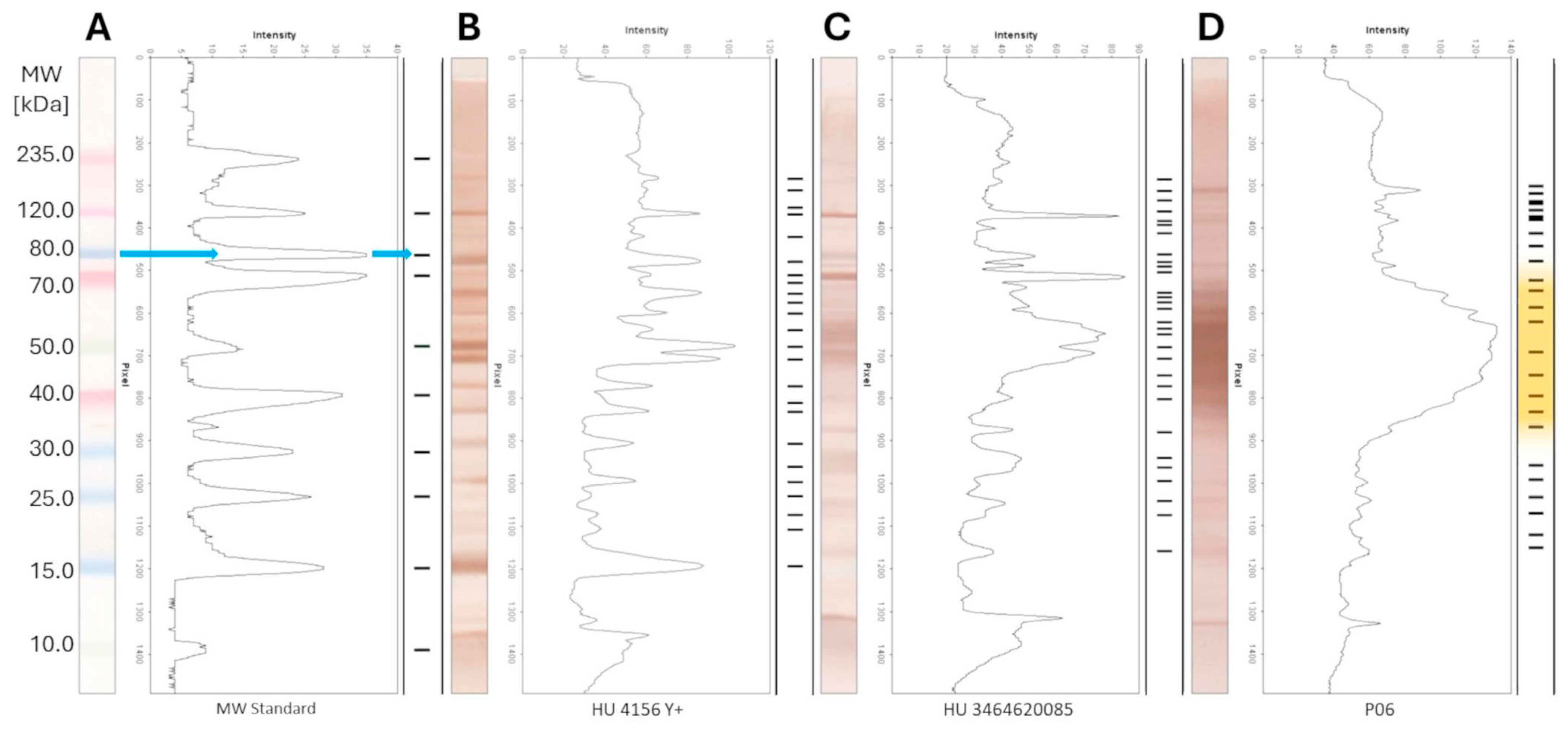
2.10. WB Panel Test—The Densitometry and Determination of the mw of the Protein Bands on the WB Lanes
2.11. WB Panel Test—Presentation of WB-Based Panel Test Results
2.12. Analytical Parameters—Routine Serology Tests
2.13. Analytical Parameters—WB Panel Test—Precision
2.14. Analytical Parameters—WB Panel Test—Precision—Repeatability
2.15. Analytical Parameters—WB Panel Test—Precision—Reproducibility
2.16. Diagnostic Parameters
3. Results
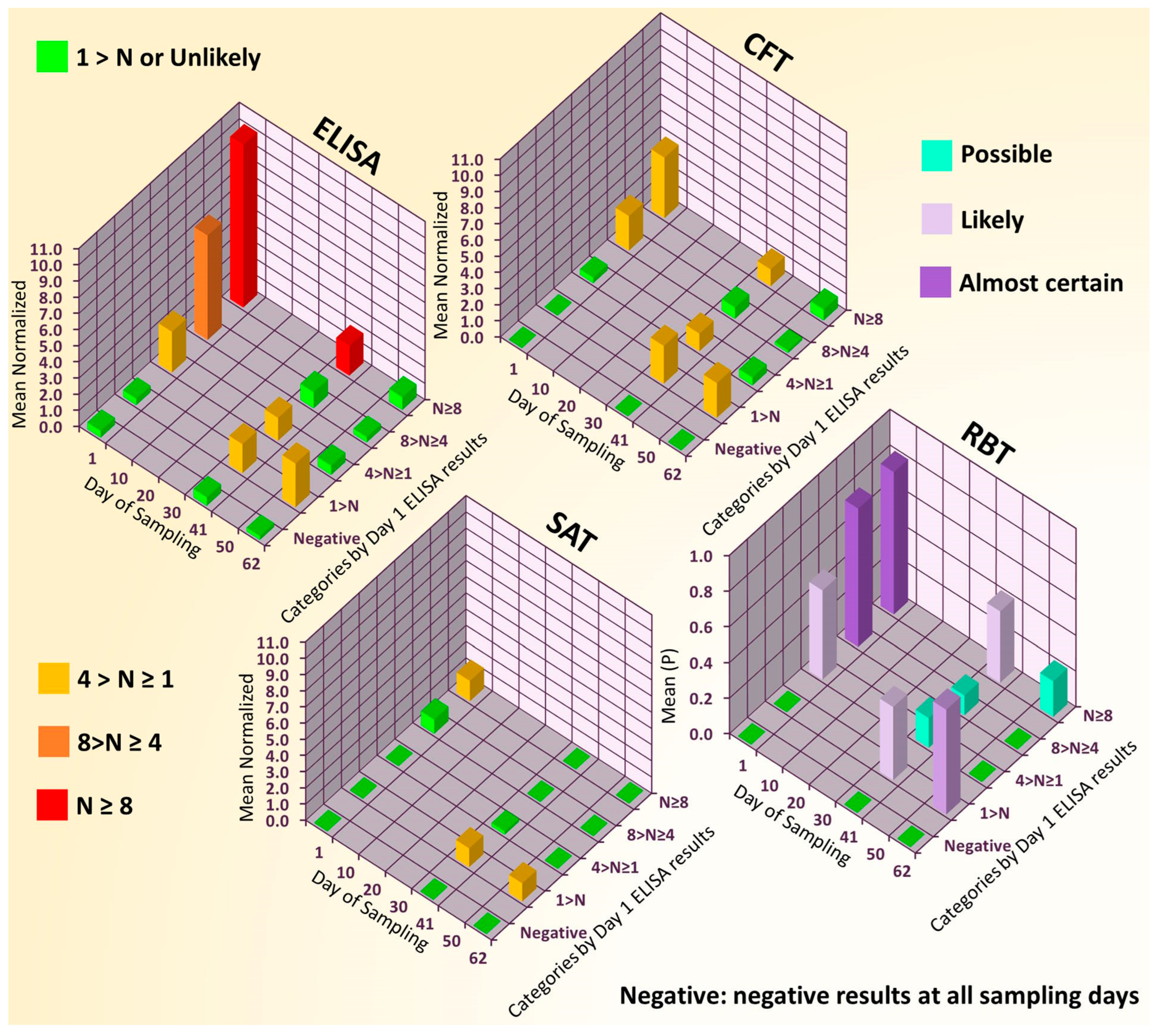
3.1. Results of Case Study Conducted with Routine Serological Tests and Swine Sera
3.2. Serological Test Results Connected to the WB Panel Test
3.3. WB Panel Test Results
3.4. Analytical Parameters of WB Panel Test
3.5. Diagnostic Parameters of Routine Brucella ELISA and WB Panel Test
4. Discussion
4.1. A Case Study Conducted with Routine Serological Tests and Swine Sera
4.2. Relevance of the Case Study Results in the Context of the WB Panel Test
4.3. Limitations of the WB Panel Test
4.4. Interpretation of the Results of the WB Panel Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, H.C.; Nöckler, K.; Göllner, C.; Bahn, P.; Vergnaud, G.; Tomaso, H.; Al Dahouk, S.; Kämpfer, P.; Cloeckaert, A.; Maquart, M.; et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 2010, 60 Pt 4, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; Chacón-Díaz, C.; Moreira-Soto, A.; Barrantes-Granados, O.; Suárez-Esquivel, M.; Viquez-Ruiz, E.; Barquero-Calvo, E.; Ruiz-Villalobos, N.; Hidalgo-Montealegre, D.; González-Barrientos, R.; et al. Virulent Brucella nosferati infecting Desmodus rotundus has emerging potential due to the broad foraging range of its bat host for humans and wild and domestic animals. mSphere 2023, 8, e0006123. [Google Scholar] [CrossRef]
- Brucellosis in Sheep and Goats—European Commission, Health & Consumer Protection Directorate—General. SANCO.C.2/AH/R23/2001. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scah_out59_en.pdf (accessed on 12 July 2001).
- Celli, J. The Intracellular Life Cycle of Brucella spp. Microbiol. Spectr. 2019, 7, 10.1128. [Google Scholar] [CrossRef]
- Martirosyan, A.; Moreno, E.; Gorvel, J.P. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 2011, 240, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzmán-Verri, C.; Chacón-Díaz, C.; Rucavado, A.; Moriyón, I.; Moreno, E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2007, 2, e631. [Google Scholar] [CrossRef]
- Leong, D.; Diaz, R.; Milner, K.; Rudbach, J.; Wilson, J.B. Some structural and biological properties of Brucella endotoxin. Infect. Immun. 1970, 1, 174–182. [Google Scholar] [CrossRef]
- Herron, J.; Alexander Thomas Dunbar, J. The British Army’s contribution to tropical medicine. Clin. Med. 2018, 18, 380–383. [Google Scholar] [CrossRef]
- Wright, A.; Smith, F. On the application of the serum test to the differential diagnosis of typhoid and Malta fever: And on the further application of the method of serum diagnosis to the elucidation of certain problems in connexion with the duration of immunity and the geographical distribution of disease. Lancet 1897, 1, 3836. [Google Scholar]
- Wright, A.E.; Semple, D. On the Employment of Dead Bacteria in the Serum Diagnosis of Typhoid and Malta Fever, and on an Easy Method of Extemporising a Blowpipe Flame for Making Capillary Sero-Sedimentation Tubes. Br. Med. J. 1897, 1, 1214–1215. [Google Scholar] [CrossRef]
- Ducrotoy, M.J.; Muñoz, P.M.; Conde-Álvarez, R.; Blasco, J.M.; Moriyón, I. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev. Vet. Med. 2018, 151, 57–72. [Google Scholar] [CrossRef]
- Bányász, B.; Antal, J.; Dénes, B. False Positives in Brucellosis Serology: Wrong Bait and Wrong Pond? Trop. Med. Infect. Dis. 2023, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, L.A.; Farahat, L.; Ramadan, E.S.; Warda, F.F. Effects of Chemically Pretreated Bovine Serum Samples on Sensitivity of Rose Bengal Test for Serodiagnosis of Bovine Brucellosis. J. Appl. Vet. Sci. 2020, 5, 6–12. [Google Scholar]
- Corbel, M.J. Studies on the mechanism of the Rose Bengal plate test for bovine brucellosis. Br. Vet. J. 1973, 129, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, A.P.; Cockrem, D.S. Reduction of non-specific reactions to the Brucella abortus serum agglutination test by the addition of EDTA. Res. Vet. Sci. 1985, 38, 288–291. [Google Scholar] [CrossRef]
- McMahon, K.J. Comparison of the 2-mercaptoethanol and dithiothreitol tests for determining Brucella immunoglobulin G agglutinating antibody in bovine serum. Can. J. Comp. Med. Rev. Can. De Med. Comp. 1983, 47, 370–372. [Google Scholar]
- Wise, B.; Craig, H.W. The Brucella Complement Fixation Reaction. J. Infect. Dis. 1942, 70, 147–151. [Google Scholar] [CrossRef]
- Brucellosis (infection with B.abortus, B. melitensis and B.suis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2022; Chapter 3.1.4.
- Muñoz, P.M.; Marín, C.M.; Monreal, D.; González, D.; Garin-Bastuji, B.; Díaz, R.; Mainar-Jaime, R.C.; Moriyón, I.; Blasco, J.M. Efficacy of several serological tests and antigens for diagnosis of bovine brucellosis in the presence of false-positive serological results due to Yersinia enterocolitica O:9. Clin. Diagn. Lab. Immunol. 2005, 12, 141–151. [Google Scholar] [CrossRef]
- Bonfini, B.; Chiarenza, G.; Paci, V.; Sacchini, F.; Salini, R.; Vesco, G.; Villari, S.; Zilli, K.; Tittarelli, M. Cross-reactivity in serological tests for brucellosis: A comparison of immune response of Escherichia coli O157:H7 and Yersinia enterocolitica O:9 vs Brucella spp. Vet. Ital. 2018, 54, 107–114. [Google Scholar] [CrossRef]
- Huber, M.; Kalis, C.; Keck, S.; Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Beutler, B.; et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur. J. Immunol. 2006, 36, 701–711. [Google Scholar] [CrossRef]
- Gammoudi, I.; Mathelie-Guinlet, M.; Morote, F.; Beven, L.; Moynet, D.; Grauby-Heywang, C.; Cohen-Bouhacina, T. Morphological and nanostructural surface changes in Escherichia coli over time, monitored by atomic force microscopy. Colloids Surf. B Biointerfaces 2016, 141, 355–364. [Google Scholar] [CrossRef]
- Vassen, V.; Valotteau, C.; Feuillie, C.; Formosa-Dague, C.; Dufrêne, Y.F.; De Bolle, X. Localized incorporation of outer membrane components in the pathogen Brucella abortus. EMBO J. 2019, 38, e100323. [Google Scholar] [CrossRef] [PubMed]
- DebRoy, C.; Fratamico, P.M.; Yan, X.; Baranzoni, G.; Liu, Y.; Needleman, D.S.; Tebbs, R.; O’Connell, C.D.; Allred, A.; Swimley, M.; et al. Comparison of O-Antigen Gene Clusters of All O-Serogroups of Escherichia coli and Proposal for Adopting a New Nomenclature for O-Typing. PLoS ONE 2016, 11, e0147434. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identifications. Oilseeds Fats Crops Lipids 2020, 27, 31. [Google Scholar] [CrossRef]
- Stenutz, R.; Weintraub, A.; Widmalm, G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 2006, 30, 382–403. [Google Scholar] [CrossRef]
- Bundle, D.R.; McGiven, J. Brucellosis: Improved Diagnostics and Vaccine Insights from Synthetic Glycans. Acc. Chem. Res. 2017, 50, 2958–2967. [Google Scholar] [CrossRef]
- Dieste-Pérez, L.; Blasco, J.M.; de Miguel, M.J.; Moriyón, I.; Muñoz, P.M. Diagnostic performance of serological tests for swine brucellosis in the presence of false positive serological reactions. J. Microbiol. Methods 2015, 111, 57–63. [Google Scholar] [CrossRef]
- Chenais, E.; Bagge, E.; Lambertz, S.T.; Artursson, K. Yersinia enterocolitica serotype O:9 cultured from Swedish sheep showing serologically false-positive reactions for Brucella melitensis. Infect. Ecol. Epidemiol. 2012, 2. [Google Scholar] [CrossRef]
- Corbel, M.J. The serological relationship between Brucella spp., Yersinia enterocolitica serotype IX and Salmonella serotypes of Kauffmann-White group N. J. Hyg. 1975, 75, 151–171. [Google Scholar] [CrossRef]
- Faria, A.R.; Dorneles, E.; Pires, S.; Andrade, H.M.; Lage, A.P. Immunoproteomics of Brucella abortus reveals potential of recombinant antigens for discriminating vaccinated from naturally infected cattle. Microb. Pathog. 2020, 147, 104345. [Google Scholar] [CrossRef]
- Nagalingam, M.; Basheer, T.J.; Balamurugan, V.; Shome, R.; Kumari, S.S.; Reddy, G.; Shome, B.R.; Rahman, H.; Roy, P.; Kingston, J.J.; et al. Comparative evaluation of the immunodominant proteins of Brucella abortus for the diagnosis of cattle brucellosis. Vet. World 2021, 14, 803–812. [Google Scholar] [CrossRef]
- Letesson, J.J.; Tibor, A.; van Eynde, G.; Wansard, V.; Weynants, V.; Denoel, P.; Saman, E. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 1997, 4, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Bulashev, A.; Akibekov, O.; Syzdykova, A.; Suranshiyev, Z.; Ingirbay, B. Use of recombinant Brucella outer membrane proteins 19, 25, and 31 for serodiagnosis of bovine brucellosis. Vet. World 2020, 13, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Bai, Q.; Wu, X.; Li, H.; Shao, J.; Sun, M.; Zhang, J. A Multi-Epitope Fusion Protein-Based p-ELISA Method for Diagnosing Bovine and Goat Brucellosis. Front. Vet. Sci. 2021, 8, 708008. [Google Scholar] [CrossRef]
- Corrente, M.; Desario, C.; Parisi, A.; Grandolfo, E.; Scaltrito, D.; Vesco, G.; Colao, V.; Buonavoglia, D. Serological diagnosis of bovine brucellosis using B. melitensis strain B115. J. Microbiol. Methods 2015, 119, 106–109. [Google Scholar] [CrossRef]
- McGiven, J.; Howells, L.; Duncombe, L.; Stack, J.; Ganesh, N.V.; Guiard, J.; Bundle, D.R. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping m epitope elements of Brucella O-polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. [Google Scholar] [CrossRef]
- Ganesh, N.V.; Sadowska, J.M.; Sarkar, S.; Howells, L.; McGiven, J.; Bundle, D.R. Molecular recognition of Brucella A and M antigens dissected by synthetic oligosaccharide glycoconjugates leads to a disaccharide diagnostic for brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269. [Google Scholar] [CrossRef] [PubMed]
- Bercovich, Z. The use of skin delayed-type hypersensitivity as an adjunct test to diagnose brucellosis in cattle: A review. Vet. Q. 2000, 22, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Degner, N.R.; Castillo-Galvan, R.; Alexander, J.; Arun, A.; de Vries, C.R.; Macintyre, A.; Perkins, B.; Ahmed, A.A.; Smollin, M. 1026. Following the Hoof Prints: Detecting Coxiella and Brucella infections with A Plasma-based Microbial Cell-Free DNA Next-generation Sequencing Test. Open Forum Infect. Dis. 2021, 8, S604. [Google Scholar] [CrossRef]
- Pan, S.-W.; Su, W.-J.; Chan, Y.-J.; Chuang, F.-Y.; Feng, J.-Y.; Chen, Y.-M. Mycobacterium tuberculosis–derived circulating cell-free DNA in patients with pulmonary tuberculosis and persons with latent tuberculosis infection. PLoS ONE 2021, 16, e0253879. [Google Scholar] [CrossRef]
- Han, D.; Li, R.; Shi, J.; Tan, P.; Zhang, R.; Li, J. Liquid biopsy for infectious diseases: A focus on microbial cell-free DNA sequencing. Theranostics 2020, 10, 5501–5513. [Google Scholar] [CrossRef]
- Peaper, D.R.; Durant, T.S. Can Circulating Cell-Free Microbial DNA Carry Us into the Future of Culture Independent Microbiology? Clin. Chem. 2020, 66, 29–32. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhao, L.; Li, M.Q.; Chen, W.G.; Xu, C.J. A sensitive and reversible staining of proteins on blot membranes. Anal. Biochem. 2020, 592, 113579. [Google Scholar] [CrossRef]
- Ashok, Y.; Nanekar, R.; Jaakola, V.P. Defining thermostability of membrane proteins by western blotting. Protein Eng. Des. Sel. 2015, 28, 539–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for the Brucellosis Laboratory; INRA Publications: Versailles Cedex, France, 1988; p. 138. [Google Scholar]
- Chrambach, A.; Rodbard, D. Polyacrylamide gel electrophoresis. Science 1971, 172, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. The basics of western blotting. Anat. Rec. 2012, 295, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994, 308, 1552. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Diagnostic tests 2: Predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef]
- Pismennõi, D.; Kattel, A.; Belouah, I.; Nahku, R.; Vilu, R.; Kobrin, E.-G. The Quantitative Measurement of Peptidoglycan Components Obtained from Acidic Hydrolysis in Gram-Positive and Gram-Negative Bacteria via Hydrophilic Interaction Liquid Chromatography Coupled with Mass Spectrometry. Microorganisms 2023, 11, 2134. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Vizcaíno, N.; Paquet, J.Y.; Bowden, R.A.; Elzer, P.H. Major outer membrane proteins of Brucella spp.: Past, present and future. Vet. Microbiol. 2002, 90, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Chodisetti, P.K.; Reddy, M. Peptidoglycan: Structure, Synthesis, and Regulation. EcoSal Plus 2021, 9, eESP-0010-2020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, W.; Zheng, K.; Liu, Z.F. Establishment of Chronic Infection: Brucella’s Stealth Strategy. Front. Cell Infect. Microbiol. 2016, 6, 30. [Google Scholar] [CrossRef] [PubMed]
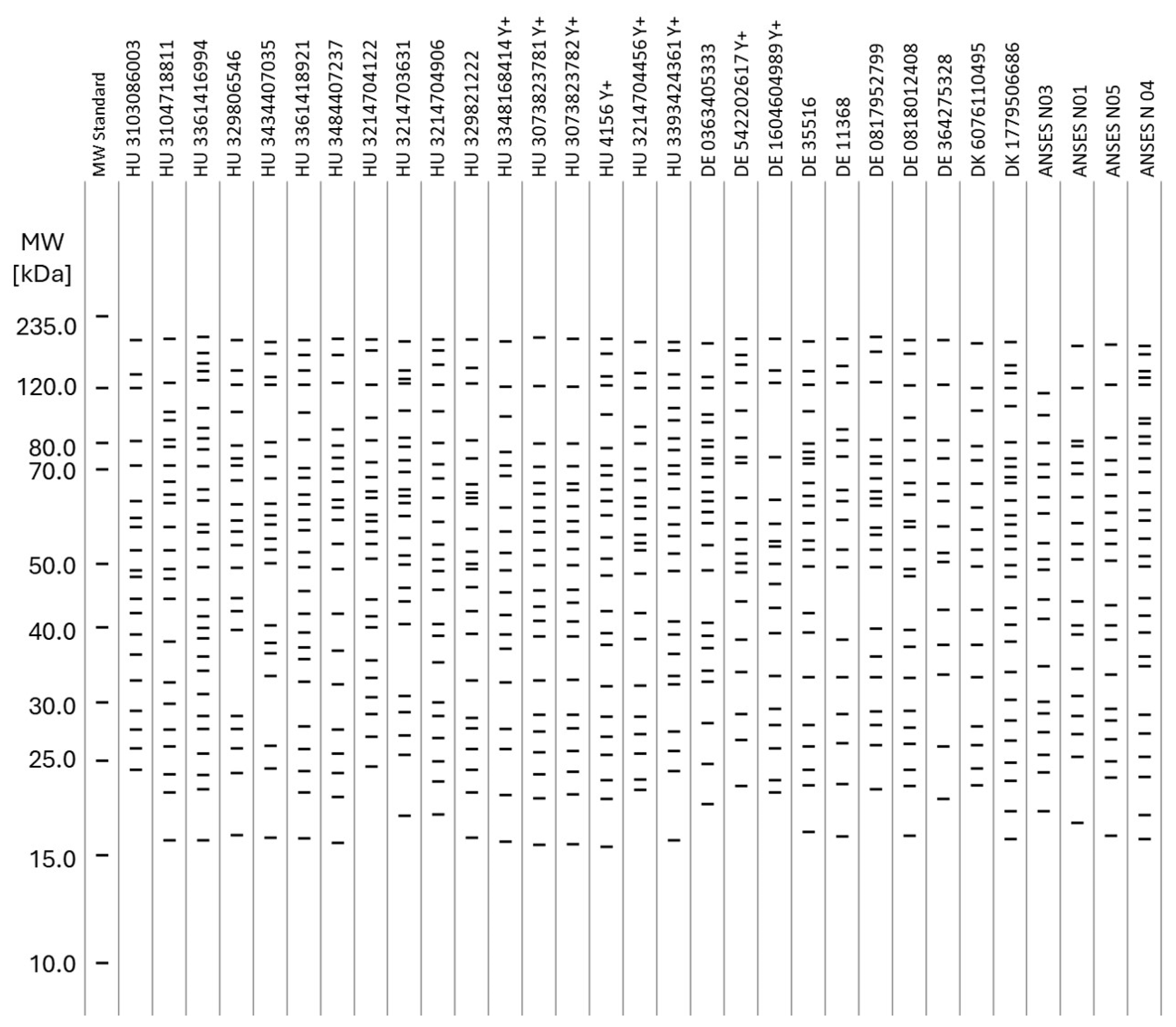
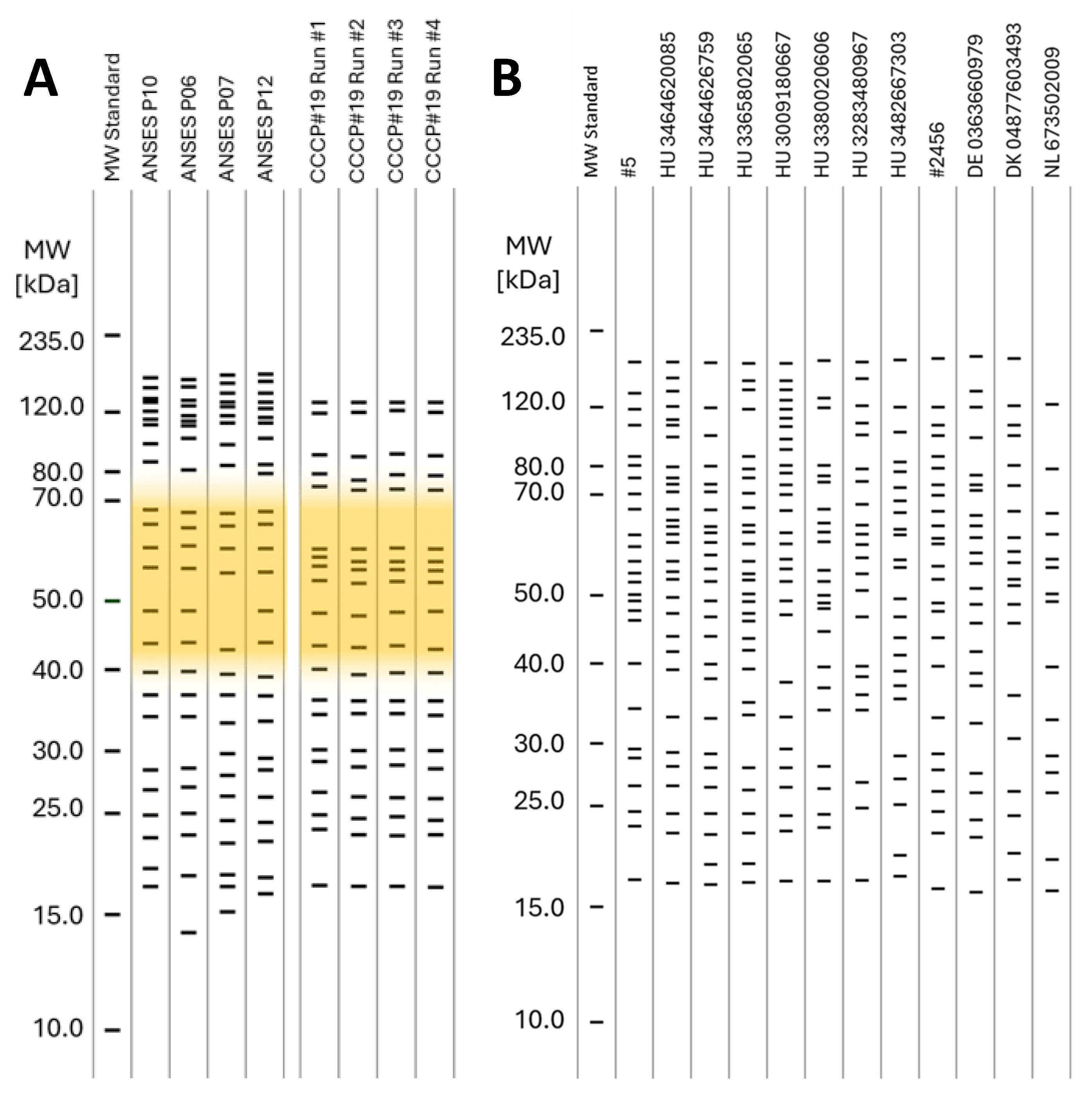
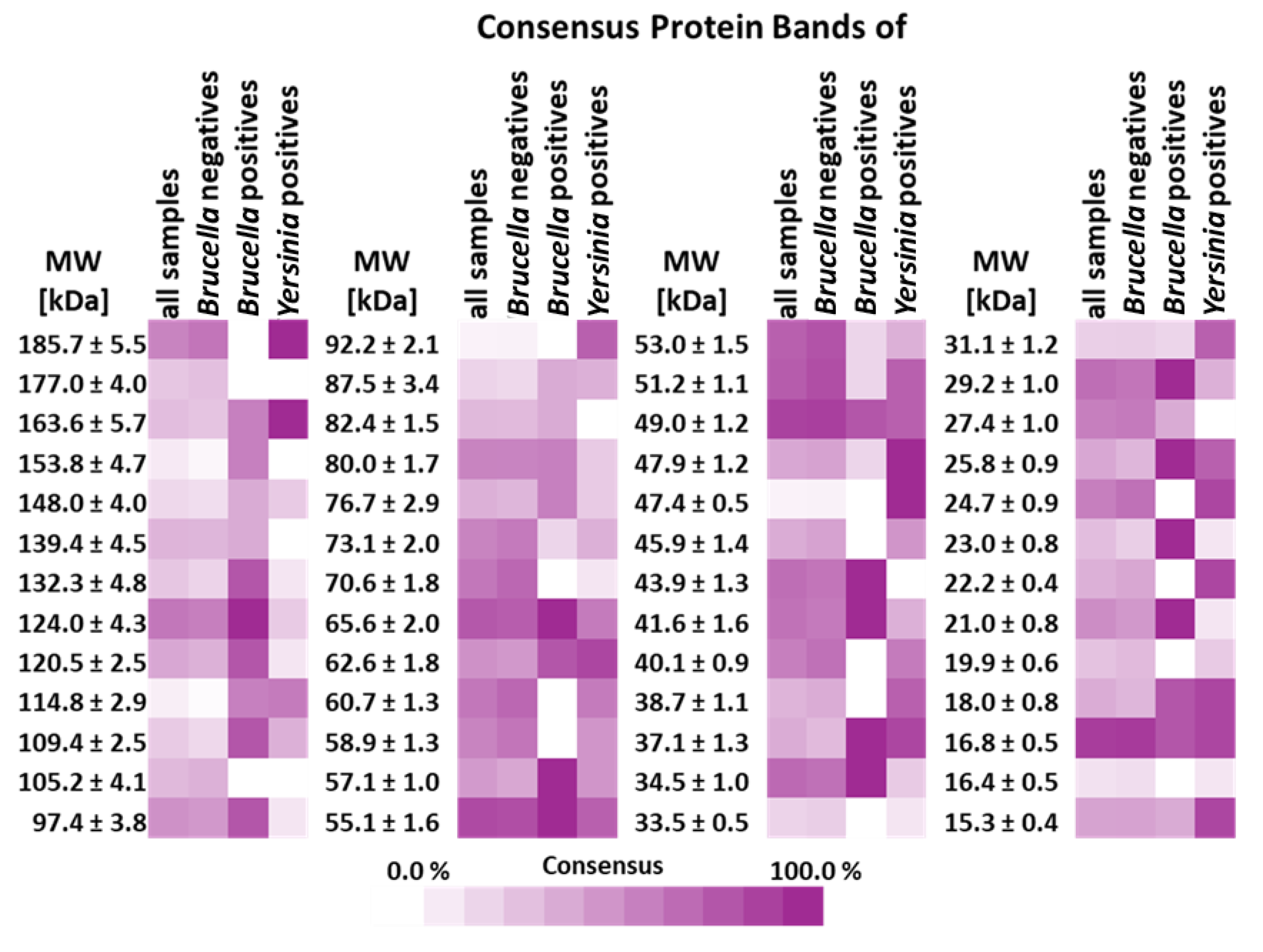
| Hungarian IDs | Reference Samples | Imported IDs | |
|---|---|---|---|
| HU 3214704456 | HU 3484407237 | CCCP#19 | DE 0363405333 |
| HU 3393424361 | HU 3214704122 | ANSES BRU POS SR06 | DE 35516 |
| HU 3348168414 | HU 3214703631 | ANSES BRU POS SR07 | DE 11368 |
| Need no explanation HU 4156 | HU 3214704906 | ANSES BRU POS SR10 | DE 0817952799 |
| HU 3073823781/1 a | HU 329821222 | ANSES BRU POS SR12 | DE 0818012408 |
| HU 3073823781/2 a | HU 3464620085 | ANSES BRU NEG SR01 | DE 364275328 |
| HU 3103086003 | HU 3464626759 | ANSES BRU NEG SR03 | DE 542202617 |
| HU 3104718811 | HU 3365802065 | ANSES BRU NEG SR04 | DE 1604604989 |
| HU 3361416994 | HU 3009180667 | ANSES BRU NEG SR05 | DE 0363660979 |
| HU 329806546 | HU 3380020606 | – | DK 6076110495 |
| HU 3434407035 | HU 3283480967 | – | DK 1779506686 |
| HU 3361418921 | #2456 | – | DK 04877603493 |
| HU 3482667303 | #5 | – | NL 673502009 |
| Reference Method | |||||
|---|---|---|---|---|---|
| Result | Positive | Negative | Total | ||
| Verified Test | Positive | TP | FP | PP = TP + FP | PPV = TP/PP |
| Negative | FN | TN | PN = TN + FN | NPV = TN/PN | |
| Total | P = TP + FN | N = TN + FP | T | ||
| DSe = TP/P | DSp = TN/N | ||||
| Positivity of Test Results [%] | ||||
|---|---|---|---|---|
| Test | Whole Herd (n = 193) | Resampled Population (n = 70) | ||
| Day of Sampling | ||||
| 1st | 41st | 62nd | ||
| ELISA | 51.8 | 41.4 | 27.1 | 24.3 |
| CFT | 13.7 | 31.4 | 32.6 | 24.3 |
| SAT | 7.4 | 15.7 | 4.3 | 14.3 |
| RBT | 13.1 | 30.0 | 17.1 | 15.7 |
| Sample ID (ELISA Negatives, Hungarian) | HU 3214704456 | HU 3393424361 | HU 3348168414 | HU 4156 | HU 3073823781/1 | HU 3073823781/2 | HU 3103086003 | HU 3104718811 | HU 3361416994 | HU 329806546 | HU 3434407035 | HU 3361418921 | HU 3484407237 | HU 3214704122 | HU 3214703631 | HU 3214704906 | HU 329821222 |
| ELISA/SER/SCR | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| CFT/SER/CON | ND | (−) | (−) | (−) | (−) | (−) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| SAT/SER/CON | ND | (−) | (−) | (−) | (−) | (−) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| RBT/SER/CON | ND | (−) | (−) | (−) | (−) | (−) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| OTR/OTR/CON | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| YER/SER/INV | (+) | (+) | (+) | (+) | (+) | (−) | ND | ND | ND | ND | ND | ND | ND | (−) | (−) | (−) | (−) |
| WBP/OTR/INV | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Sample ID (ELISA Negatives, Other EU) | DE 0363405333 | DE 35516 | DE 11368 | DE 0817952799 | DE 0818012408 | DE 364275328 | DE 542202617 | DE 1604604989 | DK 6076110495 | DK 1779506686 | ANSES BRU NEG SR01 | ANSES BRU NEG SR03 | ANSES BRU NEG SR04 | ANSES BRU NEG SR05 | |||
| ELISA/SER/SCR | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | |||
| CFT/SER/CON | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | (−) | (−) | (−) | (−) | |||
| SAT/SER/CON | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | (−) | (−) | (−) | (−) | |||
| RBT/SER/CON | ND | ND | ND | ND | ND | (−) | (−) | (−) | ND | ND | (−) | (−) | (−) | (−) | |||
| OTR/OTR/CON | ND | ND | ND | ND | ND | (−) | (−) | (−) | ND | ND | N/A | N/A | N/A | N/A | |||
| YER/SER/INV | ND | ND | ND | ND | ND | (−) | (+) | (+) | ND | ND | ND | ND | ND | ND | |||
| WBP/OTR/INV | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | |||
| Sample ID (ELISA Positives) | HU 3482667303 | HU 3464620085 | HU 3464626759 | HU 3365802065 | HU 3009180667 | HU 3380020606 | HU 3283480967 | #2456 | #5 | NL 673502009 | DK 04877603493 | DE 0363660979 | CCCP#19 | ANSES BRU POS SR06 | ANSES BRU POS SR07 | ANSES BRU POS SR10 | ANSES BRU POS SR12 |
| ELISA/SER/SCR | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| CFT/SER/CON | (−) | (+) | (+) | (+) | (−) | (+) | (+) | (−) | (−) | (+) | ND | ND | (+) | (+) | (+) | (+) | (+) |
| SAT/SER/CON | (−) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| RBT/SER/CON | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| OTR/OTR/CON | ND | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | N/A | N/A | N/A | N/A | N/A |
| YER/SER/INV | (−) | ND | ND | (−) | (+) | (+) | (−) | (+) | (+) | (+) | (−) | (−) | ND | ND | ND | ND | ND |
| WBP/OTR/INV | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) |
| Precision Parameter | ||||
|---|---|---|---|---|
| Repeatability | Reproducibility | |||
| Nominal mw | mw [kDa] | CV [%] | mw [kDa] | CV [%] |
| 235.0 kDa | 234.8 | 0.7 | 230.0 | 1.7 |
| 120.0 kDa | 120.1 | 0.5 | 120.2 | 1.2 |
| 80.0 kDa | 81.9 | 0.2 | 82.2 | 0.6 |
| 70.0 kDa | 70.4 | 0.8 | 70.9 | 0.6 |
| 50.0 kDa | 50.7 | 0.6 | 51.1 | 1.2 |
| 40.0 kDa | 42.1 | 1.3 | 42.1 | 1.0 |
| 30.0 kDa | 32.0 | 1.3 | 32.0 | 0.9 |
| 25.0 kDa | 24.2 | 1.9 | 24.3 | 1.5 |
| 15.0 kDa | 15.9 | 0.9 | 15.9 | 1.0 |
| 10.0 kDa | 10.2 | 5.0 | 10.2 | 3.1 |
| Mean | – | 1.3 | – | 1.3 |
| Reproducibility | |||||
|---|---|---|---|---|---|
| mw [kDa] | CV [%] | mw [kDa] | CV [%] | mw [kDa] | CV [%] |
| 129.5 | 0.00 | 53.6 | 0.57 | 30.8 | 0.22 |
| 118.9 | 0.89 | 51.8 | 0.31 | 28.8 | 1.34 |
| 87.8 | 0.73 | 49.0 | 0.92 | 25.0 | 1.30 |
| 78,1 | 1.52 | 47.9 | 0.50 | 22.8 | 1.07 |
| 72,4 | 0.87 | 43.8 | 0.47 | 21.1 | 1.31 |
| 64,9 | 1.12 | 40.7 | 0.68 | 17.1 | 0.25 |
| 57.0 | 0.29 | 37.1 | 0.15 | – | – |
| 54.8 | 0.67 | 35.4 | 0.31 | – | – |
| Mean | 1.3 | ||||
| Reference Method | |||||
|---|---|---|---|---|---|
| Result | Positive | Negative | Total | ||
| ELISA | Positive | 5 | 12 | PP = 17 | PPV = 0.29 |
| Negative | 0 | 31 | PN = 31 | NPV = 1.00 | |
| Total | P = 5 | P = 43 | 48 | ||
| DSe = 1.00 | DSp = 0.73 | ||||
| Reference Method | |||||
|---|---|---|---|---|---|
| Result | Positive | Negative | Total | ||
| WB PT | Positive | 5 | 0 | PP = 5 | PPV = 1.00 |
| Negative | 0 | 43 | PN = 43 | NPV = 1.00 | |
| Total | P = 5 | N = 43 | 48 | ||
| DSe = 1.00 | DSp = 1.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bányász, B.; Antal, J.; Dénes, B. Ghostbuster—A Western Blot-Based Panel Method to Resolve False-Positive Brucellosis Serology Test Results. Microorganisms 2025, 13, 574. https://doi.org/10.3390/microorganisms13030574
Bányász B, Antal J, Dénes B. Ghostbuster—A Western Blot-Based Panel Method to Resolve False-Positive Brucellosis Serology Test Results. Microorganisms. 2025; 13(3):574. https://doi.org/10.3390/microorganisms13030574
Chicago/Turabian StyleBányász, Borbála, József Antal, and Béla Dénes. 2025. "Ghostbuster—A Western Blot-Based Panel Method to Resolve False-Positive Brucellosis Serology Test Results" Microorganisms 13, no. 3: 574. https://doi.org/10.3390/microorganisms13030574
APA StyleBányász, B., Antal, J., & Dénes, B. (2025). Ghostbuster—A Western Blot-Based Panel Method to Resolve False-Positive Brucellosis Serology Test Results. Microorganisms, 13(3), 574. https://doi.org/10.3390/microorganisms13030574







