Molecular Epidemiology and Antibiotic Resistance Associated with Avian Pathogenic Escherichia coli in Shanxi Province, China, from 2021 to 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. APEC Isolation, Identification and DNA Extraction
2.3. Identification of Phylogenic Groups
2.4. Antimicrobial Susceptibility Testing
2.5. Detection of Antimicrobial Resistance Genes and Virulence Genes
2.6. Determination of Biofilm Formation Ability
- Strong biofilm formation: OD595 > 4 × ODc;
- Moderate biofilm formation: 2 × ODc ≤ OD595 ≤ 4 × ODc;
- Weak biofilm formation: ODc ≤ OD595 < 2 × ODc;
- No biofilm formation: OD595 ≤ ODc.
2.7. Statistical Analyses
3. Results
3.1. Phylogenetic Grouping
3.2. Antibiotic Susceptibility
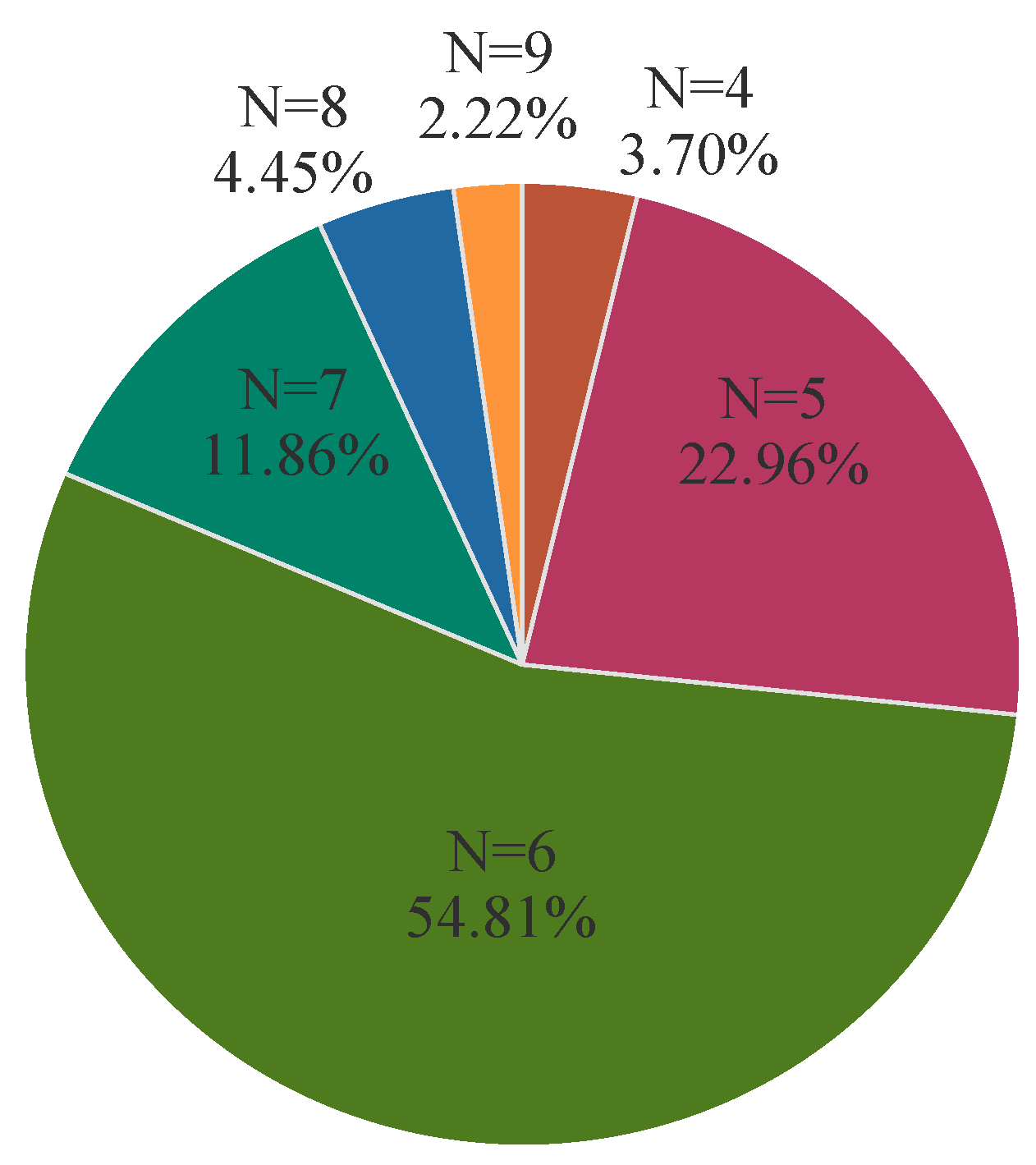
3.3. Biofilm-Forming Ability
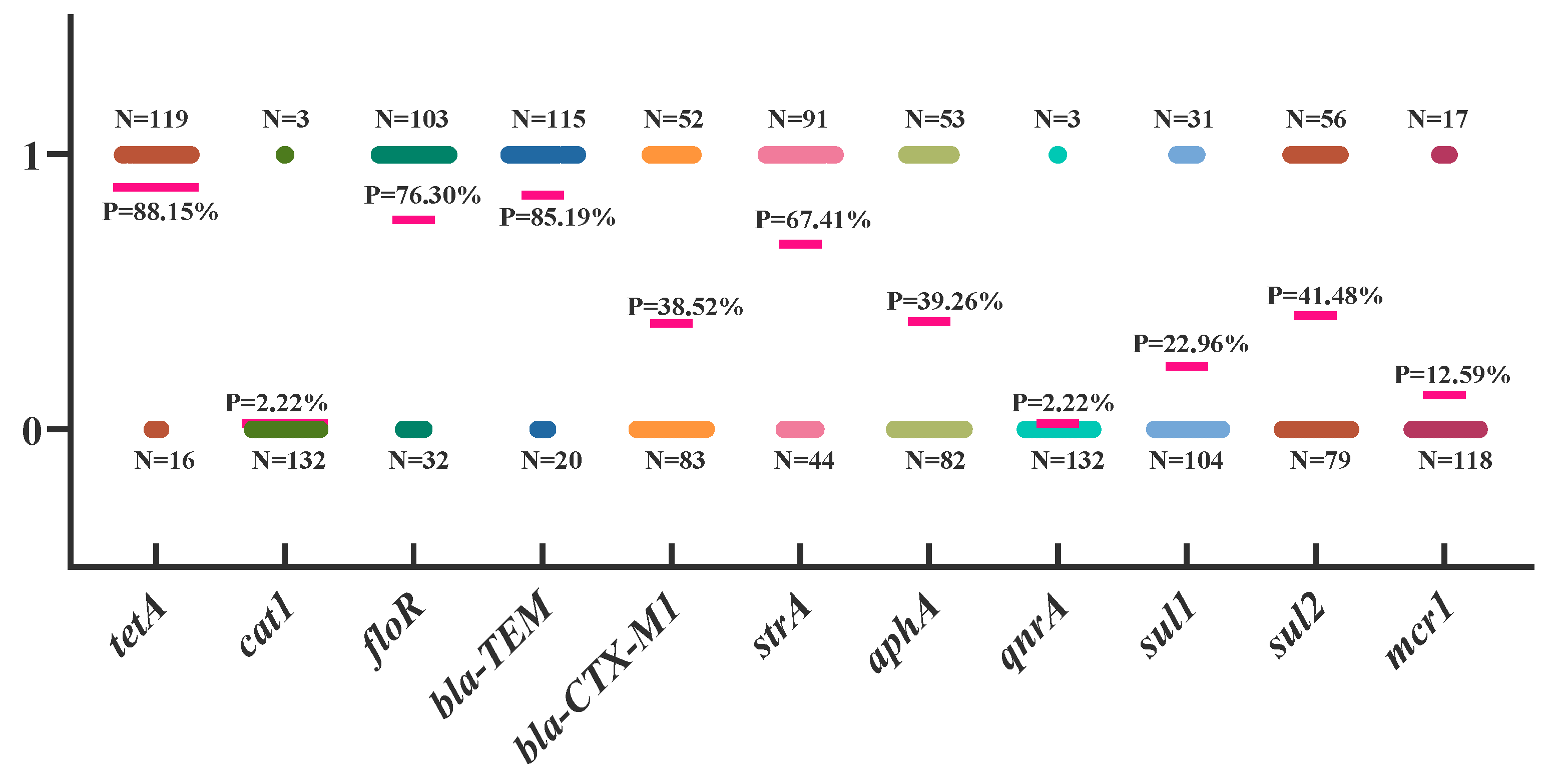
3.4. Analysis of Drug Resistance Genes in APEC
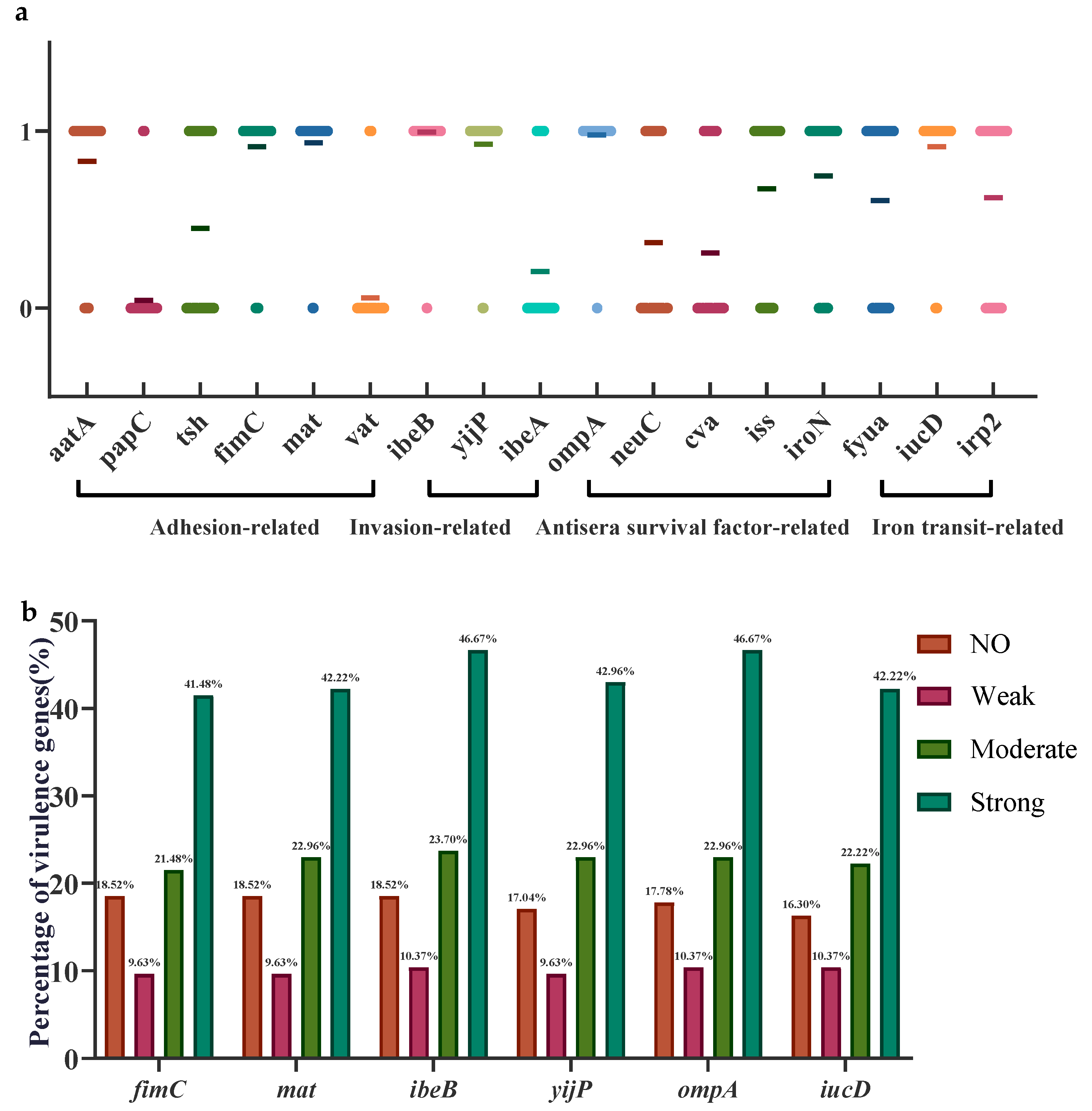
3.5. Analysis of Virulence Genes in APEC
3.6. Correlations Between Antibiotic-Resistant Phenotypes and Biofilm-Forming Ability
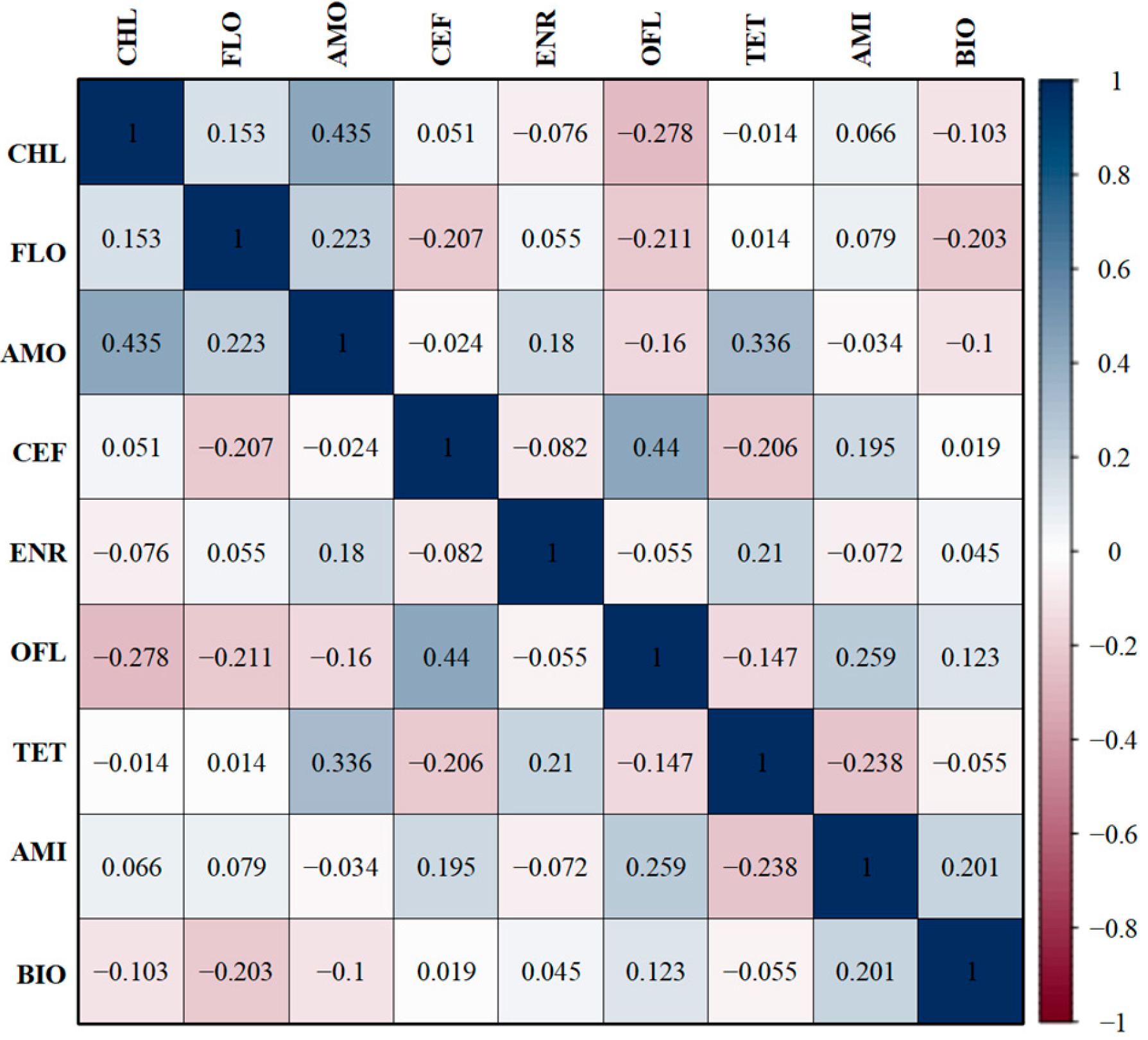
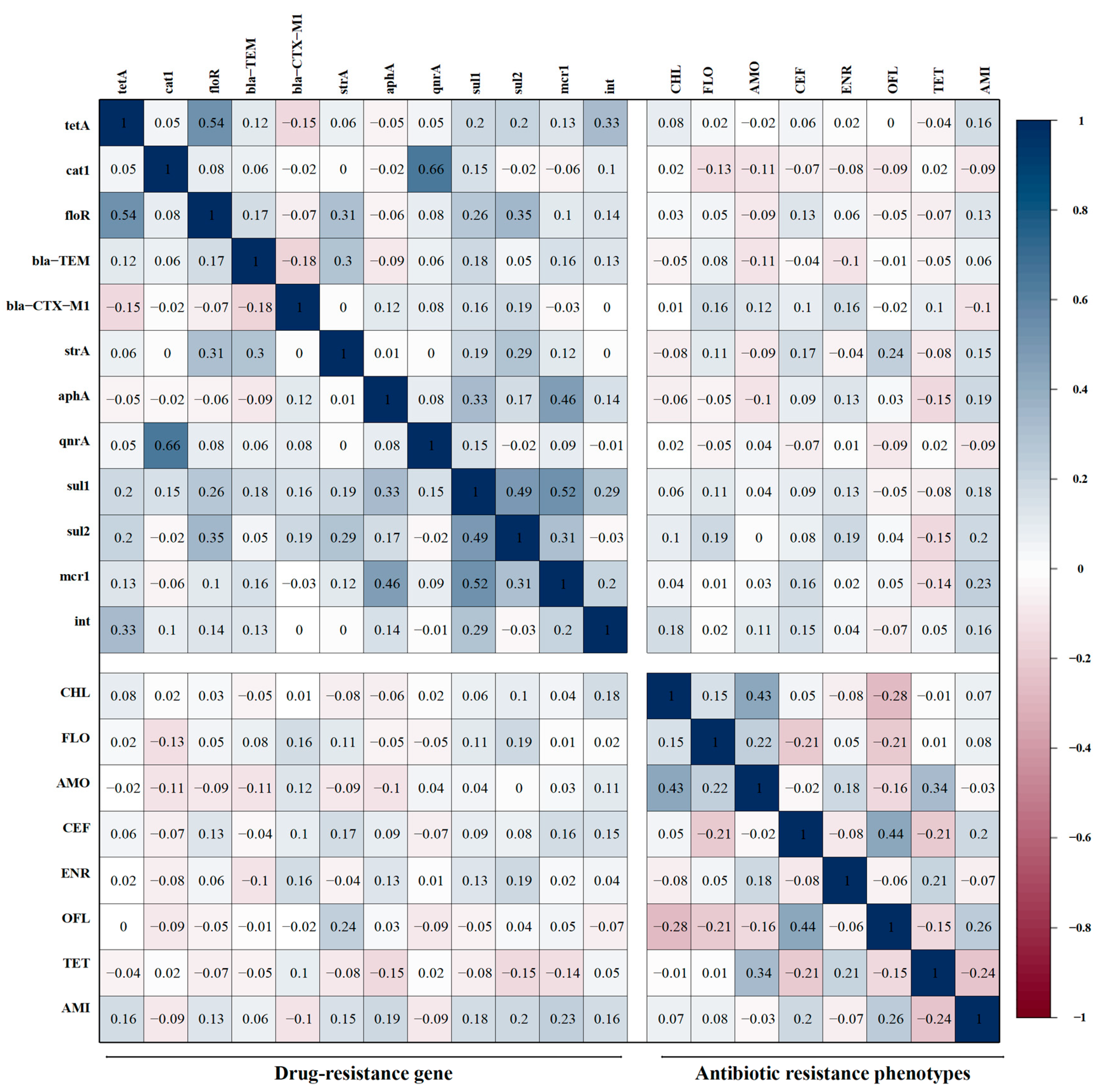
3.7. Correlations Between Antibiotic-Resistant Phenotypes and Resistant Genes
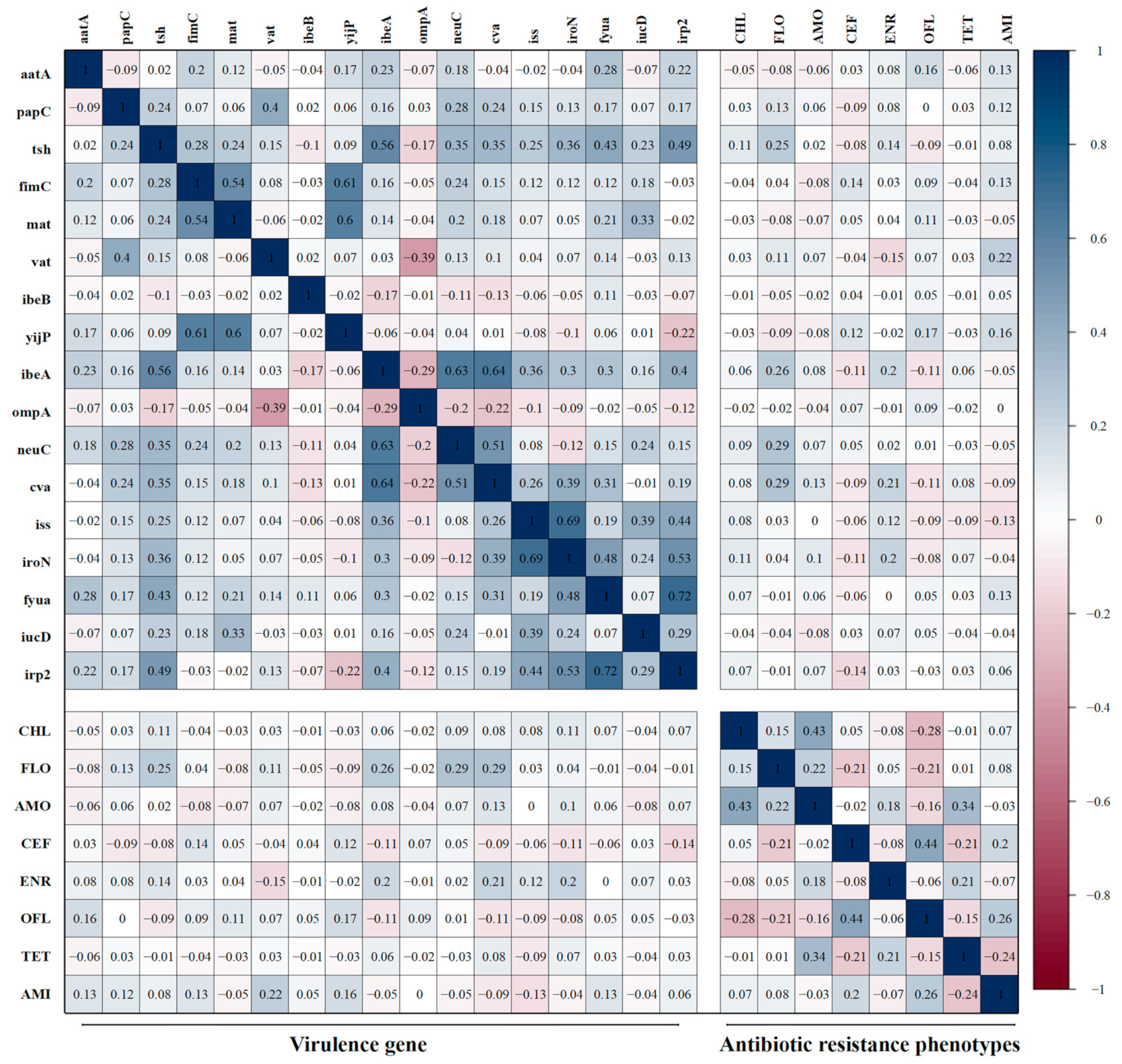
3.8. Correlations Between Antibiotic-Resistant Phenotypes and Virulence Genes
3.9. Correlations Between Biofilm Formation Ability and Virulence Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Che, C.; Jiang, W.; Chen, Z.; Tu, J.; Han, X.; Qi, K. Avian Pathogenic Escherichia coli through Pfs Affects the Transcription of Membrane Proteins to Resist β-Lactam Antibiotics. Vet. Sci. 2022, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.E.; Baig, S.; Stegger, M.; Ingmer, H.; Garmyn, A.; Butaye, P. Spontaneous Phage Resistance in Avian Pathogenic Escherichia coli. Front. Microbiol. 2021, 12, 782757. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Akrami, F.; Bouakkaz, S.; Dozois, C.M. Prevalence of Specific Serogroups, Antibiotic Resistance, and Virulence Factors of Avian Pathogenic Escherichia coli (APEC) Isolated from Clinical Cases: A Systematic Review and Meta-analysis. Microb. Pathog. 2024, 194, 106843. [Google Scholar] [CrossRef]
- Swelum, A.A.; Elbestawy, A.R.; El-Saadony, M.T.; Hussein, E.O.S.; Alhotan, R.; Suliman, G.M.; Taha, A.E.; Ba-Awadh, H.; El-Tarabily, K.A.; Abd El-Hack, M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: An updated overview. Poult. Sci. 2021, 100, 101039. [Google Scholar] [CrossRef]
- Ozaki, H.; Matsuoka, Y.; Nakagawa, E.; Murase, T. Characteristics of Escherichia coli Isolated from Broiler Chickens with Colibacillosis in Commercial Farms from a Common Hatchery. Poult. Sci. 2017, 96, 3717–3724. [Google Scholar] [CrossRef]
- Jørgensen, S.L.; Stegger, M.; Kudirkiene, E.; Lilje, B.; Poulsen, L.L.; Ronco, T.; Santos, T.P.D.; Kiil, K.; Bisgaard, M.; Pedersen, K.; et al. Diversity and Population Overlap between Avian and Human Escherichia coli Belonging to Sequence Type 95. mSphere 2019, 4, e00333-18. [Google Scholar] [CrossRef]
- Saidenberg, A.B.S.; van Vliet, A.H.M.; Stegger, M.; Johannesen, T.B.; Semmler, T.; Cunha, M.; de O. Silveira, A.C.; Anzai, E.K.; Scaletsky, I.C.A.; Dalsgaard, A.; et al. Genomic Analysis of the Zoonotic ST73 Lineage Containing Avian and Human Extraintestinal Pathogenic Escherichia coli (ExPEC). Vet. Microbiol. 2022, 267, 109372. [Google Scholar] [CrossRef]
- da Rocha, D.T.; De Oliveira Salle, F.; Borges, K.A.; Furian, T.Q.; Nascimento, V.P.D.; de Souza Moraes, H.L.; Salle, C.T.P. Avian Pathogenic Escherichia coli (APEC) and Uropathogenic Escherichia coli (UPEC): Characterization and Comparison. J. Infect. Dev. Ctries. 2021, 15, 962–971. [Google Scholar] [CrossRef]
- Cui, J.; Dong, Y.; Chen, Q.; Zhang, C.; He, K.; Hu, G.; He, D.; Yuan, L. Horizontal Transfer Characterization of ColV Plasmids in blaCTX-M-bearing Avian Escherichia coli. Poult. Sci. 2024, 103, 103631. [Google Scholar] [CrossRef]
- Hu, J.; Afayibo, D.J.A.; Zhang, B.; Zhu, H.; Yao, L.; Guo, W.; Wang, X.; Wang, Z.; Wang, D.; Peng, H.; et al. Characteristics, Pathogenic Mechanism, Zoonotic Potential, Drug Resistance, and Prevention of Avian Pathogenic Escherichia coli (APEC). Front. Microbiol. 2022, 13, 1049391. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Runcharoon, K.; Favro, M.E.; Logue, C.M. The Pathogenicity Traits of Avian Pathogenic E. coli (APEC) O25-ST131 Associated with Avian Colibacillosis in Georgia Poultry and their Genotypic and Phenotypic Overlap with other ExPEC. J. Appl. Microbiol. 2025, 136, lxaf015. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, M.; McCarthy, M.C.; Sniatynski, M.K.; Wu, L.; Dillon, J.-A.R.; Rubin, J.E.; White, A.P. Biofilm Formation and Antimicrobial Susceptibility of E. coli Associated with Colibacillosis Outbreaks in Broiler Chickens from Saskatchewan. Front. Microbiol. 2022, 13, 841516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, D.; Zhang, Y.; Xia, F.; Zhu, J.; Dai, J.; Zhuge, X. Extracellular Vesicles Produced by Avian Pathogenic Escherichia coli (APEC) Activate Macrophage Proinflammatory Response and Neutrophil Extracellular Trap (NET) Formation through TLR4 Signaling. Microb. Cell Fact. 2023, 22, 177. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, H.; Chandarajoti, K.; Jiao, Z.; Nie, L.; Lv, S.; Zuo, J.; Zhou, W.; Han, X. Design and Synthesis of Coumarin-Based Amphoteric Antimicrobials with Biofilm Interference and Immunoregulation Effects. RSC Med. Chem. 2024. [Google Scholar] [CrossRef]
- Young, M.M.; de Oliveira, A.L.; Nolan, L.K.; Barbieri, N.L.; Logue, C.M. Identification of Novel Genes Involved in the Biofilm Formation Process of Avian Pathogenic Escherichia coli (APEC). PLoS ONE 2022, 17, e0279206. [Google Scholar] [CrossRef]
- Grakh, K.; Mittal, D.; Prakash, A.; Jindal, N. Characterization and Antimicrobial Susceptibility of Biofilm-Producing Avian Pathogenic Escherichia coli from Broiler Chickens and Their Environment in India. Vet. Res. Commun. 2022, 46, 537–548. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, T.H., Jr.; Pettus, K.; Trees, D. Assessment of Etest as an Alternative to Agar Dilution for Antimicrobial Susceptibility Testing of Neisseria gonorrhoeae. J. Clin. Microbiol. 2014, 52, 1435–1440. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Feng, A.; Akter, S.; Leigh, S.A.; Wang, H.; Pharr, G.T.; Evans, J.; Branton, S.L.; Landinez, M.P.; Pace, L.; Wan, X.-F. Genomic Diversity, Pathogenicity, and Antimicrobial Resistance of Escherichia coli Isolated from Poultry in the Southern United States. BMC Microbiol. 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Ngai, D.G.; Nyamache, A.K.; Ombori, O. Prevalence and Antimicrobial Resistance Profiles of Salmonella Species and Escherichia coli Isolates from Poultry Feeds in Ruiru Sub-County, Kenya. BMC Res. 2021, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Thomrongsuwannakij, T.; Blackall, P.J.; Djordjevic, S.P.; Cummins, M.L.; Chansiripornchai, N. A Comparison of Virulence Genes, Antimicrobial Resistance Profiles and Genetic Diversity of Avian Pathogenic Escherichia coli (APEC) Isolates from Broilers and Broiler Breeders in Thailand and Australia. Avian Pathol. 2020, 49, 457–466. [Google Scholar] [CrossRef]
- Peng, L.Y.; Yuan, M.; Cui, Z.Q.; Wu, Z.M.; Yu, Z.J.; Song, K.; Tang, B.; Fu, B.D. Rutin inhibits quorum sensing, biofilm formation and virulence genes in Avian Pathogenic Escherichia coli. Microb. Pathog. 2018, 119, 54–59. [Google Scholar] [CrossRef]
- Borges, K.A.; Furian, T.Q.; de Brito, B.G.; de Brito, K.C.T.; da Rocha, D.T.; Salle, C.T.P.; Moraes, H.L.S.; do Nascimento, V.P. Characterization of Avian Pathogenic Escherichia coli isolates based on biofilm formation, ESBL production, virulence-associated genes, and phylogenetic groups. Braz. J. Microbiol. 2023, 54, 2413–2425. [Google Scholar] [CrossRef]
- Najafi, S.; Rahimi, M.; Nikousefat, Z. Extra-intestinal pathogenic Escherichia coli from human and avian origin: Detection of the most common virulence-encoding genes. Vet. Res. Forum 2019, 10, 43–46. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; pp. 567–570. [Google Scholar] [CrossRef]
- Holubova, J.; Stanek, O.; Juhasz, A.; Hamidou Soumana, I.; Makovicky, P. The Fim and FhaB adhesins play a crucial role in nasal cavity infection and Bordetella pertussis transmission in a novel mouse catarrhal infection model. PLoS Pathog. 2022, 18, e1010402. [Google Scholar] [CrossRef]
- Liu, X.; Peng, X.; Li, H. Escherichia coli Activate Extraintestinal Antibody Response and Provide Anti-Infective Immunity. Int. J. Mol. Sci. 2024, 25, 7450. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, X.; Wang, Z.; Guo, G.; Xu, Z.; Zhou, Q.; Wei, X.; Liu, Y.; Zhou, L.; Tan, Z.; et al. Whole-genome analyses of APEC carrying mcr-1 in some coastal areas of China from 2019 to 2020. J. Glob. Antimicrob. 2022, 30, 370–376. [Google Scholar] [CrossRef]
- Meng, J.; Wang, W.; Ding, J.; Gu, B.; Zhou, F.; Wu, D.; Fu, X.; Qiao, M.; Liu, J. The synergy effect of matrine and berberine hydrochloride on treating colibacillosis caused by an avian highly pathogenic multidrug-resistant Escherichia coli. Poult. Sci. 2024, 103, 104151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, X.; Guo, G.; Hu, Z.; Miao, J.; Dong, Y.; Xu, Z.; Zhou, Q.; Wei, X.; Han, X.; et al. O145 may be emerging as a predominant serogroup of Avian Pathogenic Escherichia coli (APEC) in China. Vet. Microbiol. 2022, 266, 109358. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Xie, F.; Wang, X.; Dai, L.; Wang, J.; Liu, Y.; Zhao, C.; Li, Q.; Zhang, W. Epidemiological investigation and drug resistance analysis of Avian Pathogenic Escherichia coli (APEC) of Wenchang chickens in Hainan, China. Avian Pathol. 2025, 13, 1–13. [Google Scholar] [CrossRef]
- Afayibo, D.J.A.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 9, 319. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, W.; Luo, L.; Wang, H.; Shao, H.; Zhang, T.; Luo, Q. Genetic diversity and multidrug resistance of phylogenic groups B2 and D in InPEC and ExPEC isolated from chickens in Central China. BMC Microbiol. 2022, 22, 60. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Klemm, P. FimC, a chaperone-like periplasmic protein of Escherichia coli involved in biogenesis of type 1 fimbriae. Res. Microbiol. 1992, 143, 831–838. [Google Scholar] [CrossRef]
- Hu, J.; Gu, Y.; Lu, H.; Raheem, M.A.; Yu, F.; Niu, X.; Zuo, J.; Yin, H.; Huang, C.; Song, X.; et al. Identification of novel biofilm genes in Avian Pathogenic Escherichia coli by Tn5 transposon mutant library. World J. Microbiol. Biotechnol. 2022, 38, 130. [Google Scholar] [CrossRef]
- Mondal, R.; Saldaña-Ahuactzi, Z.; Soria-Bustos, J.; Schultz, A.; Yañez-Santos, J.A.; Laguna, Y.M.; Cedillo-Ramírez, M.L.; Girón, J.A. The EcpD Tip Adhesin of the Escherichia coli Common Pilus Mediates Binding of Enteropathogenic E. coli to Extracellular Matrix Proteins. Int. J. Mol. Sci. 2022, 23, 10350. [Google Scholar] [CrossRef]
- Garnett, J.A.; Martínez-Santos, V.I.; Saldaña, Z.; Pape, T.; Hawthorne, W.; Chan, J.; Simpson, P.J.; Cota, E.; Puente, J.L.; Girón, J.A.; et al. Structural insights into the biogenesis and biofilm formation by the Escherichia coli common pilus. Proc. Natl. Acad. Sci. USA 2012, 109, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.J.; Kim, S.W.; Salaheen, S.; Hovingh, E.; Van Kessel, J.A.S. Genome-Wide Analysis of Escherichia coli Isolated from Dairy Animals Identifies Virulence Factors and Genes Enriched in Multidrug-Resistant Strains. Antibiotic 2023, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Ranabhat, G.; Subedi, D.; Karki, J.; Paudel, R.; Luitel, H.; Bhattarai, R.K. Molecular detection of Avian Pathogenic Escherichia coli (APEC) in broiler meat from retail meat shop. Heliyon 2024, 10, e35661. [Google Scholar] [CrossRef] [PubMed]
- Confer, A.W.; Ayalew, S. The OmpA family of proteins: Roles in bacterial pathogenesis and immunity. Vet. Microbiol. 2013, 163, 207–222. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Xia, Y.; Li, H.; Kou, Y.; Bao, Y.; Dai, J.; Lu, C. IbeB is involved in the invasion and pathogenicity of Avian Pathogenic Escherichia coli. Vet. Microbiol. 2012, 159, 411–419. [Google Scholar] [CrossRef]
- Yun, J.; Mao, L.; Li, J.; Hao, F.; Yang, L.; Zhang, W.; Sun, M.; Liu, M.; Wang, S.; Li, W. Molecular characterization and antimicrobial resistance profile of pathogenic Escherichia coli from goats with respiratory disease in eastern China. Microb. Pathog. 2022, 166, 105501. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.H.; Wass, C.A.; Stins, M.F.; Kim, K.S. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect. Immun. 1999, 67, 4751–4756. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, H.; Jin, Q.W.; Zhang, J. The serine protease autotransporter Tsh contributes to the virulence of Edwardsiella tarda. Vet Microbiol. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Sgariglia, E.; Aconiti Mandolini, N.; Napoleoni, M.; Medici, L.; Fraticelli, R.; Conquista, M.; Gianfelici, P.; Staffolani, M.; Fisichella, S.; Capuccella, M.; et al. Antibiotic resistance pattern and virulence genesin Avian Pathogenic Escherichia coli (APEC) from different breeding systems. Vet Ital. 2019, 55, 26–33. [Google Scholar] [CrossRef]


| Gene | Primer | Sequence | Product Length (bp) |
|---|---|---|---|
| yiaA | yiaA-F | TGAAGTGTCAGGAGACGCTG | 211 |
| yiaA-R | ATGGAGAATGCGTTCCTCAAC | ||
| chuA | chuA-F | GACGAACCAACGGTCAGGAT | 279 |
| chuA-R | TGCCGCCAGTACCAAAGACA | ||
| TspE4.C 2 | TspE4-F | GAGTAATGTCGGGGCATTCA | 152 |
| TspE4-R | CGCGCCAACAAAGTATTACG |
| Antibiotics Class | Antibiotics Name | Number of Positive Isolates | Percentage of Resistance | ||
|---|---|---|---|---|---|
| Sensitivity | Intermediate | Resistance | |||
| Amphenicols | Chloramphenicol | 1 | 1 | 133 | 98.52% |
| Florfenicol | 16 | 25 | 94 | 69.62% | |
| Beta-lactams | Amoxicillin | 2 | 8 | 125 | 92.59% |
| Ceftazidime | 111 | 20 | 4 | 2.96% | |
| Quinolones | Enrofloxacin | 24 | 92 | 19 | 14.07% |
| Ofloxacin | 96 | 33 | 6 | 4.44% | |
| Tetracyclines | Tetracycline | 2 | 0 | 133 | 98.52% |
| Sulfonamides | Cotrimoxazole | 0 | 0 | 135 | 100% |
| Aminoglycosides | Amikacin | 98 | 28 | 9 | 6.66% |
| Kanamycin | 0 | 0 | 135 | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Li, M.; Nie, L.; Zuo, J.; Fan, W.; Lian, L.; Hu, J.; Chen, S.; Jiang, W.; Han, X.; et al. Molecular Epidemiology and Antibiotic Resistance Associated with Avian Pathogenic Escherichia coli in Shanxi Province, China, from 2021 to 2023. Microorganisms 2025, 13, 541. https://doi.org/10.3390/microorganisms13030541
Li F, Li M, Nie L, Zuo J, Fan W, Lian L, Hu J, Chen S, Jiang W, Han X, et al. Molecular Epidemiology and Antibiotic Resistance Associated with Avian Pathogenic Escherichia coli in Shanxi Province, China, from 2021 to 2023. Microorganisms. 2025; 13(3):541. https://doi.org/10.3390/microorganisms13030541
Chicago/Turabian StyleLi, Fangfang, Mengya Li, Lianhua Nie, Jiakun Zuo, Wenyan Fan, Liyan Lian, Jiangang Hu, Shuming Chen, Wei Jiang, Xiangan Han, and et al. 2025. "Molecular Epidemiology and Antibiotic Resistance Associated with Avian Pathogenic Escherichia coli in Shanxi Province, China, from 2021 to 2023" Microorganisms 13, no. 3: 541. https://doi.org/10.3390/microorganisms13030541
APA StyleLi, F., Li, M., Nie, L., Zuo, J., Fan, W., Lian, L., Hu, J., Chen, S., Jiang, W., Han, X., & Wang, H. (2025). Molecular Epidemiology and Antibiotic Resistance Associated with Avian Pathogenic Escherichia coli in Shanxi Province, China, from 2021 to 2023. Microorganisms, 13(3), 541. https://doi.org/10.3390/microorganisms13030541







