A Narrative Review of the Role of S-Glutathionylation in Bacteria
Abstract
1. Introduction
2. Methods
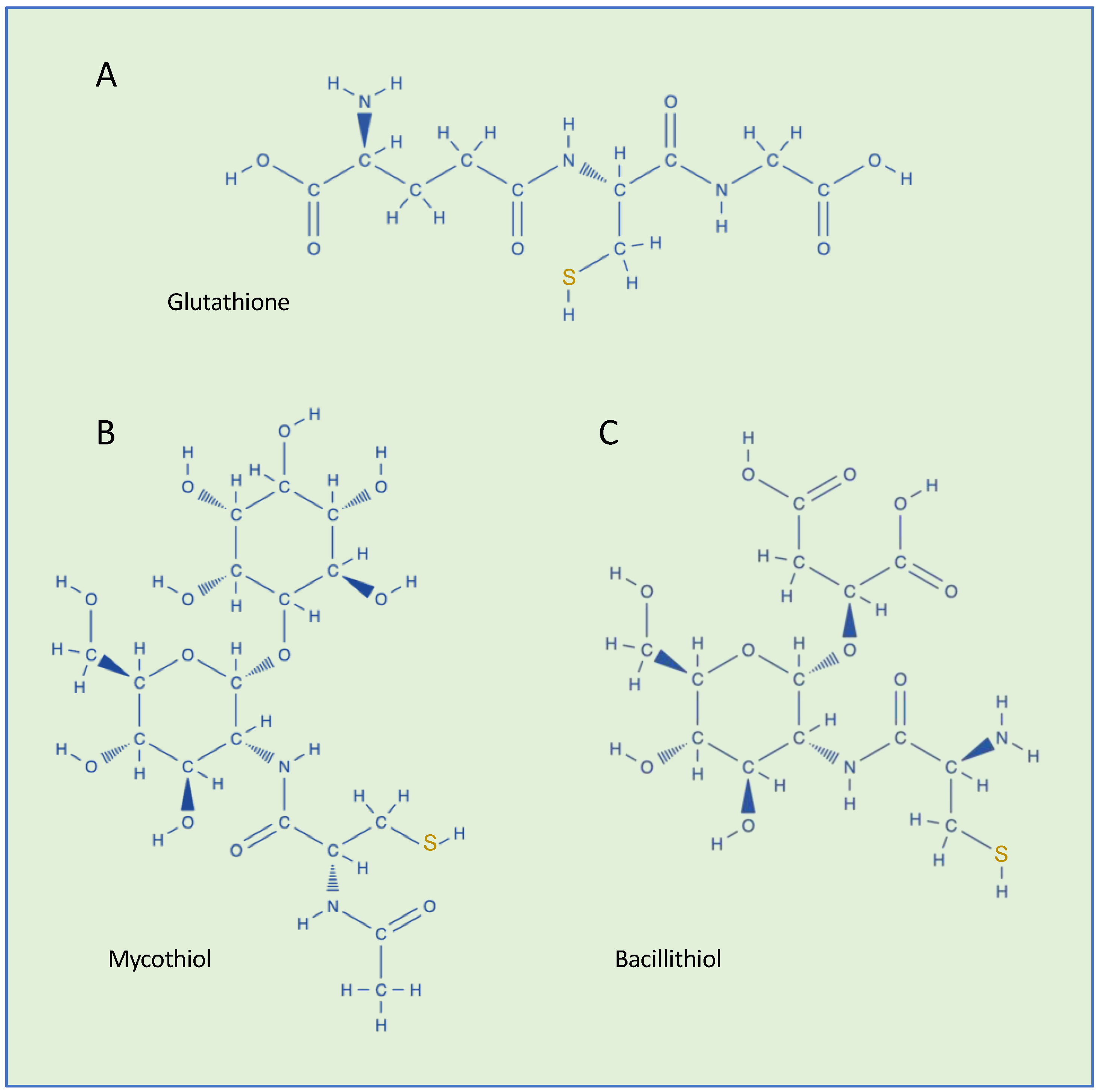
3. GS-ylation in Bacteria
3.1. Streptococcus
3.2. Escherichia coli
3.3. Yersinia pestis
3.4. Salmonella Typhimurium
3.5. Acidithiobacillus caldus
3.6. Listeria monocytogenes
3.7. Synechocystis sp.
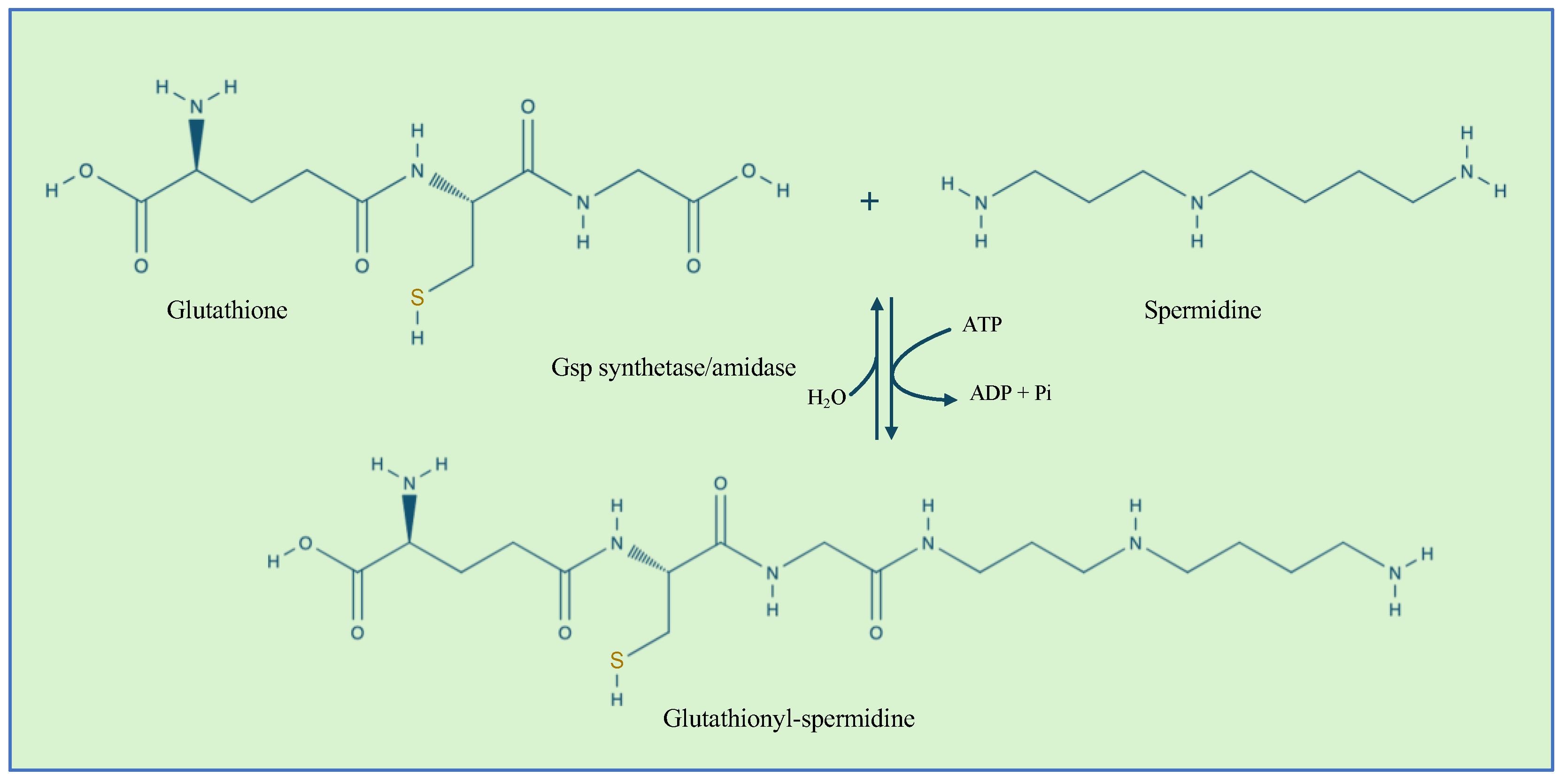
3.8. Glutathionylspermidine S-Thiolation
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramazi, S.; Zahiri, J. Post-Translational Modifications in Proteins: Resources, Tools and Prediction Methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Masulli, M.; De Laurenzi, V.; Allocati, N. The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View. Nutrients 2024, 16, 2753. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.W.K.; Gan, Y.-H. New Roles for Glutathione: Modulators of Bacterial Virulence and Pathogenesis. Redox Biol. 2021, 44, 102012. [Google Scholar] [CrossRef] [PubMed]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione Compartmentalization and Its Role in Glutathionylation and Other Regulatory Processes of Cellular Pathways. BioFactors 2019, 45, 152–168. [Google Scholar] [CrossRef]
- Pingarron-Cardenas, G.; Onkokesung, N.; Goldberg-Cavalleri, A.; Lange, G.; Dittgen, J.; Edwards, R. Selective Herbicide Safening in Dicot Plants: A Case Study in Arabidopsis. Front. Plant Sci. 2024, 14, 1335764. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; He, Y.-Q.; Ye, T.-T.; Huang, X.; Wu, H.; Ma, T.-X.; Pritchard, H.W.; Wang, X.-F.; Xue, H. Glutathionylation of a Glycolytic Enzyme Promotes Cell Death and Vigor Loss During Aging of Elm Seeds. Plant Physiol. 2024, 195, 2596–2616. [Google Scholar] [CrossRef]
- Pretzel, J.; Gehr, M.; Eisenkolb, M.; Wang, L.; Fritz-Wolf, K.; Rahlfs, S.; Becker, K.; Jortzik, E. Characterization and Redox Regulation of Plasmodium falciparum Methionine Adenosyltransferase. J. Biochem. 2016, 160, 355–367. [Google Scholar] [CrossRef]
- Schipper, S.; Wu, H.; Furdui, C.M.; Poole, L.B.; Delahunty, C.M.; Park, R.; Yates, J.R.; Becker, K.; Przyborski, J.M. Identification of Sulfenylation Patterns in Trophozoite Stage Plasmodium falciparum Using a Non-Dimedone Based Probe. Mol. Biochem. Parasitol. 2021, 242, 111362. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Lam, S.M.; Yuan, H.; Shui, G.; Yang, J. The Role of Glutathione-Mediated Triacylglycerol Synthesis in the Response to Ultra-High Cadmium Stress in Auxenochlorella protothecoides. J. Environ. Sci. 2021, 108, 58–69. [Google Scholar] [CrossRef]
- Gergondey, R.; Garcia, C.; Serre, V.; Camadro, J.M.; Auchère, F. The Adaptive Metabolic Response Involves Specific Protein Glutathionylation during the Filamentation Process in the Pathogen Candida albicans. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 1309–1323. [Google Scholar] [CrossRef]
- Shino, S.; Nasuno, R.; Takagi, H. S-Glutathionylation of Fructose-1,6-Bisphosphate Aldolase Confers Nitrosative Stress Tolerance on Yeast Cells via a Metabolic Switch. Free Radic. Biol. Med. 2022, 193, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Christiaens, R.; Janssens, P.; Collin, S. Unexpected Behavior of a Maltose-Negative Saccharomyces cerevisiae Yeast: Higher Release of Polyfunctional Thiols from Glutathionylated Than from Cysteinylated S-Conjugates. Fermentation 2024, 10, 276. [Google Scholar] [CrossRef]
- Vicente, J.; Kiene, F.; Fracassetti, D.; De Noni, I.; Shemehen, R.; Tarasov, A.; Dobrydnev, A.V.; Marquina, D.; Santos, A.; Rauhut, D.; et al. Precursors Consumption Preferences and Thiol Release Capacity of the Wine Yeasts Saccharomyces cerevisiae, Torulaspora delbrueckii, and Lachancea thermotolerans. Int. J. Food Microbiol. 2024, 425, 110858. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Glutathione Transferases in Bacteria: Bacterial GSTs. FEBS J. 2009, 276, 58–75. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Distribution of Glutathione Transferases in Gram-Positive Bacteria and Archaea. Biochimie 2012, 94, 588–596. [Google Scholar] [CrossRef]
- Sikanyika, M.; Aragão, D.; McDevitt, C.A.; Maher, M.J. The Structure and Activity of the Glutathione Reductase from Streptococcus pneumoniae. Acta Crystallogr. F Struct. Biol. Commun. 2019, 75, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox Regulation by Reversible Protein S-Thiolation in Gram-Positive Bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Maupin-Furlow, J.A. Redox and Thiols in Archaea. Antioxidants 2020, 9, 381. [Google Scholar] [CrossRef]
- Walker, E.A.; Port, G.C.; Caparon, M.G.; Janowiak, B.E. Glutathione Synthesis Contributes to Virulence of Streptococcus agalactiae in a Murine Model of Sepsis. J. Bacteriol. 2019, 201, 10-1128. [Google Scholar] [CrossRef]
- Potter, A.J.; Trappetti, C.; Paton, J.C. Streptococcus pneumoniae Uses Glutathione To Defend against Oxidative Stress and Metal Ion Toxicity. J. Bacteriol. 2012, 194, 6248–6254. [Google Scholar] [CrossRef]
- Sperandio, B.; Gautier, C.; Pons, N.; Ehrlich, D.S.; Renault, P.; Guédon, E. Three Paralogous LysR-Type Transcriptional Regulators Control Sulfur Amino Acid Supply in Streptococcus mutans. J. Bacteriol. 2010, 192, 3464–3473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Zhang, C.; Li, C.; Zhou, J.; Xu, X.; Peng, X.; Zhou, X. S-Glutathionylation Proteome Profiling Reveals a Crucial Role of a Thioredoxin-like Protein in Interspecies Competition and Cariogenecity of Streptococcus mutans. PLoS Pathog. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, V.N.; Linzner, N.; Busche, T.; Said, N.; Weise, C.; Kalinowski, J.; Wahl, M.C.; Antelmann, H. The MerR -family Regulator NmlR Is Involved in the Defense against Oxidative Stress in Streptococcus pneumoniae. Mol. Microbiol. 2023, 119, 191–207. [Google Scholar] [CrossRef]
- Potter, A.J.; Kidd, S.P.; McEwan, A.G.; Paton, J.C. The MerR/NmlR Family Transcription Factor of Streptococcus pneumoniae Responds to Carbonyl Stress and Modulates Hydrogen Peroxide Production. J. Bacteriol. 2010, 192, 4063–4066. [Google Scholar] [CrossRef]
- McEwan, A.G.; Djoko, K.Y.; Chen, N.H.; Couñago, R.L.M.; Kidd, S.P.; Potter, A.J.; Jennings, M.P. Novel Bacterial MerR-like Regulators. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 58, pp. 1–22. ISBN 978-0-12-381043-4. [Google Scholar]
- Schumacher, M.A.; Den Hengst, C.D.; Bush, M.J.; Le, T.B.K.; Tran, N.T.; Chandra, G.; Zeng, W.; Travis, B.; Brennan, R.G.; Buttner, M.J. The MerR-like Protein BldC Binds DNA Direct Repeats as Cooperative Multimers to Regulate Streptomyces Development. Nat. Commun. 2018, 9, 1139. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Mayer, M.P. The Hsp70-Chaperone Machines in Bacteria. Front. Mol. Biosci. 2021, 8, 694012. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wu, S.; Gong, W.; Chen, C.; Perrett, S. Glutathionylation of the Bacterial Hsp70 Chaperone DnaK Provides a Link between Oxidative Stress and the Heat Shock Response. J. Biol. Chem. 2016, 291, 6967–6981. [Google Scholar] [CrossRef]
- Heesemann, J.; Sing, A.; Trülzsch, K. Yersinia’s Stratagem: Targeting Innate and Adaptive Immune Defense. Curr. Opin. Microbiol. 2006, 9, 55–61. [Google Scholar] [CrossRef]
- Mitchell, A.; Tam, C.; Elli, D.; Charlton, T.; Osei-Owusu, P.; Fazlollahi, F.; Faull, K.F.; Schneewind, O. Glutathionylation of Yersinia pestis LcrV and Its Effects on Plague Pathogenesis. mBio 2017, 8, e00646-17. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Ansong, C.; Wu, S.; Meng, D.; Liu, X.; Brewer, H.M.; Deatherage Kaiser, B.L.; Nakayasu, E.S.; Cort, J.R.; Pevzner, P.; Smith, R.D.; et al. Top-down Proteomics Reveals a Unique Protein S-Thiolation Switch in Salmonella typhimurium in Response to Infection-like Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 10153–10158. [Google Scholar] [CrossRef]
- Brown, K.A.; Melby, J.A.; Roberts, D.S.; Ge, Y. Top-down Proteomics: Challenges, Innovations, and Applications in Basic and Clinical Research. Expert. Rev. Proteom. 2020, 17, 719–733. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Y.; Lin, J.; Liu, X.; Pang, X.; Lin, J. Acidithiobacillus caldus Sulfur Oxidation Model Based on Transcriptome Analysis between the Wild Type and Sulfur Oxygenase Reductase Defective Mutant. PLoS ONE 2012, 7, e39470. [Google Scholar] [CrossRef]
- Rühl, P.; Haas, P.; Seipel, D.; Becker, J.; Kletzin, A. Persulfide Dioxygenase From Acidithiobacillus caldus: Variable Roles of Cysteine Residues and Hydrogen Bond Networks of the Active Site. Front. Microbiol. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Morton, C.J.; Sani, M.-A.; Parker, M.W.; Separovic, F. Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation: Focus Review. Chem. Rev. 2019, 119, 7721–7736. [Google Scholar] [CrossRef]
- Portman, J.L.; Huang, Q.; Reniere, M.L.; Iavarone, A.T.; Portnoy, D.A. Activity of the Pore-Forming Virulence Factor Listeriolysin O Is Reversibly Inhibited by Naturally Occurring S-Glutathionylation. Infect. Immun. 2017, 85, e00959-16. [Google Scholar] [CrossRef]
- Walter, J.M.; Coutinho, F.H.; Dutilh, B.E.; Swings, J.; Thompson, F.L.; Thompson, C.C. Ecogenomics and Taxonomy of Cyanobacteria Phylum. Front. Microbiol. 2017, 8, 2132. [Google Scholar] [CrossRef]
- McIntosh, C.L.; Germer, F.; Schulz, R.; Appel, J.; Jones, A.K. The [NiFe]-Hydrogenase of the Cyanobacterium Synechocystis sp. PCC 6803 Works Bidirectionally with a Bias to H2 Production. J. Am. Chem. Soc. 2011, 133, 11308–11319. [Google Scholar] [CrossRef]
- Horch, M.; Lauterbach, L.; Lenz, O.; Hildebrandt, P.; Zebger, I. NAD(H)-Coupled Hydrogen Cycling—Structure-Function Relationships of Bidirectional [NiFe] Hydrogenases. FEBS Lett. 2012, 586, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Lindblad, P. Transcriptional Regulation of the Cyanobacterial Bidirectional Hox-Hydrogenase. Dalton Trans. 2009, 45, 9990–9996. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, J.; Saenkham, P.; Sakr, S.; Leplat, C.; Ortega-Ramos, M.; Bottin, H.; Cournac, L.; Cassier-Chauvat, C.; Chauvat, F. The AbrB2 Autorepressor, Expressed from an Atypical Promoter, Represses the Hydrogenase Operon To Regulate Hydrogen Production in Synechocystis Strain PCC6803. J. Bacteriol. 2012, 194, 5423–5433. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Dutheil, J.; Saenkham, P.; Bottin, H.; Leplat, C.; Ortega-Ramos, M.; Aude, J.-C.; Chapuis, V.; Guedeney, G.; Decottignies, P.; et al. The Activity of the Synechocystis PCC6803 AbrB2 Regulator of Hydrogen Production Can Be Post-Translationally Controlled through Glutathionylation. Int. J. Hydrogen Energy 2013, 38, 13547–13555. [Google Scholar] [CrossRef]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Maréchal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First Proteomic Study of S-Glutathionylation in Cyanobacteria. J. Proteome Res. 2015, 14, 59–71. [Google Scholar] [CrossRef]
- Lin, J.; Chiang, B.-Y.; Chou, C.-C.; Chen, T.-C.; Chen, Y.-J.; Chen, Y.-J.; Lin, C.-H. Glutathionylspermidine in the Modification of Protein SH Groups: The Enzymology and Its Application to Study Protein Glutathionylation. Molecules 2015, 20, 1452–1474. [Google Scholar] [CrossRef]
- Chiang, B.-Y.; Chen, T.-C.; Pai, C.-H.; Chou, C.-C.; Chen, H.-H.; Ko, T.-P.; Hsu, W.-H.; Chang, C.-Y.; Wu, W.-F.; Wang, A.H.-J.; et al. Protein S-Thiolation by Glutathionylspermidine (Gsp). J. Biol. Chem. 2010, 285, 25345–25353. [Google Scholar] [CrossRef]
- Nair, A.V.; Singh, A.; Rajmani, R.S.; Chakravortty, D. Salmonella Typhimurium Employs Spermidine to Exert Protection against ROS-Mediated Cytotoxicity and Rewires Host Polyamine Metabolism to Ameliorate Its Survival in Macrophages. Redox Biol. 2024, 72, 103151. [Google Scholar] [CrossRef]

| Organism | Target Protein | Target Residue | Effect of GS-Ylation | Ref. |
|---|---|---|---|---|
| Streptococcus mutants | thioredoxin-like protein | Cys41 | Protection from toxic effect of ROS | [22] |
| Streptococcus pneumoniae | NmlR | Cys52 | Redox-sensing transcriptional activator of the adhC geme | [24] |
| Escherichia coli | DnaK | Cys15 | Inactivation of DnaK | [30] |
| Yersinia pestis | LcrV protein | Cys273 | Resistance to phagocytosis | [32] |
| Acidithiobacillus caldus | Persulfide dioxygenases | Cys87/Cys224 | Protective mechanism against uncontrolled thiol oxidation | [37] |
| Listeria monocytogenes | listeriolysin O | Cys484 | Inhibition of toxin activity | [39] |
| Synechocystis sp. | ABrB2 regulator | Cys34 | Protection from oxidative stress | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federici, L.; Masulli, M.; De Laurenzi, V.; Allocati, N. A Narrative Review of the Role of S-Glutathionylation in Bacteria. Microorganisms 2025, 13, 527. https://doi.org/10.3390/microorganisms13030527
Federici L, Masulli M, De Laurenzi V, Allocati N. A Narrative Review of the Role of S-Glutathionylation in Bacteria. Microorganisms. 2025; 13(3):527. https://doi.org/10.3390/microorganisms13030527
Chicago/Turabian StyleFederici, Luca, Michele Masulli, Vincenzo De Laurenzi, and Nerino Allocati. 2025. "A Narrative Review of the Role of S-Glutathionylation in Bacteria" Microorganisms 13, no. 3: 527. https://doi.org/10.3390/microorganisms13030527
APA StyleFederici, L., Masulli, M., De Laurenzi, V., & Allocati, N. (2025). A Narrative Review of the Role of S-Glutathionylation in Bacteria. Microorganisms, 13(3), 527. https://doi.org/10.3390/microorganisms13030527







