Spontaneous Bio-Recycling: Recovering Bioactive Molecules Through Endogenous Microbial Maceration of Hemp Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Hemp Residues
2.2. The Maceration Process
2.3. The Evolution of the Microbial Community During the Hemp Maceration Process
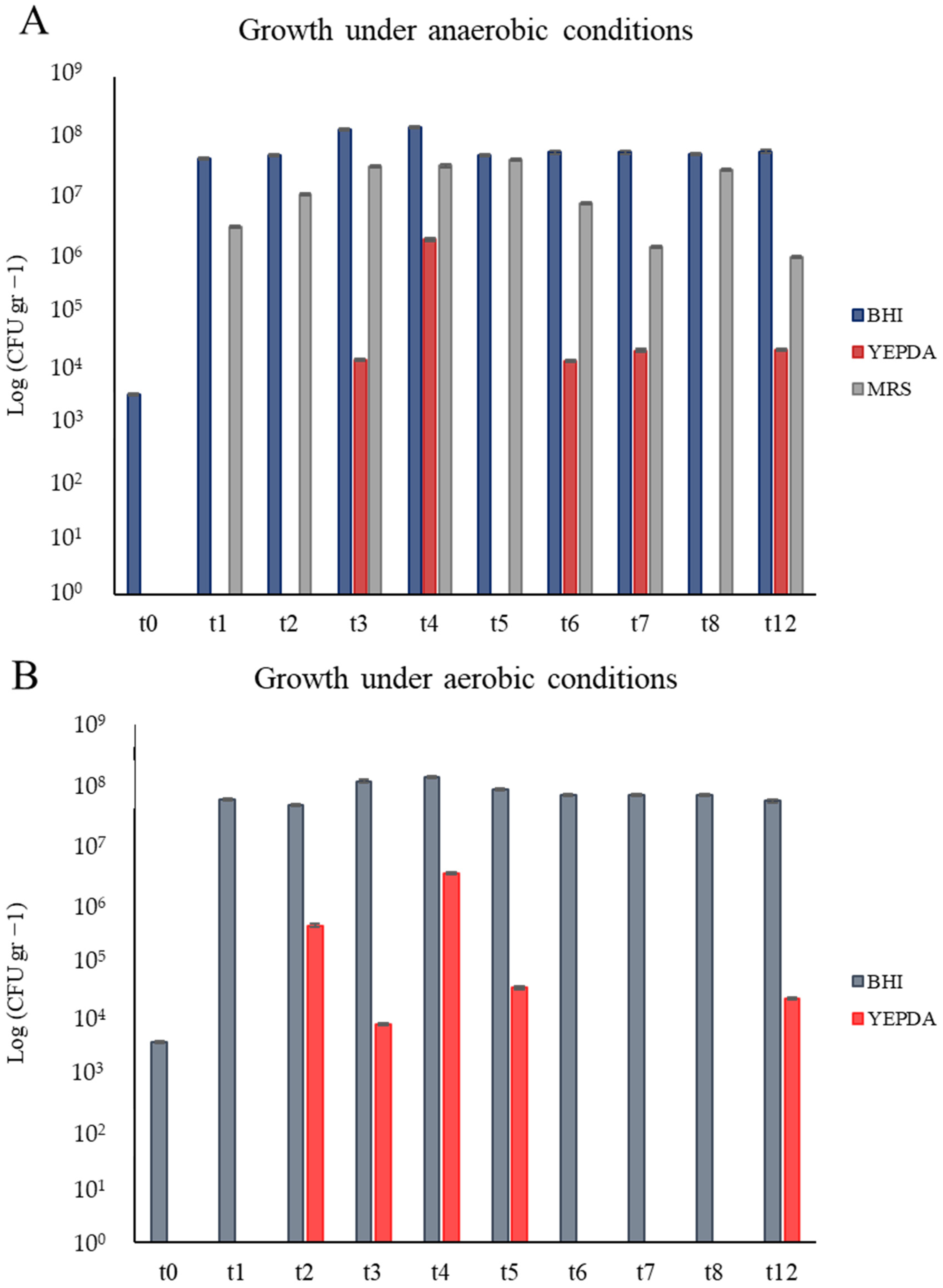
2.3.1. Viable Microbial Cell Count
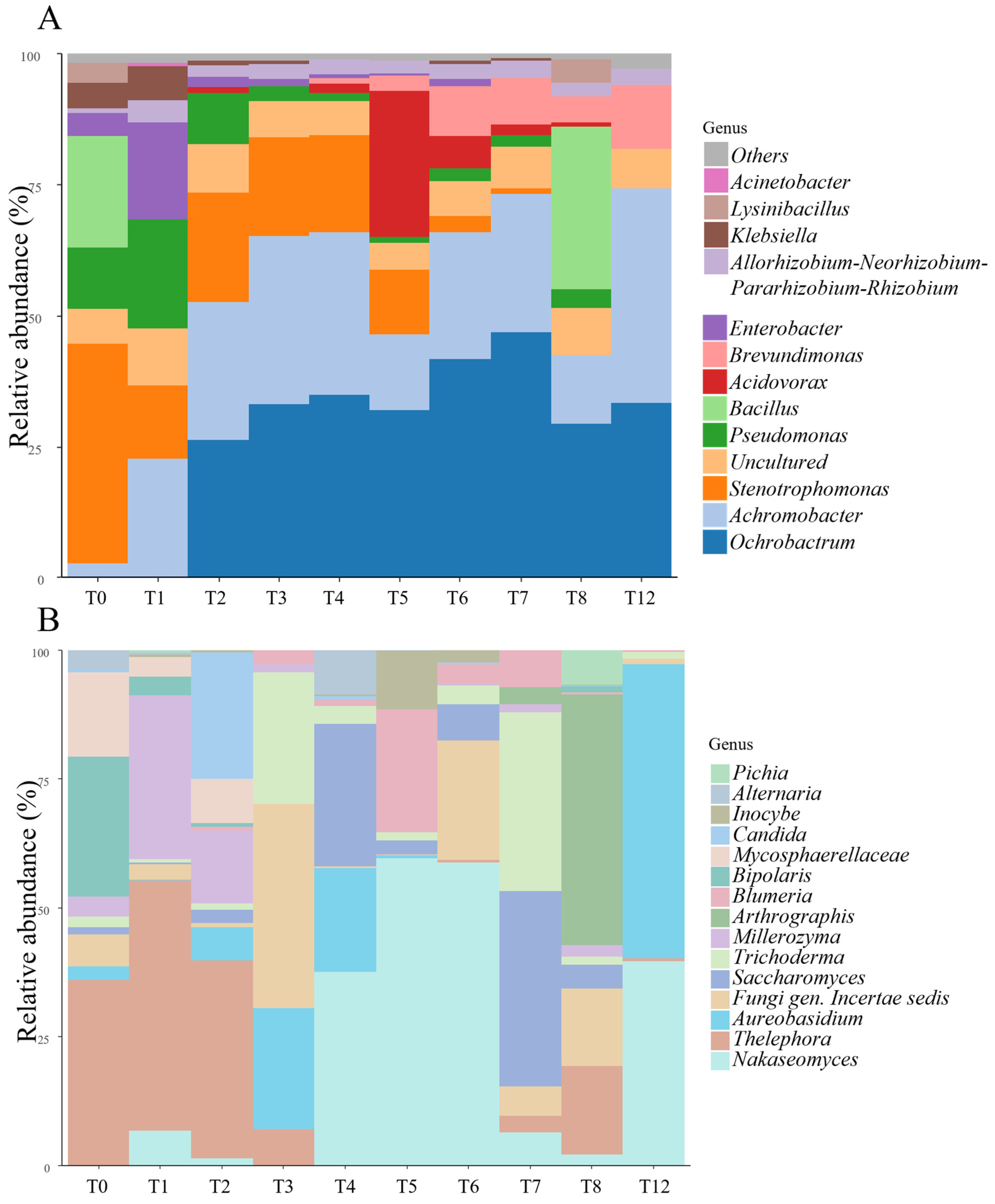
2.3.2. Taxonomic Composition with 16S rRNA Gene and ITS Metabarcoding
DNA Extraction
PCR Amplification and Oxford Nanopore (ONT) Sequencing
Sequence Analysis
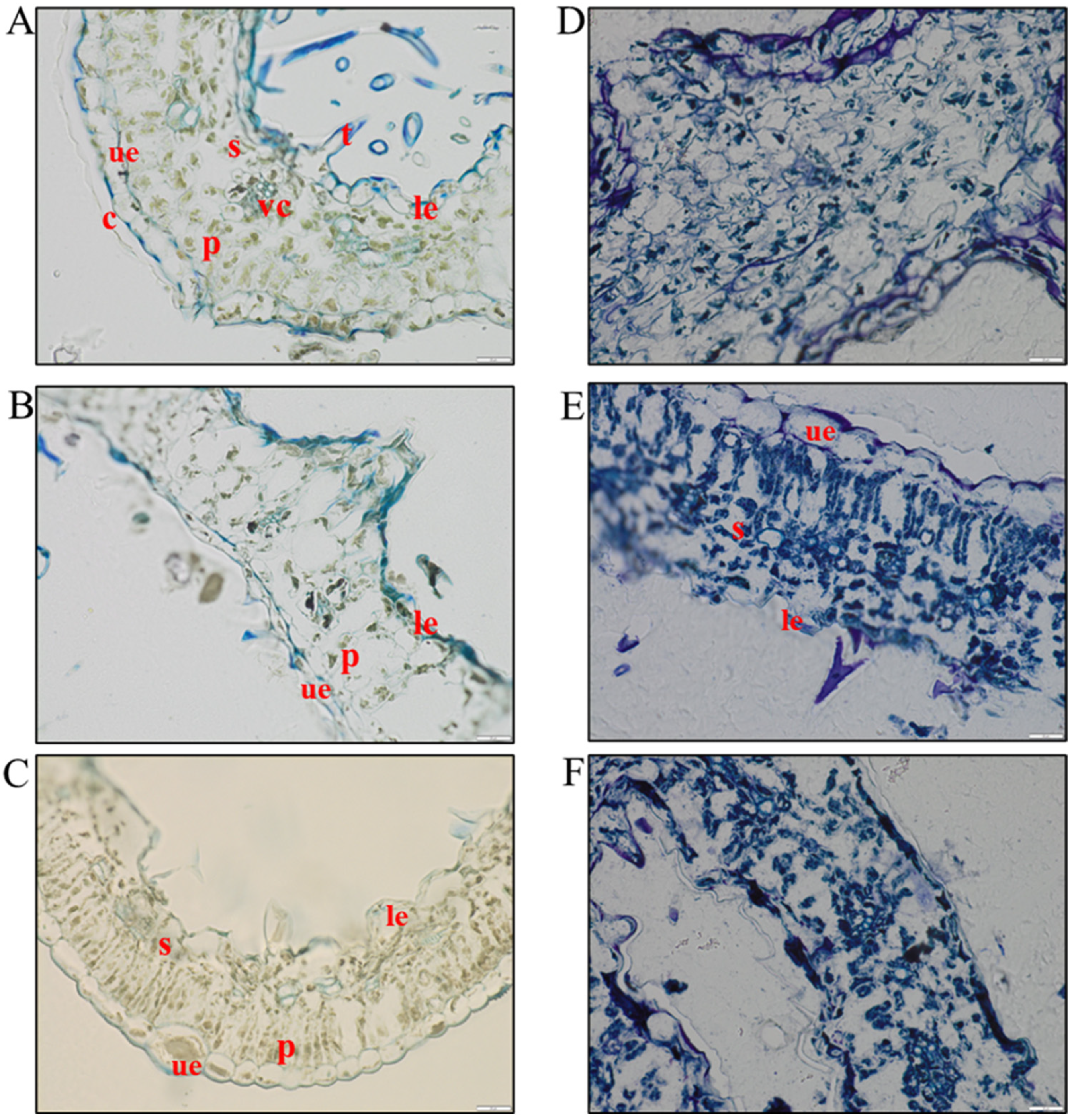
2.3.3. Light Microscopy and Image Analysis
2.4. Hemp Extracts (HWEs)
2.5. Hemp Extracts (HEs) Chemo-Physical and Biological Characterization
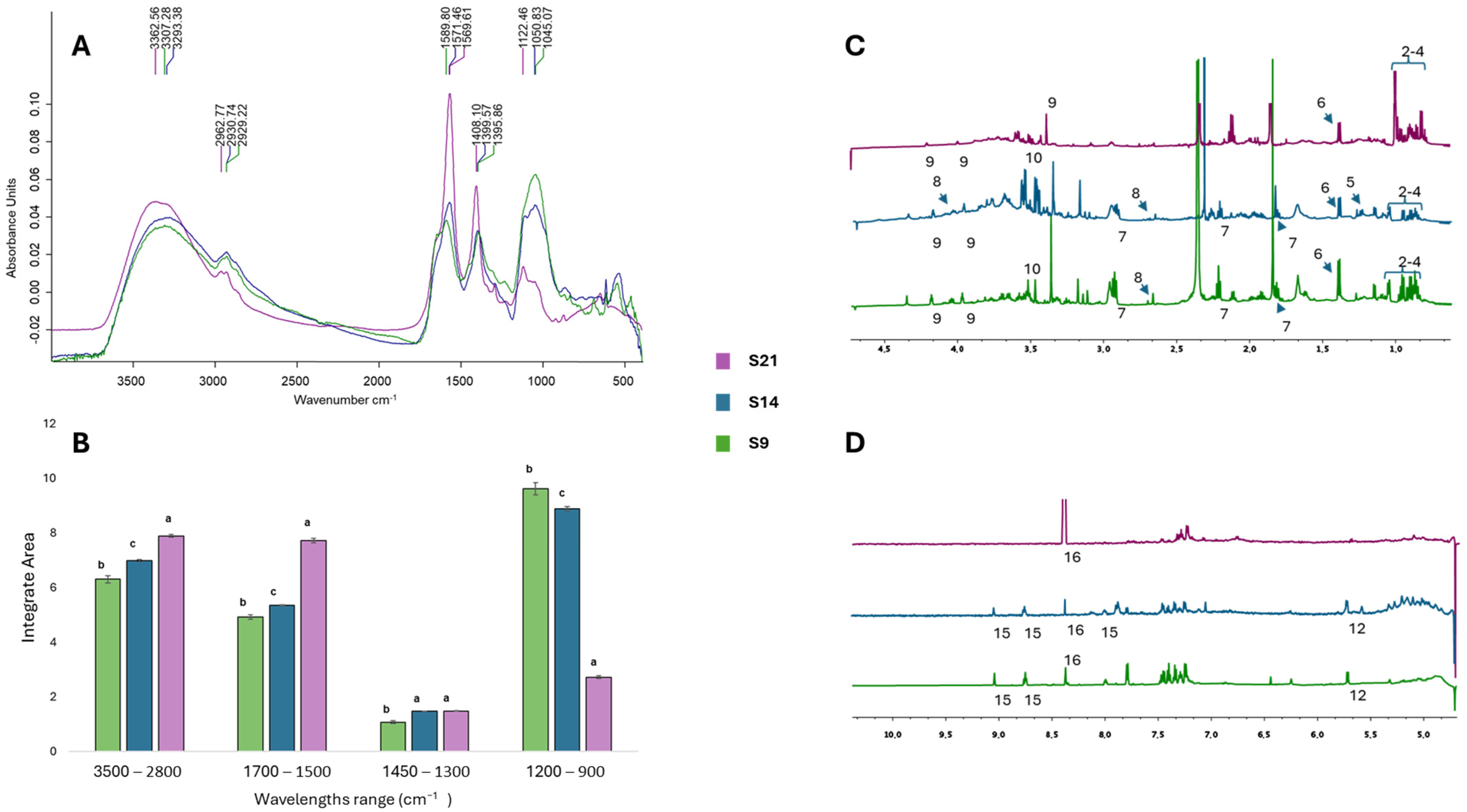
2.5.1. NMR Analysis
2.5.2. FTIR Analysis
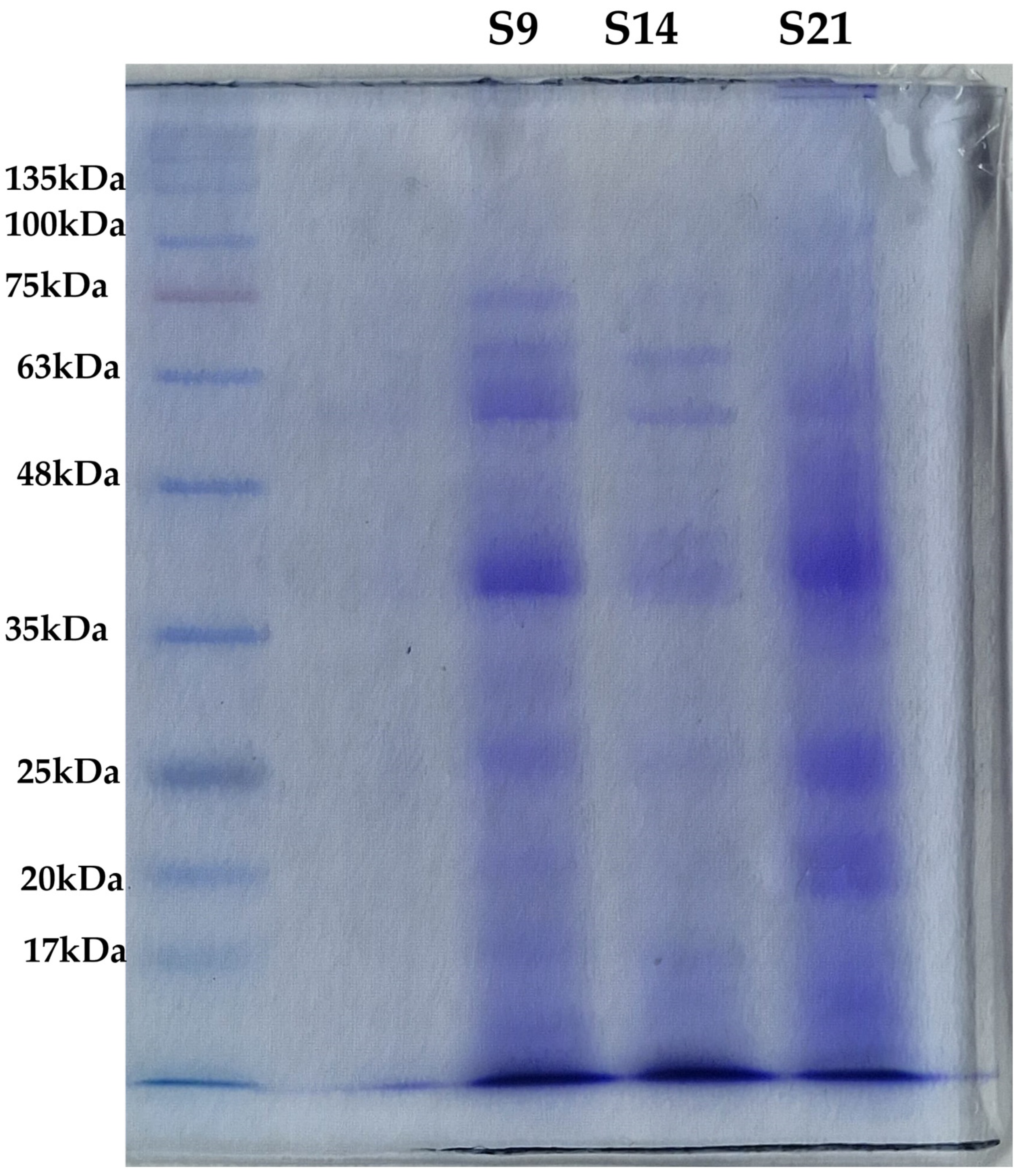
2.5.3. SDS-PAGE
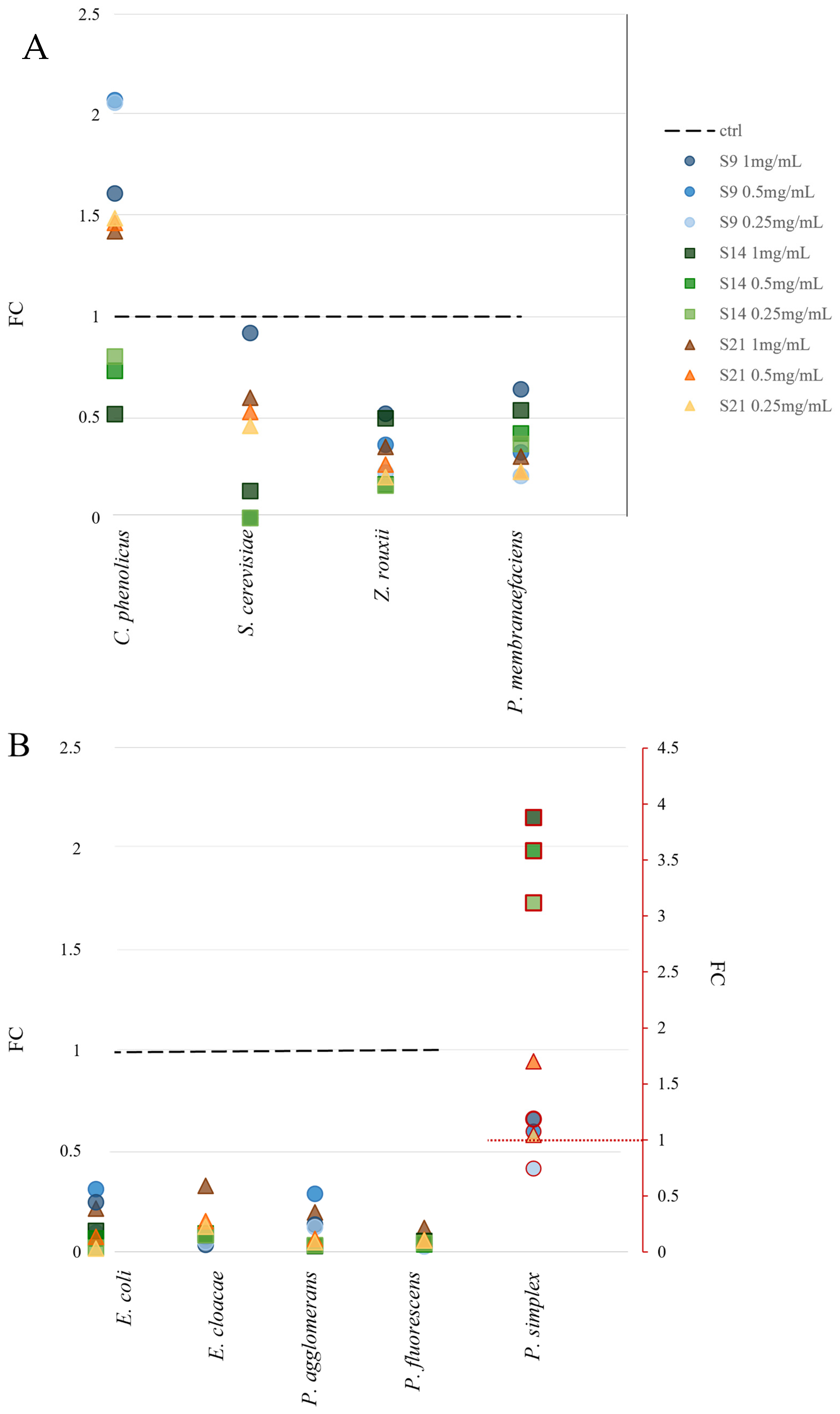
2.5.4. Growth Assay with HEs as Source of Nutrients
3. Results
3.1. Histological Analysis to Assess the Level of Hemp Degradation
3.2. Evaluating Microbial Dynamics During Hemp Degradation
3.2.1. Cultural-Dependent Analysis
3.2.2. Metabarcoding
3.3. Characterization of the Hemp Extracts
3.3.1. Chemical Characterization with NMR and FTIR
3.3.2. Biochemical Characterization with SDS-PAGE
3.4. Hemp Extracts as Nutrients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodríguez-Linzán, I.; Saquete, M.D.; Luque, R.; Osman, S.M.; Boluda-Botella, N.; Manuel, R.-D.J. Silica-derived materials from agro-industrial waste biomass: Characterization and comparative studies. Environ. Res. 2023, 231, 116002. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F.J.; Chemistry, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Valencia, G.A.; de Andrade, C.J.; Ienczak, J.L.; Monteiro, A.R.; Gutiérrez, T.J. Valorization of agri-food wastes. Fermentation 2021, 7, 111–132. [Google Scholar] [CrossRef]
- Sun, X.; Dou, Z.; Shurson, G.C.; Hu, B. Bioprocessing to upcycle agro-industrial and food wastes into high-nutritional value animal feed for sustainable food and agriculture systems. Resour. Conserv. Recycl. 2024, 201, 107325. [Google Scholar] [CrossRef]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of agro-industrial wastes into animal feed through microbial fermentation: A review of the global and Ghanaian case. Heliyon 2023, 9, e14814. [Google Scholar] [CrossRef]
- Olatunji, O. Some Non-plastic Materials and Their Environmental Impacts. In Re-envisioning Plastics Role in the Global Society: Perspectives on Food, Urbanization, and Environment; Springer Nature: Cham, Switzerland, 2024; pp. 139–169. [Google Scholar]
- Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Montesinos-Pereira, D.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Development of a green analytical methodology based on a deep eutectic solvent for the determination of pesticide residues in industrial hemp. Microchem. J. 2024, 199, 109955. [Google Scholar] [CrossRef]
- Robertson, K.J.; Brar, R.; Randhawa, P.; Stark, C.; Baroutian, S. Opportunities and challenges in waste management within the medicinal cannabis sector. Ind. Crops. Prod. 2023, 197, 116639. [Google Scholar] [CrossRef]
- Tripathi, M.; Sharma, M.; Bala, S.; Connell, J.; Newbold, J.R.; Rees, R.M.; Aminabhavi, T.M.; Thakur, V.K.; Gupta, V.K.J.S.; Technology, P. Conversion technologies for valorization of hemp lignocellulosic biomass for potential biorefinery applications. Sep. Purif. Technol. 2023, 320, 124018. [Google Scholar] [CrossRef]
- Parvez, A.M.; Lewis, J.D.; Afzal, M.T.; Reviews, S.E. Potential of industrial hemp (Cannabis sativa L.) for bioenergy production in Canada: Status, challenges and outlook. Renew. Sustain. Energy Rev. 2021, 141, 110784. [Google Scholar] [CrossRef]
- Kladar, N.; Čonić, B.S.; Božin, B.; Torović, L. European hemp-based food products–Health concerning cannabinoids exposure assessment. Food Control 2021, 129, 108233. [Google Scholar] [CrossRef]
- Cabral, E.M.; Zhu, X.; Garcia-Vaquero, M.; Pérez-Vila, S.; Tang, J.; Gómez-Mascaraque, L.G.; Poojary, M.M.; Curtin, J.; Tiwari, B.K.J.F. Recovery of Protein from Industrial Hemp Waste (Cannabis sativa, L.) Using High-Pressure Processing and Ultrasound Technologies. Foods 2023, 12, 2883. [Google Scholar] [CrossRef]
- Sciacca, F.; Virzì, N.; Pecchioni, N.; Melilli, M.G.; Buzzanca, C.; Bonacci, S.; Di Stefano, V. Functional End-Use of Hemp Seed Waste: Technological, Qualitative, Nutritional, and Sensorial Characterization of Fortified Bread. Sustainability 2023, 15, 12899. [Google Scholar] [CrossRef]
- Meffo Kemda, M.; Marchi, M.; Neri, E.; Marchettini, N.; Niccolucci, V. Environmental impact assessment of hemp cultivation and its seed-based food products. Front. Environ. Sci. 2024, 12, 1342330. [Google Scholar] [CrossRef]
- Altman, A.; Kent-Dennis, C.; Klotz, J.; McLeod, K.; Vanzant, E.; Harmon, D. Utilizing industrial hemp (Cannabis sativa L.) by-products in livestock rations. Anim. Feed. Sci. Technol. 2023, 307, 115850. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial hemp fibers: An overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Cheng, L.; Liu, L.; Zhang, R.; Zhao, S.; Ma, L. Combined effects of organic solvent and ultrasonic pretreatments on cellulose fiber extraction from hemp. Ind. Crops. Prod. 2024, 220, 119436. [Google Scholar] [CrossRef]
- Shin, S.-J.; Sung, Y.J. Improving enzymatic hydrolysis of industrial hemp (Cannabis sativa L.) by electron beam irradiation. Radiat. Phys. Chem. 2008, 77, 1034–1038. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 2014, 7, 1991–1999. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-rot fungi pretreatment combined with alkaline/oxidative pretreatment to improve enzymatic saccharification of industrial hemp. Bioresour. Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.-H.; Wang, Q.; Han, J.-R. Nutritional components and antioxidant properties of seven kinds of cereals fermented by the basidiomycete Agaricus blazei. J. Cereal Sci. 2015, 65, 202–208. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sabaratnam, V.; Kuppusamy, U.R.; Tan, Y.S. Solid-substrate fermentation of wheat grains by mycelia of indigenous species of the genus Ganoderma (higher Basidiomycetes) to enhance the antioxidant activities. Int. J. Med. Mushrooms 2014, 16, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Saharan, P.; Duhan, S.; Duhan, J.S. Bio-enrichment of phenolics and antioxidant activity of combination of Oryza sativa and Lablab purpureus fermented with GRAS filamentous fungi. Resour. Effic. Technol. 2017, 3, 347–352. [Google Scholar] [CrossRef]
- Mendez-Carmona, J.; Ramírez-Guzman, K.N.; Ascacio-Valdes, J.A.; Sepulveda, L.; Aguilar, C.N.; Technologies, E. Solid-state fermentation for recovery of carotenoids from tomato waste. Innov. Food Sci. Emerg. Technol. 2022, 80, 103108. [Google Scholar] [CrossRef]
- Arapoglou, D.; Eliopoulos, C.; Markou, G.; Langousi, I.; Saxami, G.; Haroutounian, S.A. Nutritional upgrade of olive mill stone waste, walnut shell and their mixtures by applying solid state fermentation initiated by Pleurotus ostreatus. Sci. Rep. 2024, 14, 13446. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Neacsu, M.; Christie, J.S.; Duncan, G.J.; Vaughan, N.J.; Russell, W.R. Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development. Nutraceuticals 2022, 2, 150–161. [Google Scholar] [CrossRef]
- Šťastník, O.; Mrkvicová, E.; Pavlata, L. Industrial hemp in animal feed applications. In Industrial Hemp; Pojić, M., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 341–365. [Google Scholar]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Liesack, W. Nucleic acids and classification. In The New Bacterial Systematics; Academic Press: Cambridge, MA, USA, 1993; pp. 151–194. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B.; List, F.B.C.A.; Bolchacova, E. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Cerri, M.; Reale, L.; Zadra, C. Metabolite storage in Theobroma cacao L. seed: Cyto-histological and phytochemical analyses. Front. Plant Sci. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Donati, L.; Casagrande Pierantoni, D.; Conti, A.; Calzoni, E.; Corte, L.; Santi, C.; Rosati, O.; Cardinali, G.; Emiliani, C. Water Extracts from Industrial Hemp Waste Inhibit the Adhesion and Development of Candida Biofilm and Showed Antioxidant Activity on HT-29 Colon Cancer Cells. Int. J. Mol. Sci. 2024, 25, 3979. [Google Scholar] [CrossRef] [PubMed]
- Essendoubi, M.; Toubas, D.; Bouzaggou, M.; Pinon, J.-M.; Manfait, M.; Sockalingum, G.D. Rapid identification of Candida species by FT-IR microspectroscopy. Biochim. Biophys. Acta Gen. Subj. 2005, 1724, 239–247. [Google Scholar] [CrossRef]
- Fanglian, H.E. Laemmli-SDS-PAGE. Bio-Protocol 2011, 1, e80. [Google Scholar]
- Jha, C.K.; Aeron, A.; Patel, B.V.; Maheshwari, D.K.; Saraf, M. Enterobacter: Role in plant growth promotion. Bact. Agrobiol. Plant Growth Responses 2011, 159–182. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex—A little known PGPB with anti-fungal activity—Alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013, 3, 595–620. [Google Scholar] [CrossRef]
- Shinde, S.; Cumming, J.R.; Collart, F.R.; Noirot, P.H.; Larsen, P.E. Pseudomonas fluorescens transportome is linked to strain-specific plant growth promotion in aspen seedlings under nutrient stress. Front. Plant Sci. 2017, 8, 348. [Google Scholar] [CrossRef]
- Suman, A.; Shukla, L.; Marag, P.; Verma, P.; Gond, S.; Prasad, J.S. Potential use of plant colonizing Pantoea as generic plant growth promoting bacteria for cereal crops. J. Environ. Biol. 2020, 41, 987–994. [Google Scholar] [CrossRef]
- Ahmadi, F.; Najafpour, G.D.; Mohammadi, M. Production of bio-fungicide from sugarcane bagasse using Pichia membranifaciens yeast and its activity against post-harvest pathogenic fungi. Biointerface Res. Appl. Chem. 2021, 11, 10435–10445. [Google Scholar]
- Nimsi, K.; Manjusha, K.; Kathiresan, K.; Arya, H. Plant growth-promoting yeasts (PGPY), the latest entrant for use in sustainable agriculture: A review. J. Appl. Microbiol. 2023, 134, lxac088. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.E.-S.; El-Nady, M.F. Application of Saccharomyces cerevisiae as a biocontrol agent against Fusarium infection of sugar beet plants. Acta Biol. Szeged. 2008, 52, 271–275. [Google Scholar]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microbiol. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef] [PubMed]
- Vadkertiová, R.; Dudášová, H.; Balaščáková, M. Yeasts in agricultural and managed soils. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.-A., Yurkov, A., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 117–144. [Google Scholar]
- Geminiani, L.; Campione, F.P.; Corti, C.; Luraschi, M.; Motella, S.; Recchia, S.; Rampazzi, L. Differentiating between natural and modified cellulosic fibres using ATR-FTIR spectroscopy. Heritage 2022, 5, 4114–4139. [Google Scholar] [CrossRef]
- Olaru, A.; Malutan, T.; Ursescu, C.M.; Geba, M.; Stratulat, L. Structural changes in hemp fibers following temperature, humidity and UV or gamma-ray radiation exposure. Cellul. Chem. Technol. 2016, 50, 31–39. [Google Scholar]
- Zhang, H.; Zhang, R.; Cai, Y.; Li, X. Research on infrared spectral characteristics and rapid detection technology of industrial hemp. Spectrosc. Lett. 2024, 1–11. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Pinske, C.; Sawers, R.G. Anaerobic formate and hydrogen metabolism. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Popović, S.; Biliaderis, C. Hempseed meal protein isolates prepared by different isolation techniques. Part, I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Traditional and New Applications of Hemp. In Sustainable Agriculture Reviews 42; Crini, G., Lichtfouse, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 37–87. [Google Scholar]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Grase, L.; Gaidukova, G.; Thakur, V.K. Sustainable hemp-based bioplastics with tunable properties via reversible thermal crosslinking of cellulose. Int. J. Biol. Macromol. 2023, 242, 125055. [Google Scholar] [CrossRef] [PubMed]
- Beluns, S.; Platnieks, O.; Jurinovs, M.; Buss, R.; Gaidukovs, S.; Orlova, L.; Starkova, O.; Thakur, V.K. Sustainable foams from hemp, lignin, xylan, pectin, and glycerol: Tunable via reversible citric acid crosslinking for absorption and insulation applications. Giant 2024, 19, 100295. [Google Scholar] [CrossRef]
- Pereira, J.F.; Núñez, E.; Reyes, A.; Mali, S.; Lopez-Rubio, A.; Fabra, M.J. On the use of lignocellulosic hemp fibers to produce biodegradable cost-efficient biocomposites. Future Foods 2024, 10, 100507. [Google Scholar]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of bioactive compounds from Cannabis sativa L. flowers and/or leaves using deep eutectic solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Bioactive Compound Extraction of Hemp (Cannabis sativa L.) Leaves through Response Surface Methodology Optimization. Agriengineering 2024, 6, 1300–1318. [Google Scholar] [CrossRef]
- Rydberg, J. Solvent Extraction Principles and Practice, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Sarmah, M.; Mondal, M.; Bora, U.J.C. Agro-waste extract based solvents: Emergence of novel green solvent for the design of sustainable processes in catalysis and organic chemistry. ChemistrySelect 2017, 2, 5180–5188. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Technology. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Chang, S.H.; Research, P. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—A review. Environ. Sci. Pollut. Res. 2020, 27, 32371–32388. [Google Scholar] [CrossRef]
- Sharma, M.; Sridhar, K.; Gupta, V.K.; Dikkala, P.K.; Chemistry, S. Greener technologies in agri-food wastes valorization for plant pigments: Step towards circular economy. Curr. Res. Green Sustain. Chem. 2022, 5, 100340. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased natural deep eutectic system as versatile solvents: Structure, interaction and advanced applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef]
- Olson, N.E.; Neher, D.A.; Holden, V.I. Utilization. On-Farm Conversion of Cannabis Sativa Waste Biomass into an Organic Fertilizer by Microbial Digestion. Compos. Sci. Util. 2024, 31, 38–54. [Google Scholar] [CrossRef]
- Kiruba, N.J.M.; Saeid, A. An insight into microbial inoculants for bioconversion of waste biomass into sustainable “bio-organic” fertilizers: A bibliometric analysis and systematic literature review. Int. J. Mol. Sci. 2022, 23, 13049. [Google Scholar] [CrossRef] [PubMed]
- Carrazco, A.M.; Díaz-Rodríguez, A.M.; Cota, F.I.P.; de los Santos Villalobos, S. Legal framework for the development of microbial inoculants. In New Insights, Trends, and Challenges in the Development and Applications of Microbial Inoculants in Agriculture; Academic Press: Cambridge, MA, USA, 2024; pp. 143–151. [Google Scholar]
- Balasubramani, V.; Nagarajan, K.; Karthic, M.; Pandiyarajan, R. Extraction of lignocellulosic fiber and cellulose microfibrils from agro waste-palmyra fruit peduncle: Water retting, chlorine-free chemical treatments, physio-chemical, morphological, and thermal characterization. Int. J. Biol. Macromol. 2024, 259, 129273. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Zhen, F.; Wang, Z.; Song, Z.; Chen, J.; Hrynsphan, D.; Tatsiana, S.J.C. Mechanisms of flame retardant tris (2-ethylhexyl) phosphate biodegradation via novel bacterial strain Ochrobactrum tritici WX3-8. Chemosphere 2023, 311, 137071. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Commichau, F.M.; Benndorf, D.; Hertel, R.; Holzer, K.; Hoelzle, L.E.; Mardoukhi, M.S.Y.; Noack, L.E.; Martienssen, M. Biodegradation of selected aminophosphonates by the bacterial isolate Ochrobactrum sp. BTU1. Microbiol. Res. 2024, 280, 127600. [Google Scholar] [CrossRef] [PubMed]
- Marzec-Grządziel, A.; Gałązka, A.J.A. Sequencing of the Whole Genome of a Bacterium of the Genus Achromobacter Reveals Its Potential for Xenobiotics Biodegradation. Agriculture 2023, 13, 1519. [Google Scholar] [CrossRef]
- Wisplinghoff, H. Pseudomonas spp., Acinetobacter spp. and miscellaneous Gram-negative bacilli. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1579–1599. [Google Scholar]
- Steinberg, J.P.; Burd, E. 238-Other Gram-negative and Gram-variable bacilli. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3015–3033. [Google Scholar]
- Yokota, S.; Taniguchi, T.; Takayanagi, S. Brucella anthropi bacteremia: Persistent bacteremia with minimal symptoms. J. Infect. Chemother. 2025, 31, 102595. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Antoniadou, A.; Kanellakopoulou, K. Acinetobacter baumannii: A universal threat to public health? Int. J. Antimicrob. Agents 2008, 32, 106–119. [Google Scholar] [CrossRef]
- Veschetti, L.; Boaretti, M.; Saitta, G.M.; Mantovani, R.P.; Lleò, M.M.; Sandri, A.; Malerba, G. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol. Res. 2022, 263, 127140. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, K.R.J.; López-Hernández, J.; García-Cárdenas, E.; Pelagio-Flores, R.; Lopez-Bucio, J.S.; Texon, A.C.; Ibarra-Laclette, E.; López-Bucio, J. The plant growth promoting rhizobacterium Achromobacter sp. 5B1, rescues Arabidopsis seedlings from alkaline stress by enhancing root organogenesis and hormonal responses. Microbiol. Res. 2024, 281, 127594. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Yu, P.; Ni, B. Complete genome sequence of Achromobacter sp. strain E1, an endophyte from Zea mays L. cultivar (Zheng dan 958). Microbiol. Resour. Announc. 2024, 13, e00560-24. [Google Scholar] [CrossRef] [PubMed]
- Ventorino, V.; Chouyia, F.E.; Romano, I.; Mori, M.; Pepe, O. Water retting process with hemp pre-treatment: Effect on the enzymatic activities and microbial populations dynamic. Appl. Microbiol. Biotechnol. 2024, 108, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, R.; Chen, J.; Chen, J.; Feng, X. Seawater-retting treatment of hemp and characterization of bacterial strains involved in the retting process. Process. Biochem. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Abid, K.; Jabri, J.; Yaich, H.; Malek, A.; Rekhis, J.; Kamoun, M. In vitro study on the effects of exogenic fibrolytic enzymes produced from Trichoderma longibrachiatum on ruminal degradation of olive mill waste. Arch. Anim. Breed. 2022, 65, 79–88. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, M.; Wang, Y.; Wang, L.; Liu, H.; Shi, M.; Zhong, Y. Constitutive overexpression of cellobiohydrolase 2 in Trichoderma reesei reveals its ability to initiate cellulose degradation. Eng. Microbiol. 2023, 3, 100059. [Google Scholar] [CrossRef]
- Devasia, S.; Anand, S.; Nair, A.J.; Management, W. Laccase mediated bioremediation of industrial dyes by a potent strain of Arthrographis sp. Int. J. Environ. Waste Manag. 2022, 29, 278–290. [Google Scholar] [CrossRef]
- Abid, K.; Jabri, J.; Yaich, H.; Malek, A.; Rekhis, J.; Kamoun, M. Improving the nutritional value and rumen fermentation characteristics of sesame seed coats through bioconversion approach using exogenous fibrolytic enzymes produced by Trichoderma longibrachiatum. Biomass Convers. Biorefin. 2023, 13, 14917–14925. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X.; Cao, Y.; Wang, X.; Lu, J.; Xie, L.; Liu, K.; Li, X. The neuroprotective and antidiabetic effects of trigonelline: A review of signaling pathways and molecular mechanisms. Biochimie 2023, 206, 93–104. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional values of industrial hemp byproducts for dairy cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef] [PubMed]
| Sample ID | Plant Variety | Waste Material | Maceration Time | Maceration Method |
|---|---|---|---|---|
| S14 | Cannabis sativa L. cv. Strawberry | Dry flowers and leaves | 72 h | Short-term |
| S9 | Cannabis sativa L. cv. Strawberry | Dry flowers and leaves | 1 month | Long-term |
| S21 | Cannabis sativa L. cv. Strawberry | Dry flowers and leaves | 2 months | Long-term |
| SPECIES | Collection Id | Source of Isolation |
|---|---|---|
| Cryptococcus phenolicus | CMC 1668 | Soil |
| Pichia membranaefaciens | CMC 206 | Soil |
| Saccharomyces cerevisiae | CMC 207 | CBS Type strain |
| Zygosaccharomyces rouxii | CMC 259 | Soil |
| Peribacillus simplex | CMC 85 | Seed |
| Enterobacter cloacae | B 89 | Seed |
| Escherichia coli | B 6 | Faecis |
| Pantoea agglomerans | B 35 | Seed |
| Pseudomonas fluorescens | B 57 | Seed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, L.; Conti, A.; Casagrande Pierantoni, D.; Ruspi, C.; Cerri, M.; Emiliani, C.; Cardinali, G.; Corte, L. Spontaneous Bio-Recycling: Recovering Bioactive Molecules Through Endogenous Microbial Maceration of Hemp Residues. Microorganisms 2025, 13, 455. https://doi.org/10.3390/microorganisms13020455
Donati L, Conti A, Casagrande Pierantoni D, Ruspi C, Cerri M, Emiliani C, Cardinali G, Corte L. Spontaneous Bio-Recycling: Recovering Bioactive Molecules Through Endogenous Microbial Maceration of Hemp Residues. Microorganisms. 2025; 13(2):455. https://doi.org/10.3390/microorganisms13020455
Chicago/Turabian StyleDonati, Leonardo, Angela Conti, Debora Casagrande Pierantoni, Chiara Ruspi, Martina Cerri, Carla Emiliani, Gianluigi Cardinali, and Laura Corte. 2025. "Spontaneous Bio-Recycling: Recovering Bioactive Molecules Through Endogenous Microbial Maceration of Hemp Residues" Microorganisms 13, no. 2: 455. https://doi.org/10.3390/microorganisms13020455
APA StyleDonati, L., Conti, A., Casagrande Pierantoni, D., Ruspi, C., Cerri, M., Emiliani, C., Cardinali, G., & Corte, L. (2025). Spontaneous Bio-Recycling: Recovering Bioactive Molecules Through Endogenous Microbial Maceration of Hemp Residues. Microorganisms, 13(2), 455. https://doi.org/10.3390/microorganisms13020455









