Molecular Insights into Cell-Mediated Immunity in Atypical Non-Ulcerated Cutaneous Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
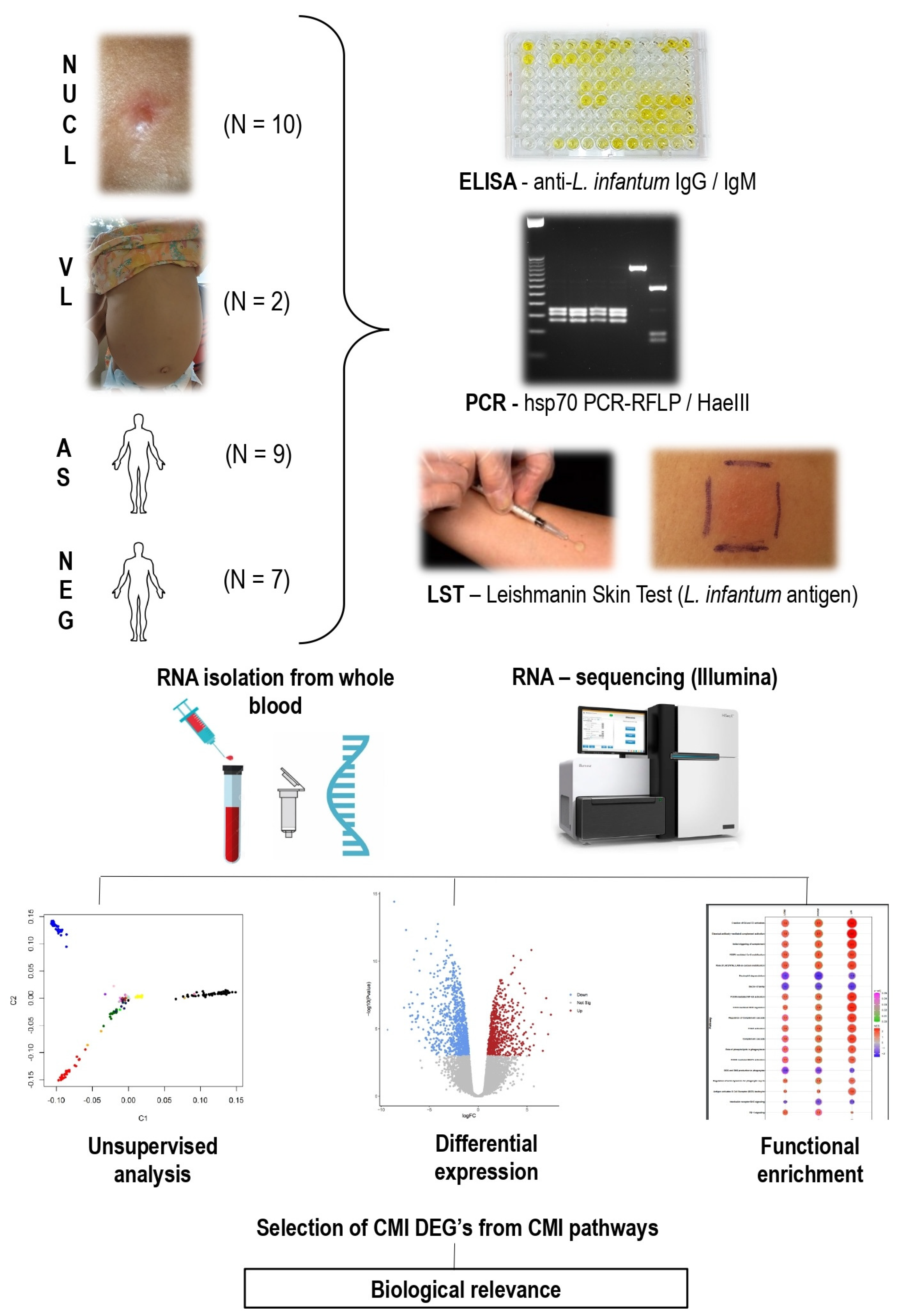
2.1. Sampling
2.2. Immunological and Molecular Diagnosis
2.3. Steps of RNA Sequencing, Differential Expression and Functional Enrichment Analysis
2.4. Comparison of Biomarkers of Immune Response Profiles and Lymphocyte Exhaustion
2.5. Immunohistochemistry Study
3. Results
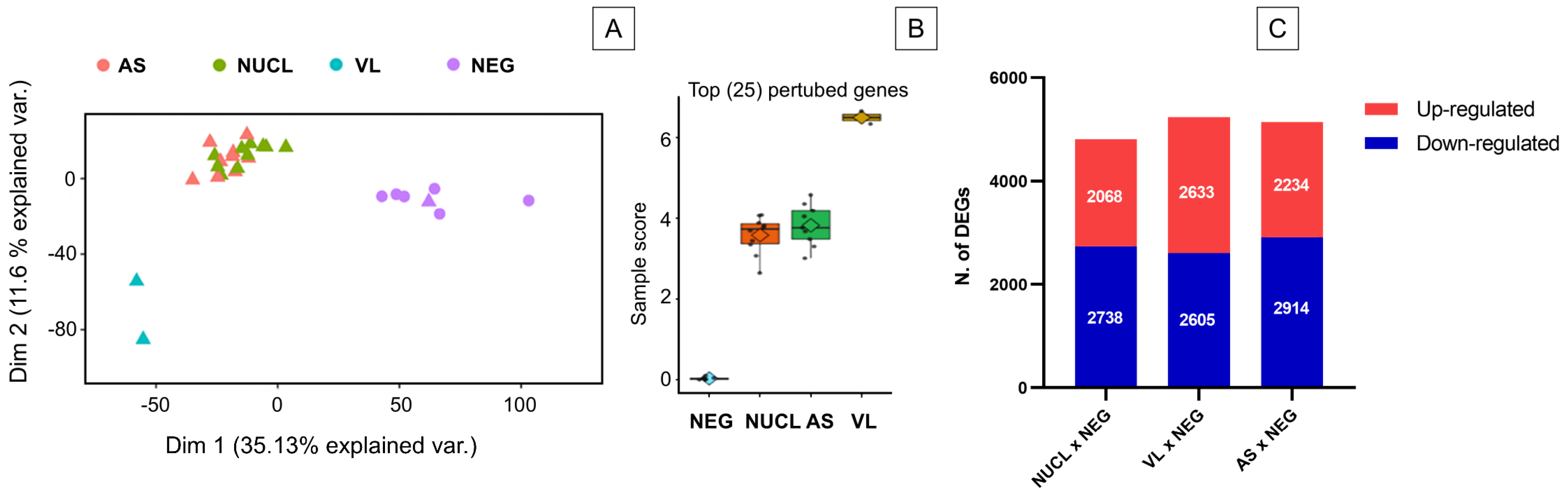
3.1. RNAseq Analysis and Number of Differentially Expressed Genes (DEGs)
3.2. Identifying Gene Signature of Cell-Mediated Immunity in NUCL
3.3. Pathways Enrichment Analysis
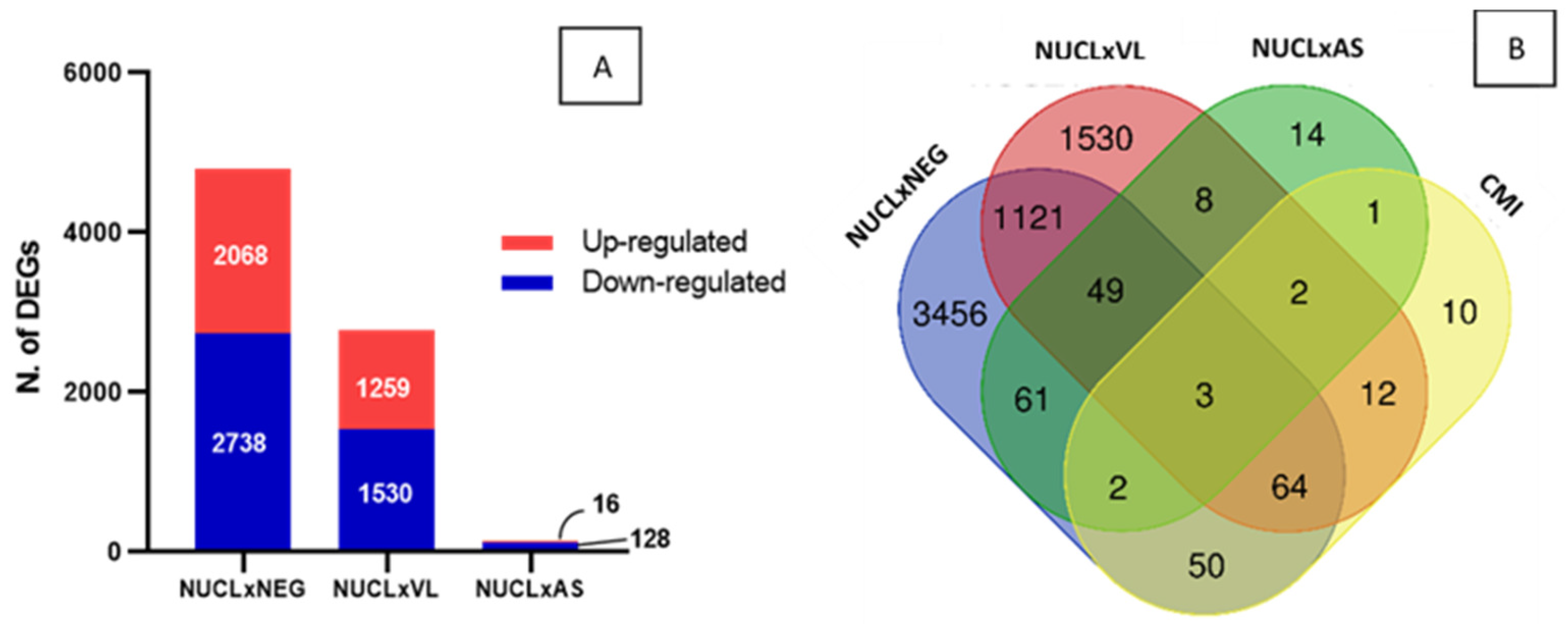
3.4. Comparison of DEGs for CMI Between NUCL and VL
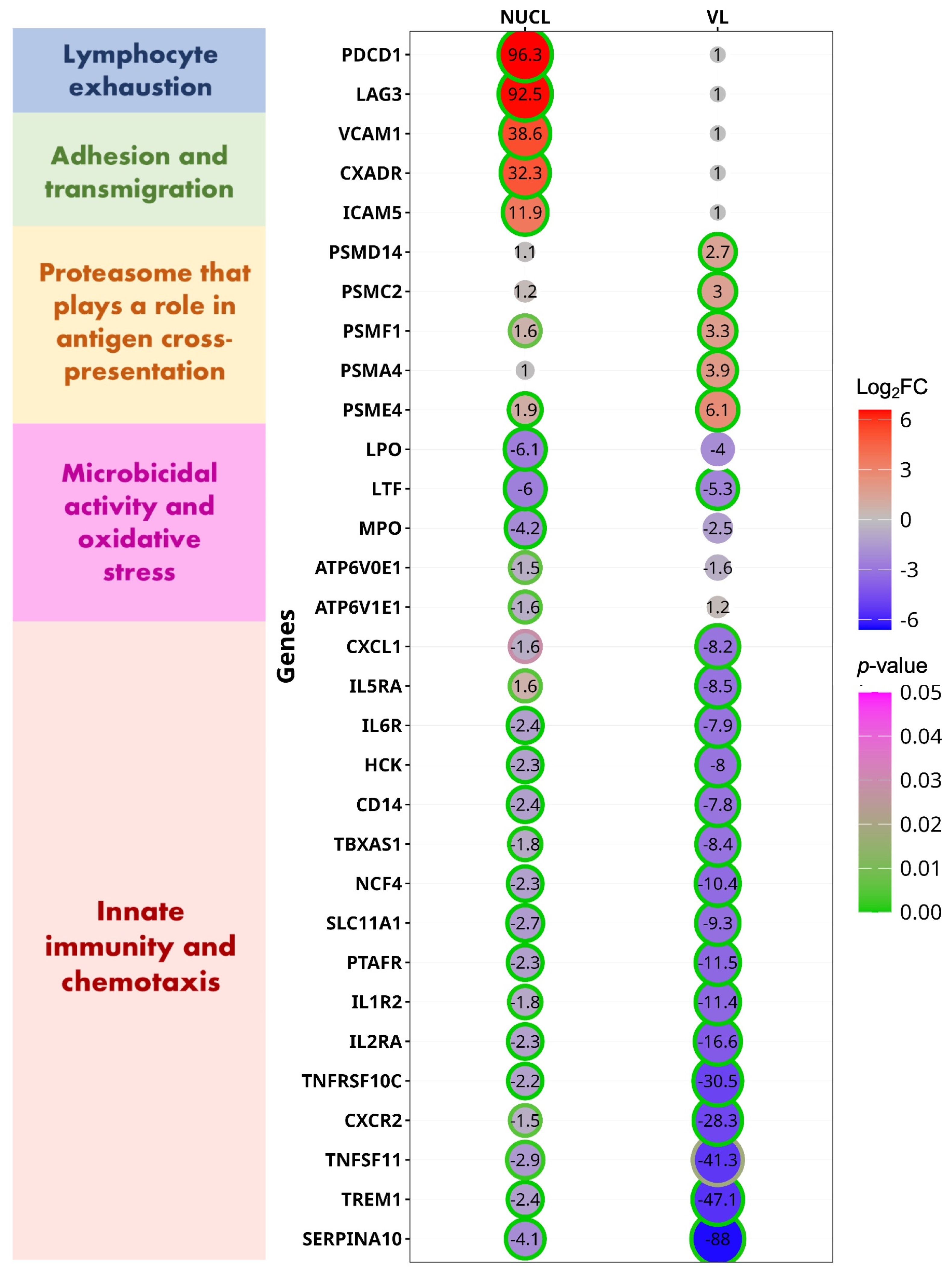
3.5. Blood Gene Expression Shows a Stronger Pro-Inflammatory Response and Regulation in NUCL than in VL
3.6. In Vivo Cell-Mediated Immunity Confirms a Stronger Pro-Inflammatory Response in NUCL
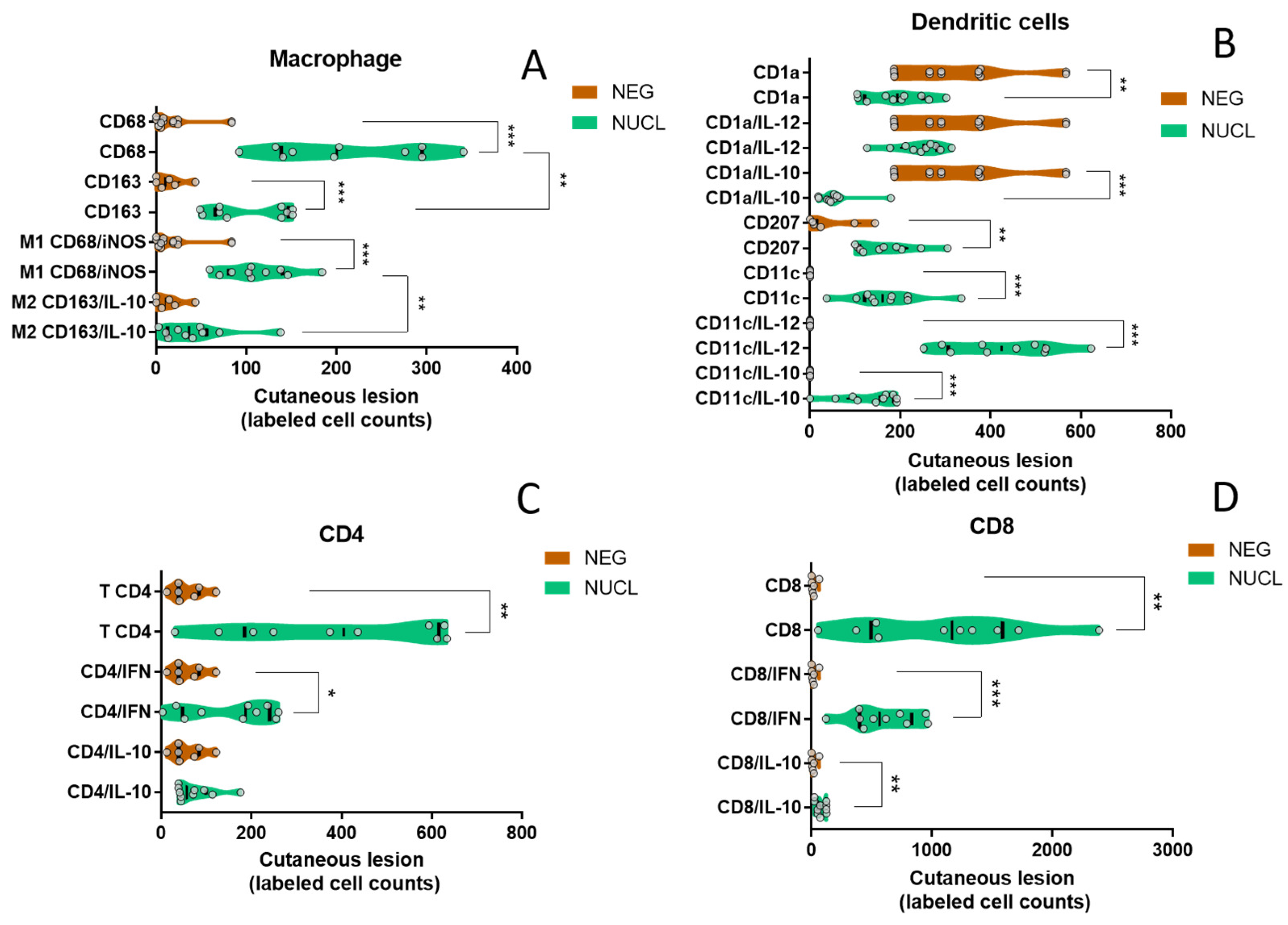
3.7. In Situ Cutaneous Evaluation Corroborates That NUCL Immune Response Is Predominantly Pro-Inflammatory
4. Discussion
4.1. T Lymphocyte Exhaustion
4.2. Innate IMMUNE Response
4.3. CD8+ T Lymphocytes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponce, C.; Ponce, E.; Morrison, A.; Cruz, A.; Kreutzer, R.; McMahon-Pratt, D.; Neva, F. Leishmania donovani chagasi: New clinical variant of cutaneous leishmaniasis in Honduras. Lancet 1991, 337, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.; Chance, M.; Ponce, C.; Ponce, E.; Maingon, R. Leishmania chagasi: Genotypically similar parasites from Honduras cause both visceral and cutaneous leishmaniasis in humans. Exp. Parasitol. 1997, 85, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Ferreira, A.F.; da Matta, V.L.R.; de Castro Gomes, C.M.; Sosa-Ochoa, W.H.; Zúniga, C.; Silveira, F.T.; Corbett, C.E.P.; Laurenti, M.D. Role of antigen-presenting cells in non-ulcerated skin lesions caused by Leishmania (Leishmania) infantum chagasi. Parasite Immunol. 2023, 45, e12971. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.D.; Sousa, A.D.Q. Clinical Spectrum of Leishmaniasis. Clin. Infect. Dis. 1996, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- da Matta, V.L.R.; Gonçalves, A.N.; Gomes, C.M.C.; Chouman, I.H.; Ferreira, F.M.; Campos, M.B.; Lima, L.V.; Vasconcelos Dos Santos, T.; Ramos, P.K.; Furtado, R.R.; et al. Gene Signatures of Symptomatic and Asymptomatic Clinical-Immunological Profiles of Human Infection by Leishmania (L.) chagasi in Amazonian Brazil. Microorganisms 2023, 11, 653. [Google Scholar] [CrossRef]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Favero Ferreira, A.; Sosa Ochoa, W.; Ribeiro da Matta, V.L.; Zúniga Valeriano, C.; Pereira Corbett, C.E.; Dalastra Laurenti, M. Histopathological features of skin lesions in patients affected by non-ulcerated or atypical cutaneous leishmaniasis in Honduras, Central America. Int. J. Exp. Pathol. 2018, 99, 249–257. [Google Scholar] [CrossRef]
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023, 12, 297. [Google Scholar] [CrossRef]
- Morgan, D.J.; Guimaraes, L.H.; Machado, P.R.; D’Oliveira, A., Jr.; Almeida, R.P.; Lago, E.L.; Faria, D.R.; Tafuri, W.L.; Dutra, W.O.; Carvalho, E.M. Cutaneous leishmaniasis during pregnancy: Exuberant lesions and potential fetal complications. Clin. Infect. Dis. 2007, 45, 478–482. [Google Scholar] [CrossRef]
- Lago, T.; Carvalho, L.P.; Nascimento, M.; Guimarães, L.H.; Lago, J.; Castellucci, L.; Carvalho, A.M.; Lago, A.; Carvalho, E.M. Influence of Obesity on Clinical Manifestations and Response to Therapy in Cutaneous Leishmaniasis Caused by Leishmania braziliensis. Clin. Infect. Dis. 2021, 73, 1020–1026. [Google Scholar] [CrossRef]
- Lago, A.S.; Lima, F.R.; Carvalho, A.M.; Sampaio, C.; Lago, N.; Guimarães, L.H.; Lago, J.; Machado, P.R.L.; Carvalho, L.P.; Arruda, S.; et al. Diabetes Modifies the Clinic Presentation of Cutaneous Leishmaniasis. Open Forum Infect. Dis. 2020, 7, ofaa491. [Google Scholar] [CrossRef]
- Aoun, K.; Bouratbine, A. Cutaneous leishmaniasis in North Africa: A review. Parasite 2014, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- del Giudice, P.; Marty, P.; Lacour, J.P.; Perrin, C.; Pratlong, F.; Haas, H.; Dellamonica, P.; Le Fichoux, Y. Cutaneous leishmaniasis due to Leishmania (Leishmania) infantum chagasi: Case reports and literature review. Arch. Dermatol. 1998, 134, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lyra, M.R.; Pimentel, M.I.; Madeira Mde, F.; Antonio Lde, F.; Lyra, J.P.; Fagundes, A.; Schubach Ade, O. First report of cutaneous leishmaniasis caused by Leishmania (Leishmania) infantum chagasi in an urban area of rio de janeiro, brazil. Rev. Inst. Med. Trop. São Paulo 2015, 57, 451–454. [Google Scholar] [CrossRef]
- Castro, L.S.; França Ade, O.; Ferreira Ede, C.; Hans Filho, G.; Higa Júnior, M.G.; Gontijo, C.M.; Pereira, A.A.; Dorval, M.E. Leishmania (Leishmania) infantum chagasi as a causative agent of cutaneous leishmaniasis in the State of Mato Grosso do Sul, Brazil. Rev. Inst. Med. Trop. São Paulo 2016, 58, 23. [Google Scholar] [CrossRef]
- Lypaczewski, P.; Thakur, L.; Jain, A.; Kumari, S.; Paulini, K.; Matlashewski, G.; Jain, M. An intraspecies Leishmania donovani hybrid from the Indian subcontinent is associated with an atypical phenotype of cutaneous disease. iScience 2022, 25, 103802. [Google Scholar] [CrossRef]
- Lypaczewski, P.; Matlashewski, G. Leishmania donovani hybridisation and introgression in nature: A comparative genomic investigation. Lancet Microbe 2021, 2, e250–e258. [Google Scholar] [CrossRef] [PubMed]
- Farias Amorim, C.; O’Novais, F.; Nguyen, B.T.; Nascimento, M.T.; Lago, J.; Lago, A.S.; Carvalho, L.P.; Beiting, D.P.; Scott, P. Localized skin inflammation during cutaneous leishmaniasis drives a chronic, systemic IFN-gamma signature. PLoS Negl. Trop. Dis. 2021, 15, e0009321. [Google Scholar] [CrossRef]
- Sanz, C.R.; Miró, G.; Sevane, N.; Reyes-Palomares, A.; Dunner, S. Modulation of Host Immune Response during Leishmania (Leishmania) infantum chagasi Natural Infection: A Whole-Transcriptome Analysis of the Popliteal Lymph Nodes in Dogs. Front. Immunol. 2022, 12, 794627. [Google Scholar] [CrossRef]
- Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Tomokane, T.Y.; Sosa Ochoa, W.; Zúniga Valeriano, C.; Castro Gomes, C.M.; Corbett, C.E.P.; Laurenti, M.D. Evaluation of Regulatory Immune Response in Skin Lesions of Patients Affected by Nonulcerated or Atypical Cutaneous Leishmaniasis in Honduras, Central America. Mediat. Inflamm. 2018, 2018, 3487591. [Google Scholar] [CrossRef]
- Sosa-Ochoa, W.; Zúniga, C.; Chaves, L.F.; Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Ribeiro da Matta, V.L.; Pereira Corbett, C.E.; Tobias Silveira, F.; Dalastra Laurenti, M. Clinical and Immunological Features of Human Leishmania (L.) infantum-Infection, Novel Insights Honduras, Central America. Pathogens 2020, 9, 554. [Google Scholar] [CrossRef]
- Sandoval, C.; Araujo, G.; Sosa, W.; Avalos, S.; Silveira, F.; Corbett, C.; Zúniga, C.; Laurenti, M. In situ cellular immune response in non-ulcerated skin lesions due to Leishmania (L.) infantum chagasi infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200149. [Google Scholar] [CrossRef] [PubMed]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2019. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G.; et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef]
- Russo, P.S.T.; Ferreira, G.R.; Cardozo, L.E.; Bürger, M.C.; Arias-Carrasco, R.; Maruyama, S.R.; Hirata, T.D.C.; Lima, D.S.; Passos, F.M.; Fukutani, K.F.; et al. CEMiTool: A Bioconductor package for performing comprehensive modular co-expression analyses. BMC Bioinform. 2018, 19, 56. [Google Scholar] [CrossRef]
- Steen, C.B.; Liu, C.L.; Alizadeh, A.A.; Newman, A.M. Profiling Cell Type Abundance and Expression in Bulk Tissues with CIBERSORTx. Methods Mol. Biol. 2020, 2117, 135–157. [Google Scholar] [CrossRef]
- Gonçalves, A.N.A.; Lever, M.; Russo, P.S.T.; Gomes-Correia, B.; Urbanski, A.H.; Pollara, G.; Noursadeghi, M.; Maracaja-Coutinho, V.; Nakaya, H.I. Assessing the Impact of Sample Heterogeneity on Transcriptome Analysis of Human Diseases Using MDP Webtool. Front Genet. 2019, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Prigent, P.; Pagès, F.; Bruniquel, D.; Triebel, F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur. J. Immunol. 1996, 26, 1180–1186. [Google Scholar] [CrossRef]
- Costa-Madeira, J.C.; Trindade, G.B.; Almeida, P.H.P.; Silva, J.S.; Carregaro, V. T Lymphocyte Exhaustion During Human and Experimental Visceral Leishmaniasis. Front. Immunol. 2022, 13, 835711. [Google Scholar] [CrossRef]
- Amprey, J.L.; Späth, G.F.; Porcelli, S.A. Inhibition of CD1 expression in human dendritic cells during intracellular infection with Leishmania donovani. Infect. Immun. 2004, 72, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Jayakumar, A.; McDowell, M.A. Inhibition of groups 1 and 2 CD1 molecules on human dendritic cells by Leishmania species. Parasite Immunol. 2007, 29, 515–524. [Google Scholar] [CrossRef]
- Belo, R.; Santarém, N.; Pereira, C.; Pérez-Cabezas, B.; Macedo, F.; Leite-de-Moraes, M.; Cordeiro-da-Silva, A. Leishmania (Leishmania) infantum chagasi Exoproducts Inhibit Human Invariant NKT Cell Expansion and Activation. Front. Immunol. 2017, 8, 710. [Google Scholar] [CrossRef]
- Marchese, M.E.; Berdnikovs, S.; Cook-Mills, J.M. Distinct sites within the vascular cell adhesion molecule-1 (VCAM-1) cytoplasmic domain regulate VCAM-1 activation of calcium fluxes versus Rac1 during leukocyte transendothelial migration. Biochemistry 2012, 51, 8235–8246. [Google Scholar] [CrossRef]
- McGraw, J.M.; Thelen, F.; Hampton, E.N.; Bruno, N.E.; Young, T.S.; Havran, W.L.; Witherden, D.A. JAML promotes CD8 and γδ T cell antitumor immunity and is a novel target for cancer immunotherapy. J. Exp. Med. 2021, 218, e20202644. [Google Scholar] [CrossRef]
- Liu, L.; Sun, M.; Song, D.; Wang, Z. The genetic polymorphisms of intercellular cell adhesion molecules and breast cancer susceptibility: A meta-analysis. Mol. Biol. Rep. 2013, 40, 1855–1860. [Google Scholar] [CrossRef]
- Toma, V.A.; Tigu, A.B.; Farcaș, A.D.; Sevastre, B.; Taulescu, M.; Gherman, A.M.R.; Roman, I.; Fischer-Fodor, E.; Pârvu, M. New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors. Int. J. Mol. Sci. 2019, 20, 3627. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Q.; Shu, P.; Lin, X.; Gao, X.; Shen, K. COLEC12 Promotes Tumor Progression and Is Correlated with Poor Prognosis in Gastric Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231218163. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, J.F.; Kavanagh, D.G.; Lirvall, M.; Sanders, C.; Cover, T.L.; Bhardwaj, N.; Larsson, M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood 2003, 102, 4448–4455. [Google Scholar] [CrossRef]
- Singh, B.; Bhushan Chauhan, S.; Kumar, R.; Singh, S.S.; Ng, S.; Amante, F.; de Labastida Rivera, F.; Singh, O.P.; Rai, M.; Nylen, S.; et al. A molecular signature for CD8+ T cells from visceral leishmaniasis patients. Parasite Immunol. 2019, 41, e12669. [Google Scholar] [CrossRef]
- Wijkstrom-Frei, C.; El-Chemaly, S.; Ali-Rachedi, R.; Gerson, C.; Cobas, M.A.; Forteza, R.; Salathe, M.; Conner, G.E. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell Mol. Biol. 2003, 29, 206–212. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Obinger, C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry 1998, 37, 17923–17930. [Google Scholar] [CrossRef]
- Wang, L.; Wu, D.; Robinson, C.V.; Wu, H.; Fu, T.M. Structures of a Complete Human V-ATPase Reveal Mechanisms of Its Assembly. Mol. Cell 2020, 80, 501–511.e3. [Google Scholar] [CrossRef]
- Hu, F.; Mei, R.; Zhang, H.; Hao, D.; Li, W. Bioinformatics analysis of prognostic value and immune cell infiltration of SERPINA1 gene in cutaneous melanoma. Ann. Transl. Med. 2022, 10, 966. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Zhang, Y.; Cheng, S.; Wu, M.; Gu, S.; Xu, S.; Wu, Y.; Sheng, J.; Voon, D.C.; et al. Clinical significance and immune infiltration analyses of a novel coagulation-related signature in ovarian cancer. Cancer Cell Int. 2023, 23, 232. [Google Scholar] [CrossRef]
- Ford, J.W.; Gonzalez-Cotto, M.; MacFarlane, A.W., 4th; Peri, S.; Howard, O.M.Z.; Subleski, J.J.; Ruth, K.J.; Haseebuddin, M.; Al-Saleem, T.; Yang, Y.; et al. Tumor-Infiltrating Myeloid Cells Co-Express TREM1 and TREM2 and Elevated TREM-1 Associates with Disease Progression in Renal Cell Carcinoma. Front. Oncol. 2022, 11, 662723. [Google Scholar] [CrossRef]
- Rethi, B.; Eidsmo, L. FasL and TRAIL signaling in the skin during cutaneous leishmaniasis—Implications for tissue immunopathology and infectious control. Front. Immunol. 2012, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Araujo Flores, G.V.; Sandoval Pacheco, C.M.; Sosa Ochoa, W.H.; Gomes, C.M.C.; Zúniga, C.; Corbett, C.P.; Laurenti, M.D. Th17 lymphocytes in atypical cutaneous leishmaniasis caused by Leishmania (L.) infantum chagasi in Central America. Parasite Immunol. 2020, 42, e12772. [Google Scholar] [CrossRef] [PubMed]
- González-Tafoya, E.; Diupotex, M.; Zamora-Chimal, J.; Salaiza-Suazo, N.; Ruiz-Remigio, A.; Becker, I. TNF contributes to T-cell exhaustion in chronic L. mexicana infections of mice through PD-L1 up-regulation. Cell. Immunol. 2020, 358, 104196. [Google Scholar] [CrossRef]
- Nylén, S.; Maurya, R.; Eidsmo, L.; Manandhar, K.D.; Sundar, S.; Sacks, D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 2007, 204, 805–817. [Google Scholar] [CrossRef]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4+CD25−Foxp3+ Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef]
- Castellucci, L.C.; Almeida, L.; Cherlin, S.; Fakiola, M.; Francis, R.W.; Carvalho, E.M.; Santos da Hora, A.; do Lago, T.S.; Figueiredo, A.B.; Cavalcanti, C.M.; et al. A Genome-wide Association Study Identifies SERPINB10, CRLF3, STX7, LAMP3, IFNG-AS1, and KRT80 As Risk Loci Contributing to Cutaneous Leishmaniasis in Brazil. Clin. Infect. Dis. 2021, 72, e515–e525. [Google Scholar] [CrossRef]
- Kaushal, H.; Bras-Gonçalves, R.; Negi, N.S.; Lemesre, J.L.; Papierok, G.; Salotra, P. Role of CD8+ T cells in protection against Leishmania donovani infection in healed Visceral Leishmaniasis individuals. BMC Infect. Dis. 2014, 14, 653. [Google Scholar] [CrossRef]
- Ruiz, J.H.; Becker, I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol. 2007, 29, 671–678. [Google Scholar] [CrossRef]
- Papin, S.; Cuenin, S.; Agostini, L.; Martinon, F.; Werner, S.; Beer, H.D.; Grütter, C.; Grütter, M.; Tschopp, J. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007, 14, 1457–1466. [Google Scholar] [CrossRef]
- Kimura, T.; Jain, A.; Choi, S.W.; Mandell, M.A.; Schroder, K.; Johansen, T.; Deretic, V. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 2015, 210, 973–989. [Google Scholar] [CrossRef]
- Lee, B.C.; Avraham, S.; Imamoto, A.; Avraham, H.K. Identification of the nonreceptor tyrosine kinase MATK/CHK as an essential regulator of immune cells using Matk/CHK-deficient mice. Blood 2006, 108, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Khan, S.N.; Radic, M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008, 180, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.M.; Datta, P.; Bell, S.E.; Kitamura, D.; Turner, M.; Butcher, G.W. GIMAP1 Is Essential for the Survival of Naive and Activated B Cells In Vivo. J. Immunol. 2016, 196, 207–216. [Google Scholar] [CrossRef]
- Datta, P.; Webb, L.M.; Avdo, I.; Pascall, J.; Butcher, G.W. Survival of mature T cells in the periphery is intrinsically dependent on GIMAP1 in mice. Eur. J. Immunol. 2017, 47, 84–93. [Google Scholar] [CrossRef]
- Giannakakis, A.; Karapetsas, A.; Dangaj, D.; Lanitis, E.; Tanyi, J.; Coukos, G.; Sandaltzopoulos, R. Overexpression of SMARCE1 is associated with CD8+ T-cell infiltration in early stage ovarian cancer. Int. J. Biochem. Cell Biol. 2014, 53, 389–398. [Google Scholar] [CrossRef]
- Meininger, I.; Krappmann, D. Lymphocyte signaling and activation by the CARMA1-BCL10-MALT1 signalosome. Biol. Chem. 2016, 397, 1315–1333. [Google Scholar] [CrossRef]
- Anderson, S.K.; Gallinger, S.; Roder, J.; Frey, J.; Young, H.A.; Ortaldo, J.R. A cyclophilin-related protein involved in the function of natural killer cells. Proc. Natl. Acad. Sci. USA 1993, 90, 542–546. [Google Scholar] [CrossRef]
- Wu, J.; Groh, V.; Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002, 169, 1236–1240. [Google Scholar] [CrossRef]
- Fallas, J.L.; Tobin, H.M.; Lou, O.; Guo, D.; Sant’Angelo, D.B.; Denzin, L.K. Ectopic expression of HLA-DO in mouse dendritic cells diminishes MHC class II antigen presentation. J. Immunol. 2004, 173, 1549–1560. [Google Scholar] [CrossRef]

| CMI DEG | LogFCAS | LogFCVL | LogFCNEG | Function |
|---|---|---|---|---|
| BATF2 | 1.25 | NS | NS | Enhances CD8+ T-cell infiltration and activation. |
| SH3PXD2B | 1.15 | −1.77 | 4.49 | Allows NOX1- or NOX3-dependent reactive oxygen species (ROS) generation. |
| CD274 | 1.10 | NS | 0.99 | Ligand for the inhibitory receptor PDCD1/PD-1. |
| GBP1 | 0.84 | −1.41 | NS | Inflammasome assembly in response to infection. |
| CCR3 | 0.83 | 5.92 | 1.89 | Receptor for C-C type chemokine. |
| CR1 | 0.8 | 1.32 | NS | Receptor that plays a critical role in the capture and clearance of complement-opsonized pathogens. |
| MARCO | −1.13 | −1.46 | 6.62 | Pattern recognition receptor (PRR) which binds bacteria. |
| COLEC12 | −1.61 | NS | 3.37 | Scavenger receptor correlated to infiltration of suppressor immune cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, L.F.S.; Sandoval Pacheco, C.M.; Flores, G.V.A.; Ferreira, F.M.; Gonçalves, A.N.A.; Sosa-Ochoa, W.H.; da Matta, V.L.R.; Gomes, C.M.C.; Zúniga, C.; Corbett, C.E.P.; et al. Molecular Insights into Cell-Mediated Immunity in Atypical Non-Ulcerated Cutaneous Leishmaniasis. Microorganisms 2025, 13, 413. https://doi.org/10.3390/microorganisms13020413
Batista LFS, Sandoval Pacheco CM, Flores GVA, Ferreira FM, Gonçalves ANA, Sosa-Ochoa WH, da Matta VLR, Gomes CMC, Zúniga C, Corbett CEP, et al. Molecular Insights into Cell-Mediated Immunity in Atypical Non-Ulcerated Cutaneous Leishmaniasis. Microorganisms. 2025; 13(2):413. https://doi.org/10.3390/microorganisms13020413
Chicago/Turabian StyleBatista, Luís Fábio S., Carmen M. Sandoval Pacheco, Gabriela V. Araujo Flores, Frederico M. Ferreira, André N. A. Gonçalves, Wilfredo H. Sosa-Ochoa, Vânia L. R. da Matta, Claudia M. C. Gomes, Concepción Zúniga, Carlos E. P. Corbett, and et al. 2025. "Molecular Insights into Cell-Mediated Immunity in Atypical Non-Ulcerated Cutaneous Leishmaniasis" Microorganisms 13, no. 2: 413. https://doi.org/10.3390/microorganisms13020413
APA StyleBatista, L. F. S., Sandoval Pacheco, C. M., Flores, G. V. A., Ferreira, F. M., Gonçalves, A. N. A., Sosa-Ochoa, W. H., da Matta, V. L. R., Gomes, C. M. C., Zúniga, C., Corbett, C. E. P., Jeffares, D. C., Nakaya, H. I., Silveira, F. T., & Laurenti, M. D. (2025). Molecular Insights into Cell-Mediated Immunity in Atypical Non-Ulcerated Cutaneous Leishmaniasis. Microorganisms, 13(2), 413. https://doi.org/10.3390/microorganisms13020413







