Taxane-Producing Fungi Isolated from Taxus globosa Tree Bark
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Collection
2.1.1. Endophytic Fungi Isolation
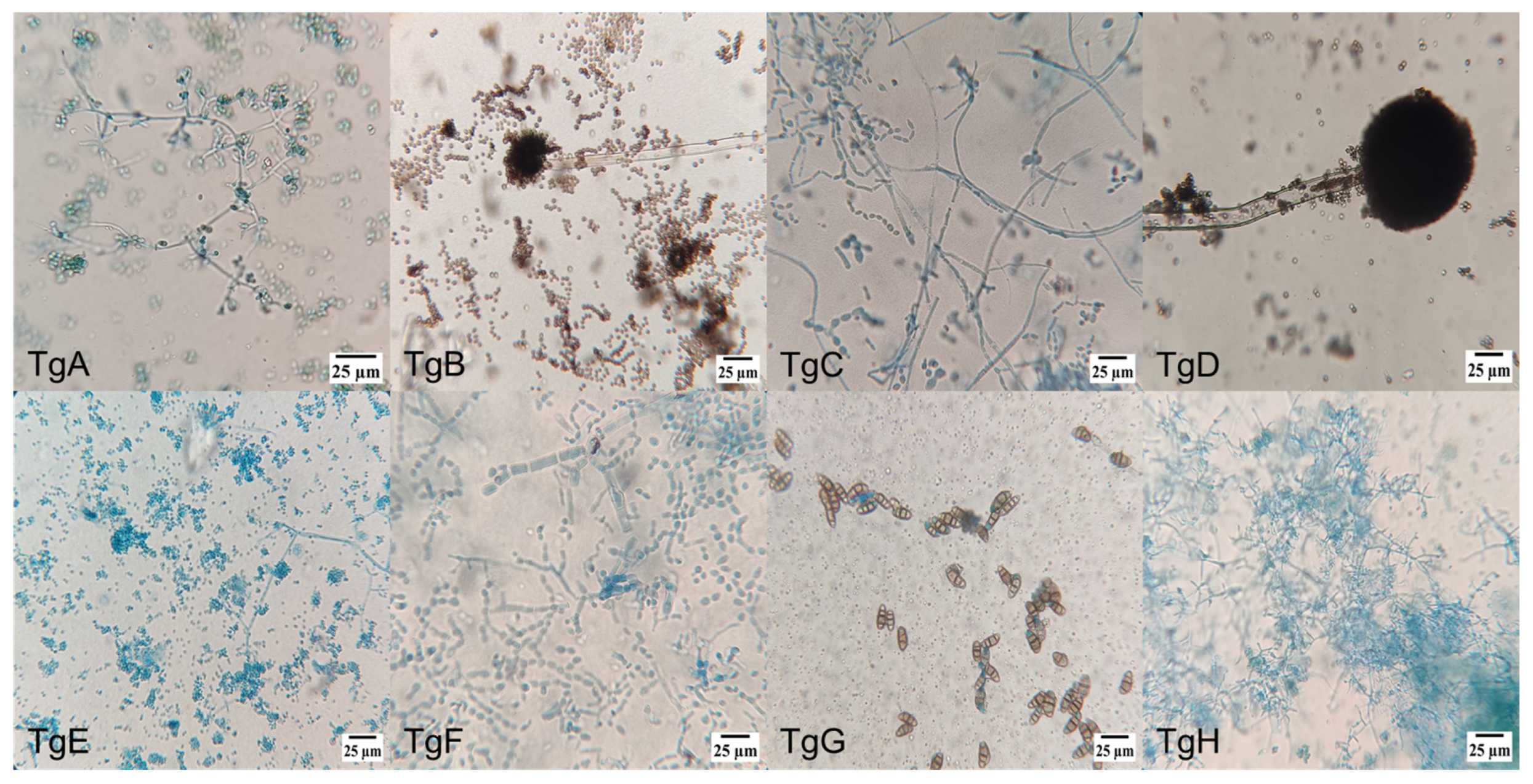
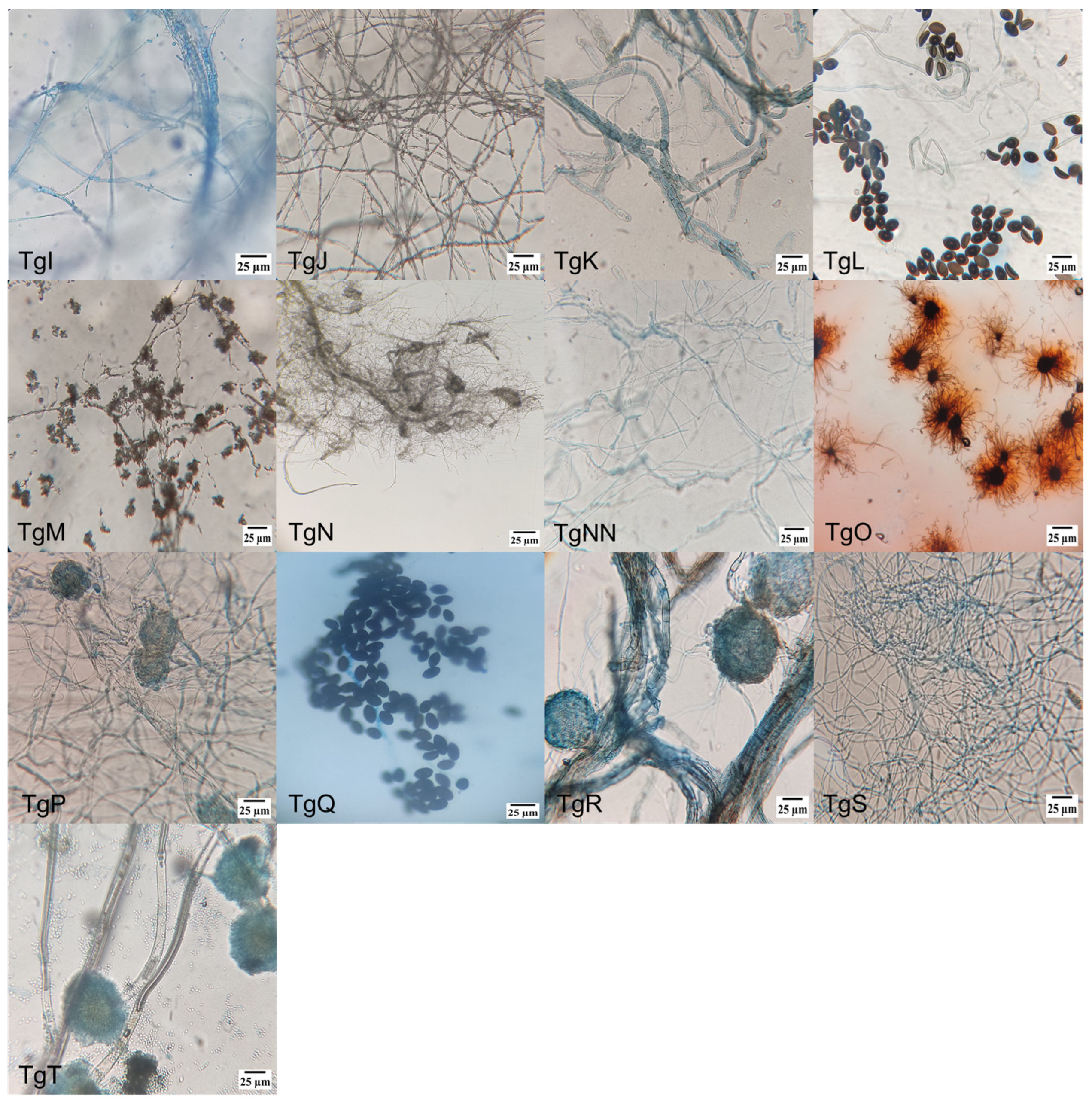
2.1.2. Microscopical Morphology Characterization
2.1.3. Genomic DNA Extraction
2.1.4. Molecular Identification Through ITS
2.1.5. Phylogenetic Analysis
2.1.6. Identification of Taxane-Producing Fungi by LC–QTOF MS/MS
2.1.7. Taxane Extraction
2.1.8. Taxane Purification
2.1.9. Taxane Spectrometrical Analysis
3. Results and Discussion
3.1. Endophytic Fungus Strain Isolation, Identification, and Classification
3.2. Phylogenetic Analysis of the Fungus Islolates
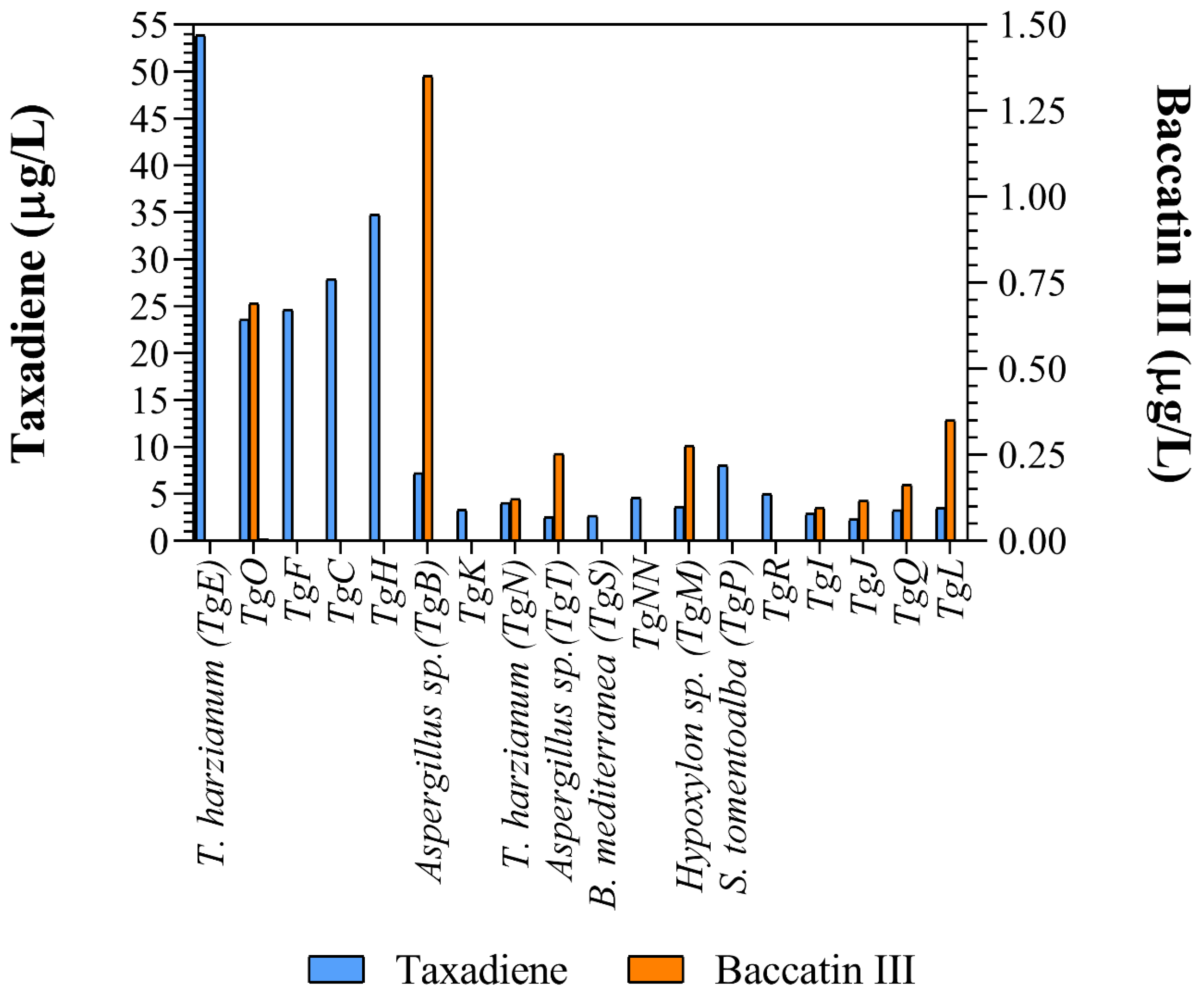
3.3. Taxane-Producing Endophytic Fungus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kok, M.; Gielen, R.-J.; Adams, S.; Lennerz, J.K.; Sharma, P.; Loibl, S.; Reardon, E.; Sonke, G.; Linn, S.; Delaloge, S.; et al. Academic Uphill Battle to Personalize Treatment for Patients With Stage II/III Triple-Negative Breast Cancer. J. Clin. Oncol. 2024, 42, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, L.; Luzzi, L.; Ghiribelli, C.; Paladini, P.; Di Bisceglie, M.; Gotti, G. Results of induction chemotherapy followed by surgical resection in patients with stage IIIA (N2) non-small cell lung cancer: The importance of the nodal down-staging after chemotherapy. Eur. J. Cardiothorac. Surg. 2001, 20, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Manish, L.; Mangesh, B.; Sabale, V. Taxus—The panacea for cancer treatment. Anc. Sci. Life 2005, 24, 152–159. [Google Scholar]
- Ruiz-Sanchez, J.; Flores-Bustamante, Z.R.; Dendooven, L.; Favela-Torres, E.; Soca-Chafre, G.; Galindez-Mayer, J.; Flores-Cotera, L.B. A comparative study of Taxol production in liquid and solid-state fermentation with Nigrospora sp. a fungus isolated from Taxus globosa. J. Appl. Microbiol. 2010, 109, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.T.; De La Peña, R.; Tocol, C.; Sattely, E.S. Reconstitution of early paclitaxel biosynthetic network. Nat. Commun. 2024, 15, 1419. [Google Scholar] [CrossRef]
- Nurullah, M.; Usmani, Z.; Ahmad, S.; Panda, B.P.; Amin, S.; Mir, S.R. Purification and characterization of Taxol and 10-Deacetyl baccatin III from the bark, needles, and endophytes of Taxus baccata by preparative high-performance liquid chromatography, ultra-high-performance liquid chromatography-mass spectrometry, and nuclear magnetic resonance. J. Sep. Sci. 2023, 46, 2200841. [Google Scholar] [CrossRef]
- Cusidó, R.M.; Palazón, J.; Bonfill, M.; Navia-Osorio, A.; Morales, C.; Piñol, M.T. Improved Paclitaxel and Baccatin III Production in Suspension Cultures of Taxusmedia. Biotechnol. Prog. 2002, 18, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Jondoaghleboob, A.; Ghasemnezhad, A.; Khoshhal, M.S.; Rahnama, K. Callus induction, growth, and metabolite variations in two Taxus spp. under in vitro conditions. Indian J. Hortic. 2024, 81, 223–230. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhu, X.; Liu, J.; Zhang, L.; Ahmed, N.; Qi, J.; Chen, B.; Tang, D.; Yu, J.; et al. Biochemical synthesis of taxanes from mevalonate. Synth. Syst. Biotechnol. 2024, 9, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.L.; Parker, W.L.; Patel, R.N. Enzymatic C-4 deacetylation of 10-deacetylbaccatin III. Biotechnol. Appl. Biochem. 2006, 45, 81–85. [Google Scholar] [CrossRef]
- Bestoso, F.; Ottaggio, L.; Armirotti, A.; Balbi, A.; Damonte, G.; Degan, P.; Mazzei, M.; Cavalli, F.; Ledda, B.; Miele, M. In vitro cell cultures obtained from different explants of Corylus avellana produce Taxol and taxanes. BMC Biotechnol. 2006, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Moieni, A.; Safaie, N. Elicitors Derived from Hazel (Corylus avellana L.) Cell Suspension Culture Enhance Growth and Paclitaxel Production of Epicoccum nigrum. Sci. Rep. 2018, 8, 12053. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Tsay, H.-S. Plant Cell Cultures an Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. J. Appl. Sci. Eng. Tech. 2004, 2, 29–48. [Google Scholar]
- Patil, R.A.; Kolewe, M.E.; Roberts, S.C. Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures. Plant Cell Tissue Organ Cult. 2013, 112, 303–310. [Google Scholar] [CrossRef]
- Yu, C.; Hou, K.; Zhang, H.; Liang, X.; Chen, C.; Wang, Z.; Wu, Q.; Chen, G.; He, J.; Bai, E.; et al. Integrated mass spectrometry imaging and single-cell transcriptome atlas strategies provide novel insights into taxoid biosynthesis and transport in Taxus mairei stems. Plant J. 2023, 115, 1243–1260. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014, 32, 304–311. [Google Scholar] [CrossRef]
- Cope, E.A. Taxaceae: The genera and cultivated species. Bot. Rev. 1998, 64, 291–322. [Google Scholar] [CrossRef]
- López-Upton, J.; Garcia-Martí, X. Taxus globosa Schltdl (Taxaceae). Distribution and diagnosis of an endangered yew. Earth Sci. 2015, 4, 80. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Osuna, L.; Moyano, E.; Bonfill, M.; Tapia, N.; Cusido, R.M.; Palazon, J. Changes in gene transcription and taxane production in elicited cell cultures of Taxus × media and Taxus globosa. Phytochemistry 2015, 117, 174–184. [Google Scholar] [CrossRef]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 2nd ed.; Tsuneo, W., Ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Larone, D.H.; Hayden, R.T.; Thomas, J.W. Larone’s Medically Important Fungi: A Guide to Identification; ASM Press: Washington, DC, USA, 2018. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Yaghmour, R.M.R. General mycological laboratory procedures. In Mycology Manual; Department of Biological Sciences: Nablus, State of Palestine, 1998; pp. 1–9. [Google Scholar]
- Zaiyou, J.; Li, M.; Guifang, X.; Xiuren, Z. Isolation of an endophytic fungus producing baccatin III from Taxus wallichiana var. mairei. J. Ind. Microbiol. Biotechnol. 2013, 40, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, K. A new endophytic taxol-and baccatin III-producing fungus isolated from Taxus chinensis var. mairei. AFRICAN J. Biotechnol. 2011, 10, 1–15. [Google Scholar] [CrossRef]
- Guo, B.H.; Kai, G.Y.; Jin, H.B.; Tang, K.X. Taxol synthesis. Afr. J. Biotechnol. 2006, 5, 15–20. [Google Scholar]
- Kasaei, A.; Mobini-Dehkordi, M.; Mahjoubi, F.; Saffar, B. Isolation of Taxol-Producing Endophytic Fungi from Iranian Yew Through Novel Molecular Approach and Their Effects on Human Breast Cancer Cell Line. Curr. Microbiol. 2017, 74, 702–709. [Google Scholar] [CrossRef]
- Chakravarthi, B.V.S.K.; Singh, S.; Kamalraj, S.; Gupta, V.K.; Jayabaskaran, C. Evaluation of spore inoculum and confirmation of pathway genetic blueprint of T13αH and DBAT from a Taxol-producing endophytic fungus. Sci. Rep. 2020, 10, 21139. [Google Scholar] [CrossRef]
- Cai, L.; Jeewon, R.; Hyde, K.D. Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycol. Res. 2006, 110 Pt 2, 137–150. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Soca-Chafre, G.; Rivera-Orduña, F.N.; Hidalgo-Lara, M.E.; Hernandez-Rodriguez, C.; Marsch, R.; Flores-Cotera, L.B. Molecular phylogeny and paclitaxel screening of fungal endophytes from Taxus globosa. Fungal. Biol. 2011, 115, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Orduña, F.N.; Suarez-Sanchez, R.A.; Flores-Bustamante, Z.R. Diversity of endophytic fungi of Taxus globosa (Mexican yew). Fungal Divers. 2011, 47, 65–74. [Google Scholar] [CrossRef]
- Flores-Bustamante, Z.R. Aislamiento e Identificación de Microorganismos Asociados al Tejo Mexicano (Taxus globosa) y Estudio de su Capacidad Para Producir Taxol; Instituto Politécnico Nacional: Mexico City, Mexico, 2009. [Google Scholar]
- García, D.; Stchigel, A.M.; Cano, J.; Guarro, J.; Hawksworth, D.L. A synopsis and re-circumscription of Neurospora (syn. Gelasinospora) based on ultrastructural and 28S rDNA sequence data. Mycol. Res. 2004, 108 Pt 10, 1119–1142. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Nugent, L.K.; Sihanonth, P.; Thienhirun, S.; Whalley, A.J.S. Biscogniauxia: A genus of latent invaders. Mycologist 2005, 19, 40–43. [Google Scholar] [CrossRef]
- Ming-Jen Cheng, A.; Ming-Der Wu, A.; Hiromasa Yanai, B.; Yung-Shun Su, C.; Ih-Sheng Chen, D.; Gwo-Fang Yuan, A.; Sung-Yuan Hsieh, A.; Jih-Jung Chen, E. Secondary metabolites from the endophytic fungus Biscogniauxia formosana and their antimycobacterial activity. Phytochem. Lett. 2012, 5, 467–472. [Google Scholar] [CrossRef]
- Silva-Hughes, A.F.; Wedge, D.E.; Cantrell, C.L.; Carvalho, C.R.; Pan, Z.; Moraes, R.M.; Madoxx, V.L.; Rosa, L.H. Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol. Res. 2015, 175, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef]
- MacMillan, J. Occurrence of Gibberellins in Vascular Plants, Fungi, and Bacteria. J. Plant Growth Regul. 2001, 20, 387–442. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khana, A.L.; Hamayuna, M.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of penicillium citrinum and aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Nisar, M.; Khan, I.; Ahmad, B.; Ali, I.; Ahmad, W.; Choudhary, M.I. Antifungal and antibacterial activities of Taxus wallichiana Zucc. J. Enzyme. Inhib. Med. Chem. 2008, 23, 256–260. [Google Scholar] [CrossRef]
- Toghueo, R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Izquierdo-Bueno, I.; González-Rey, E.; González-Rodríguez, V.E.; Cantoral, J.M.; Collado, I.G.; Garrido, C. In Vitro Analysis of the Antagonistic Biological and Chemical Interactions between the Endophyte Sordaria tomento-alba and the Phytopathogen Botrytis cinerea. Int. J. Mol. Sci. 2024, 25, 1022. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, R.E.; Sánchez-Fuentes, R.; Rangel-Sánchez, H.; Hernández-Ortega, S.; López-Cortés, J.G.; Macías-Rubalcava, M.L. Antifungal and antioomycete activities and modes of action of isobenzofuranones isolated from the endophytic fungus Hypoxylon anthochroum strain Gseg1. Pestic. Biochem. Physiol. 2020, 169, 104670. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Tsao, R.; Raizada, M.N. Chemical inhibitors suggest endophytic fungal paclitaxel is derived from both mevalonate and non-mevalonate-like pathways. J. Nat. Prod. 2011, 74, 2497–2504. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ahmed, A.S.; Hassan, I.A.; El-Sayed, E.S.R.; Karam El-Din, A.Z.A. Production of paclitaxel with anticancer activity by two local fungal endophytes, Aspergillus fumigatus and Alternaria tenuissima. Appl. Microbiol. Biotechnol. 2017, 101, 5831–5846. [Google Scholar] [CrossRef]
- Qiao, W.; Ling, F.; Yu, L.; Huang, Y.; Wang, T. Enhancing taxol production in a novel endophytic fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis var. mairei. Fungal Biol. 2017, 121, 1037–1044. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Raizada, M.N. Sites of biosynthesis and storage of Taxol in Taxus media (Rehder) plants: Mechanism of accumulation. Phytochemistry 2020, 175, 112369. [Google Scholar] [CrossRef]

| Strain | Detection Method | Baccatin III Production | Reference |
|---|---|---|---|
| Diaporthe phaseolorum | HPLC | Baccatin III content of full and drained PDB culture is 0.219 mg/L and 0.193 mg/L, respectively, and 0.014 mg/g of dry mass. | [26] |
| Didymostilbe sp. | LC-MS | 8–15 µg/L | [27] |
| Ozonium sp. | HPLC-MS | 12–18 µg/L | [28] |
| Cladosporium, Alternaria, Fusarium | HPLC | 23.5–139.2 mg/kg dw | [29] |
| F. solani | Enzymatic Competitive inhibition immunoassay (CIEIA) | 4.9–128.3 µg/L | [30] |
| Isolate TgO and Aspergillus sp. | LC–QTOF MS/MS | 0.7–1.35 µg/L | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara-Sánchez, J.G.; Aguilar-Uscanga, M.G.; Ledesma-Escobar, C.A.; Castro-Martínez, C.; Condé, R.; Sachman-Ruíz, B.; del Moral, S. Taxane-Producing Fungi Isolated from Taxus globosa Tree Bark. Microorganisms 2025, 13, 300. https://doi.org/10.3390/microorganisms13020300
Guevara-Sánchez JG, Aguilar-Uscanga MG, Ledesma-Escobar CA, Castro-Martínez C, Condé R, Sachman-Ruíz B, del Moral S. Taxane-Producing Fungi Isolated from Taxus globosa Tree Bark. Microorganisms. 2025; 13(2):300. https://doi.org/10.3390/microorganisms13020300
Chicago/Turabian StyleGuevara-Sánchez, Jocelyn Guadalupe, María Guadalupe Aguilar-Uscanga, Carlos Augusto Ledesma-Escobar, Claudia Castro-Martínez, Renaud Condé, Bernardo Sachman-Ruíz, and Sandra del Moral. 2025. "Taxane-Producing Fungi Isolated from Taxus globosa Tree Bark" Microorganisms 13, no. 2: 300. https://doi.org/10.3390/microorganisms13020300
APA StyleGuevara-Sánchez, J. G., Aguilar-Uscanga, M. G., Ledesma-Escobar, C. A., Castro-Martínez, C., Condé, R., Sachman-Ruíz, B., & del Moral, S. (2025). Taxane-Producing Fungi Isolated from Taxus globosa Tree Bark. Microorganisms, 13(2), 300. https://doi.org/10.3390/microorganisms13020300







