Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture
Abstract
1. Introduction
- Soil Health: Restoring and maintaining soil fertility, minimising reliance on synthetic fertilisers while maintaining productivity.
- Climate Change Mitigation: Enhancing carbon sequestration in soils and reducing greenhouse gas (GHG) emissions from agriculture.
- Water Management: Improving water use efficiency and quality through microbial interventions.
- Pest and Disease Control: Developing microbial alternatives and adjuncts to chemical pesticides.
- Nutrient Recycling: Facilitating the efficient use and recycling of nutrients in agricultural systems.
2. Soil Health and Fertility Through Microbial Partnerships
2.1. Microbial Mediation in Nutrient Cycling and Productivity Enhancement
- Nitrogen-fixing bacteria: Convert atmospheric nitrogen into plant-available forms.
- Nitrifying and denitrifying bacteria: Regulate nitrogen transformation and recycling.
- Phosphate and potassium solubilising microbes: Convert insoluble nutrients into soluble forms.
2.2. Microbial Influence on Soil Properties and Structure
2.2.1. Soil Aggregation and Structure
2.2.2. Organic Matter Decomposition
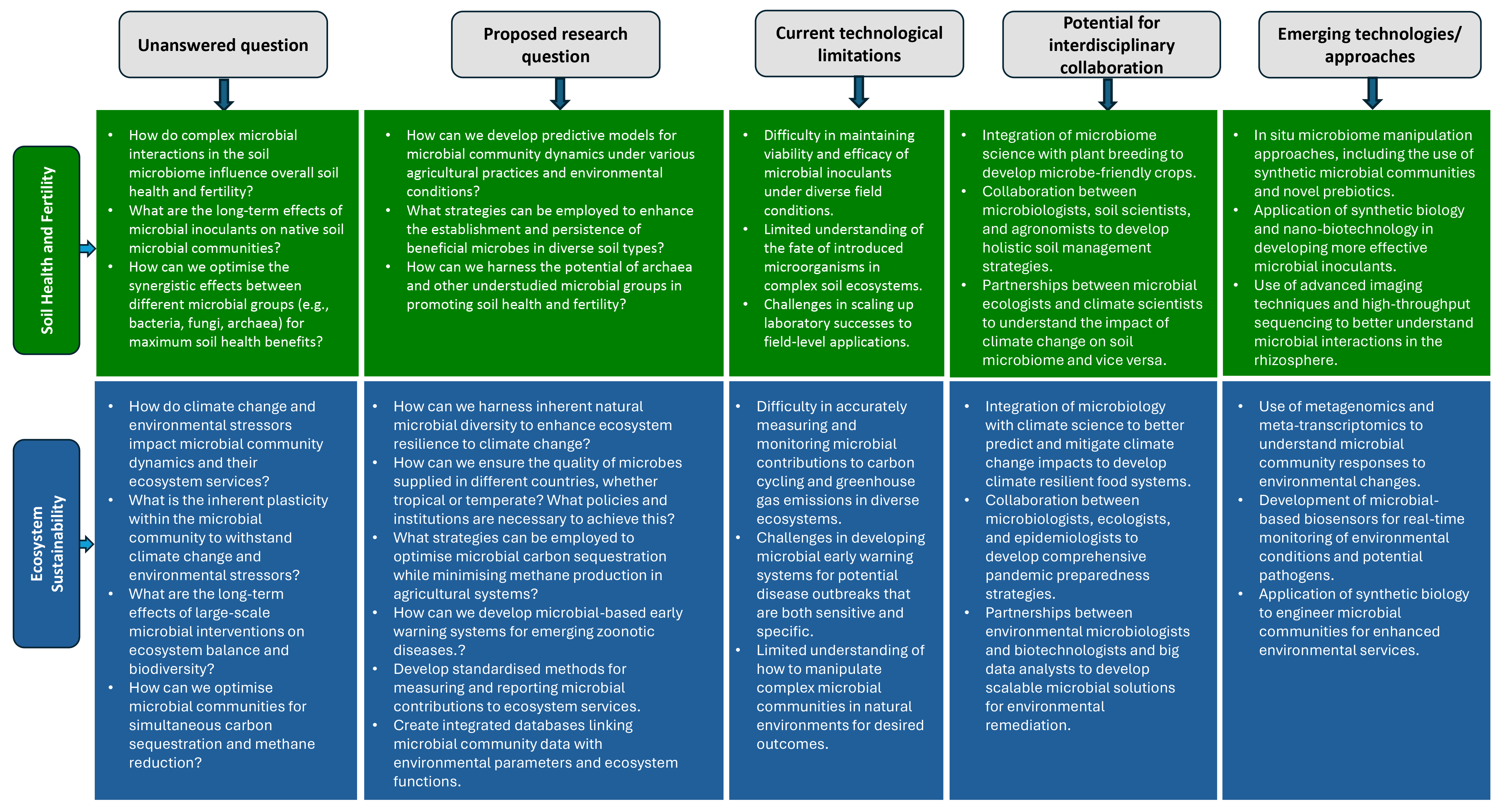
2.3. Research Gaps and Future Directions
3. Ecosystem Sustainability Through Microbial Functions
3.1. Microbes as Carbon Sequesters
3.2. Microbes as Soil Erosion Protectors
3.3. Microbes in Waste Management and Bioremediation
3.4. Microbes in Water Management and Wastewater Treatment
3.5. Microbes in Climate Change Mitigation
3.6. Microbes in Agricultural Pandemic Preparedness
3.7. Research Gaps and Future Directions
4. Plant Health, Productivity and Food Security Through Microbial Innovations
4.1. Biofertilisers, Biostimulants and Bioprotectants: The 3Bs
4.1.1. Plant Growth-Promoting Rhizobacteria (PGPR)
4.1.2. Rhizobia and Other Biofertilisers
| Microbe | Crop | Role | Reference |
|---|---|---|---|
| Pseudomonas protegens | Wheat (Triticum aestivum) | Increased the IAA/ABA ratio, enhanced grain yield | [112] |
| Bacillus cereus ZnSB13 | Chickpea (Cicer arietinum L.) | Increment in fresh and dry nodule weights, shoot and root dry weight, effective grain yield | [113] |
| Pseudomonas spp., VBZ4 | Tomato (Solanum lycopersicum L.) | Taller plants, broader stems, higher fresh and dry shoot and root weights, and a greater number of fruits per plant | [114] |
| Rhizobium tropici + Bacillus subtilis | Common bean (Phaseolus vulgaris L.) | Improvement in shoot dry matter and grain yield | [115] |
| Gluconacetobacter diazotrophicus | Rice (Oryza sativa) | Promoting various root growth and developmental mechanisms | [116] |
| Pseudomonas fluorescens | Foxtail millet (Setaria italica) | Increase in germination rate, improved soil adherence to root tissue, dry mass | [117] |
| Pseudomonas jessenni MP1 and Pseudomonas palleroniana N26 | Kidney bean (Phaseolus vulgaris L.) | Improved grain yield, nutritional status, and crop growth | [12] |
| Streptomyces griseus CAI-24 | Chilli and tomato | Enhanced fruit yield | [118] |
| Rhizobiapusense IC-59 and Paraburkholderia kururiensis IC-76a | Chickpea (Cicer arietinum L.) | Enhanced nodule number and nodule weight and IAA production | [119] |
| Streptomyces avermitilis CAI-85 and Streptomyces albus CAI-93 | Rice (Oryza sativa) | Root and shoot development and crop productivity | [120] |
| Streptomyces spp., CAI-26 and Streptomyces griseus MMA-32 | Pigeonpea (Cajanus cajan) | Enhanced stover and grain yield | [121] |
| Streptomyces griseus CAI-24 and Streptomyces griseus MMA-32 | Pearl millet (Pennisetum glaucum) | Enhanced the stover and grain yield and improved nutrient content (Iron and Zinc) | [122] |
| Streptomyces griseus CAI-68 and Streptomyces griseus MMA-32 | Chickpea (Cicer arietinum L.) | Increased seed mineral content | [123] |
| Streptomyces africanus KAI-32 | (Sorghum) Sorghum bicolor | Significantly enhanced stover yield | [124] |
| Trichoderma spp. | Sugarcane, rice, vegetables | Biocontrol; enhanced nutrient uptake | [125] |
| AMF | Cotton | Enhanced phosphorus acquisition, growth, seed cotton yield and fiber quality | [126] |
| Rhizobia | Legumes | Biological nitrogen fixation, phytohormone production and stress resilience | [127] |
4.1.3. Arbuscular Mycorrhizal Fungi (AMF)
4.1.4. Microbes as Bioprotectants
| Microbe | Test Plant/Disease/Pest | Target Pathogen/Pest | Mechanisms | Reference |
|---|---|---|---|---|
| Bacillus amyloliquefaciens | Rhizome rot disease of turmeric (Curcuma longa) | Rhizoctonia solani | Antifungal lipopeptides Synthesis | [154] |
| Pseudomonas sp. CMR12a | Root rot of cocoyam (Colocasia esculenta) | Pythium myriotylum | Phenazines and cyclic lipopeptides synthesis | [155] |
| Bacillus thuringiensis | Sclerotiniose in mustard (Brassica campestris) | Sclerotinia sclerotiorum and Plutella xylostella | ISR in plants by simultaneously activating SA, JA, and ET signaling pathway | [156] |
| Pseudomonas fluorescens Q2-87 | Arabidopsis thaliana | Botrytis cinerea | ISR, phenolic compound synthesis | [157] |
| Pseudomonas chlororaphis R47 | Late blight of potato (Solanum tuberosum) | Phytophthora infestans | Production of HCN inhibiting mycelium and zoospore germination inhibition | [158] |
| Paenibacillus polymyxa | Charcoal rot of soybean (Glycine max) | Rhizoctonia bataticola | Antifungal lipopeptides Synthesis | [159] |
| Paenibacillus polymyxa | Fusarium wilt of cucumber (Cucumis sativus) | Fusarium oxysporum f. sp. cucumerinum | Antimicrobial compounds & hydrolytic enzyme synthesis | [160] |
| Serratia marcescens ETR17 | Root rot of tea (Camellia sinensis) | Lasiodiplodia theobromae | Production of hydrolytic enzymes | [161] |
| Streptomyces violaceusniger AC12AB | Common scab of potato (Solanum tuberosum) | Streptomyces scabies | Antibiotic production | [162] |
| Trichoderma asperellum FJ035 | Fusarium wilt of cucumber (Cucumis sativus) | Fusarium oxysporium | Antagonism and spatiotemporal competition | [163] |
| Trichodermaharzianum | Curvularia leaf spot of maize (Zea mays) | Curvularia lunata | ISR to fungal disease | [164] |
| Beauveria bassiana | Cotton leafworm in cotton (Gossypium sp.) | Spodoptera litura | Pupal & adult deformities | [165] |
| Metarhizium anisopliae | Fusarium head blight of wheat (Triticum aestivum) | Fusarium graminearum | Produces fungistatic secondary metabolites | [166] |
| Podoviruses | Soft rot of potato (Solanum tuberosum) | Pectobacterium carotovorum | Cell lysis | [167] |
| Streptomyces spp., AUR-2 | Botrytis gray mold (BGM) of chickpea | Botrytis cinerea | Anti-fungal activity and host plant resistance | [168] |
| Streptomyces africanus KAI-32 and Streptomyces griseus CAI-127 | Fusarium wilt of chickpea | Fusarium oxysporum | Anti-fungal activity and host plant resistance | [169] |
| Streptomyces albus CAI-21 | Charcoal rot of sorghum | Macrophomina phaseolina | Anti-fungal secondary metabolites synthesis | [170] |
| Streptomyces griseus CAI-155 | Legume pod borer in chickpea | Helicoverpa armigera | Antifeedant, larvicidal activity | [149] |
4.1.5. Economic Impact and Market Growth of 3Bs
4.2. Insect Microbiomes as Allies in Agricultural Ecosystems
4.3. Microbes as Drought-Stress Alleviators
| Microbe | Crop | Stress Type | Function | Reference |
|---|---|---|---|---|
| Species of Gracilibacillus, Staphylococcus, Virgibacillus, Salinicoccus, Bacillus, Zhihengliuella, Brevibacterium, Oceanobacillus, Exiguobacterium, Pseudomonas, Arthrobacter, Halomonas | Maize (Zea mays) | Salinity | ACC deaminase, IAA and exo-polysaccharide production; biofilm formation and phosphate solubilization | [187] |
| Azospirillum and Rhizobia | Soybean (Glycine max) | Moderate Drought | Production of phytohormones and improving root architecture | [188] |
| Bacillus aryabhattai | Cowpea (Vigna unguiculata) | Drought and Paraquat pesticide residue | Production of phytohormones and improving root architecture | [189] |
| Gigaspora margarita, Funneliformis mosseae , Funneliformis fasciculatus | Soybean (Glycine max) | Water stress | Vesicular-arbuscular mycorrhizal fungi provided low resistance pathway for water movement across the root | [190] |
| B. thuringiensis | French lavender Lavandula dentata | Drought | Production of IAA by the bacterium improving nutrition, physiology, and metabolic activities of plant by inducing higher proline and K-content, and decreased oxidative stress-related enzymes | [191] |
| Pseudomonas putida GAP-P45 | Arabidopsis thaliana | Water stress | Changes in proline metabolic gene expression profile—up-regulation of the expression of genes involved in proline biosynthesis, i.e., ornithine-Δ-aminotransferase (OAT), Δ 1 -pyrroline-5-carboxylate synthetase1 (P5CS1), Δ 1 -pyrroline-5-carboxylate reductase (P5CR), as well as proline catabolism, i.e., proline dehydrogenase1 (PDH1) and Δ 1-pyrroline-5-carboxylate dehydrogenase (P5CDH). | [192] |
| Burkholderia cepacia, Promicromonospora sp. and Acinetobacter calcoaceticus | Cucumis sativus | Salinity and Drought | Enhanced leaf biomass and chlorophyll | [193] |
| PGPR consortia (Pseudomonas composti SDT3, Aeromonas aquariorum SDT13, Bacillus zhangzhouensis HPJ40, Pseudarthrobacter oxydans SRT15, B.methylotrophicus SMT38 and B. aryabhattai SMT48 | Grapevine (Vitis vinifera) | Heat stress | Osmoprotectant promotion, improved antioxidant mechanisms and membrane stability, improved light-harvesting capabilities | [194] |
| Microbacterium sp. (AR-ACC2), Methylophaga sp. (AR-ACC3), and Paenibacillus sp. (ANR-ACC3) | Rice (Oryza sativa) | Flooding stress | Enhanced germination, seedling vigour index, root and shoot length and total chlorophyll contents, reduced ethylene production | [195] |
| Bacillus cereus VBE23 | Maize (Zea mays) | Drought stress | EPS and ACC deaminase production; phosphate and zinc solubilization | [196] |
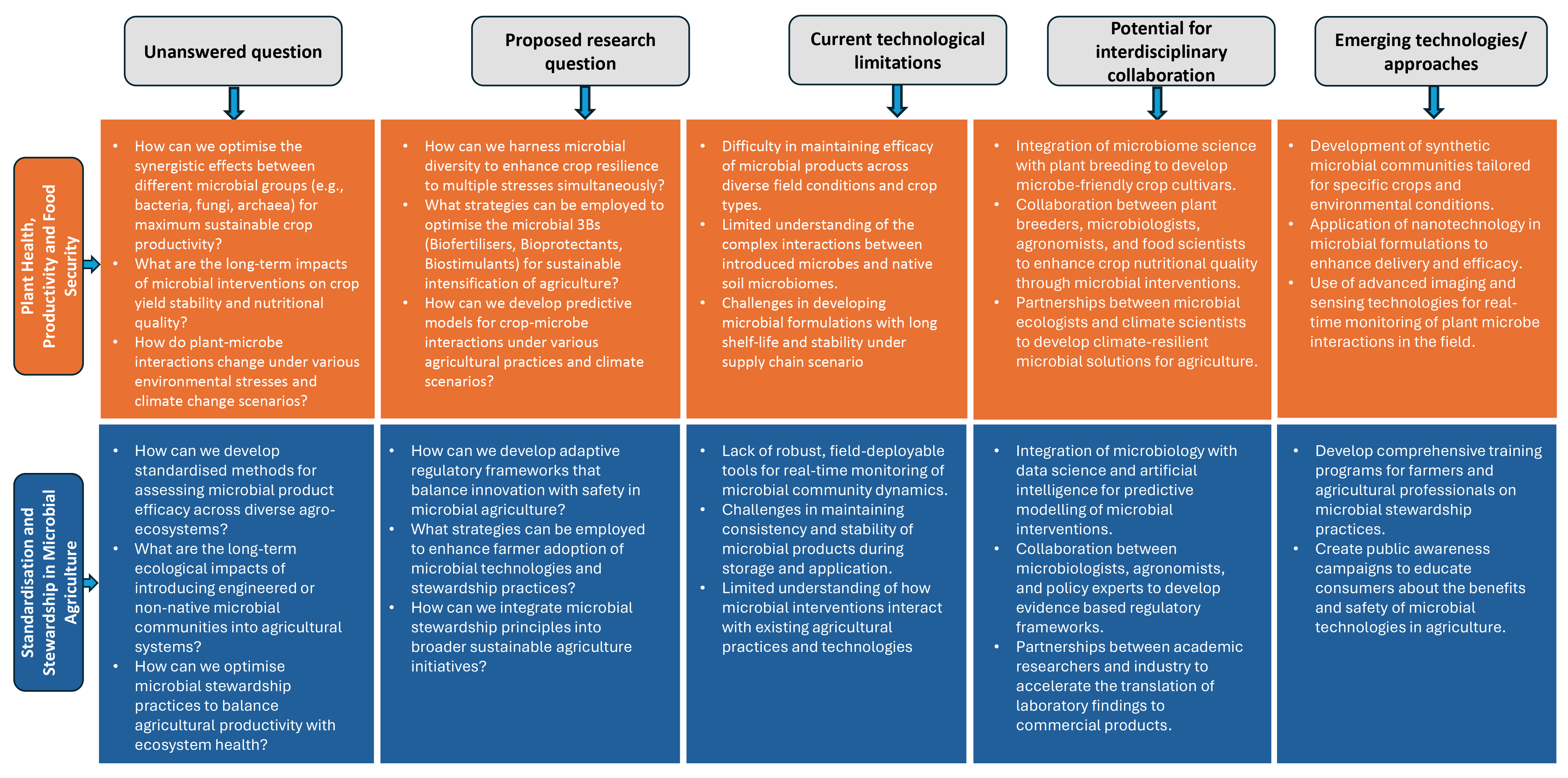
4.4. Research Gaps and Future Directions
5. Advancing Standardisation and Stewardship in Microbial Agriculture
5.1. Reimagining Standardisation for Microbial Production, Storage, and Utilisation
5.1.1. Adaptive Standardisation Frameworks
5.1.2. Standardisation of Complex Microbial Consortia
5.1.3. Blockchain-Enabled Traceability
5.1.4. AI-Driven Quality Assurance
5.1.5. Global Microbial Product Database
5.2. Microbial Stewardship
5.2.1. Microbial Ecosystem Engineering
5.2.2. Monitoring Unintended Ecological Consequences
5.2.3. Synthetic Biology and Gene Editing in Microbial Stewardship
5.2.4. Global Microbial Diversity Bank
5.2.5. Cross-Kingdom Interaction Studies
5.3. Research Gaps and Future Directions
6. Conclusions
6.1. Key Insights
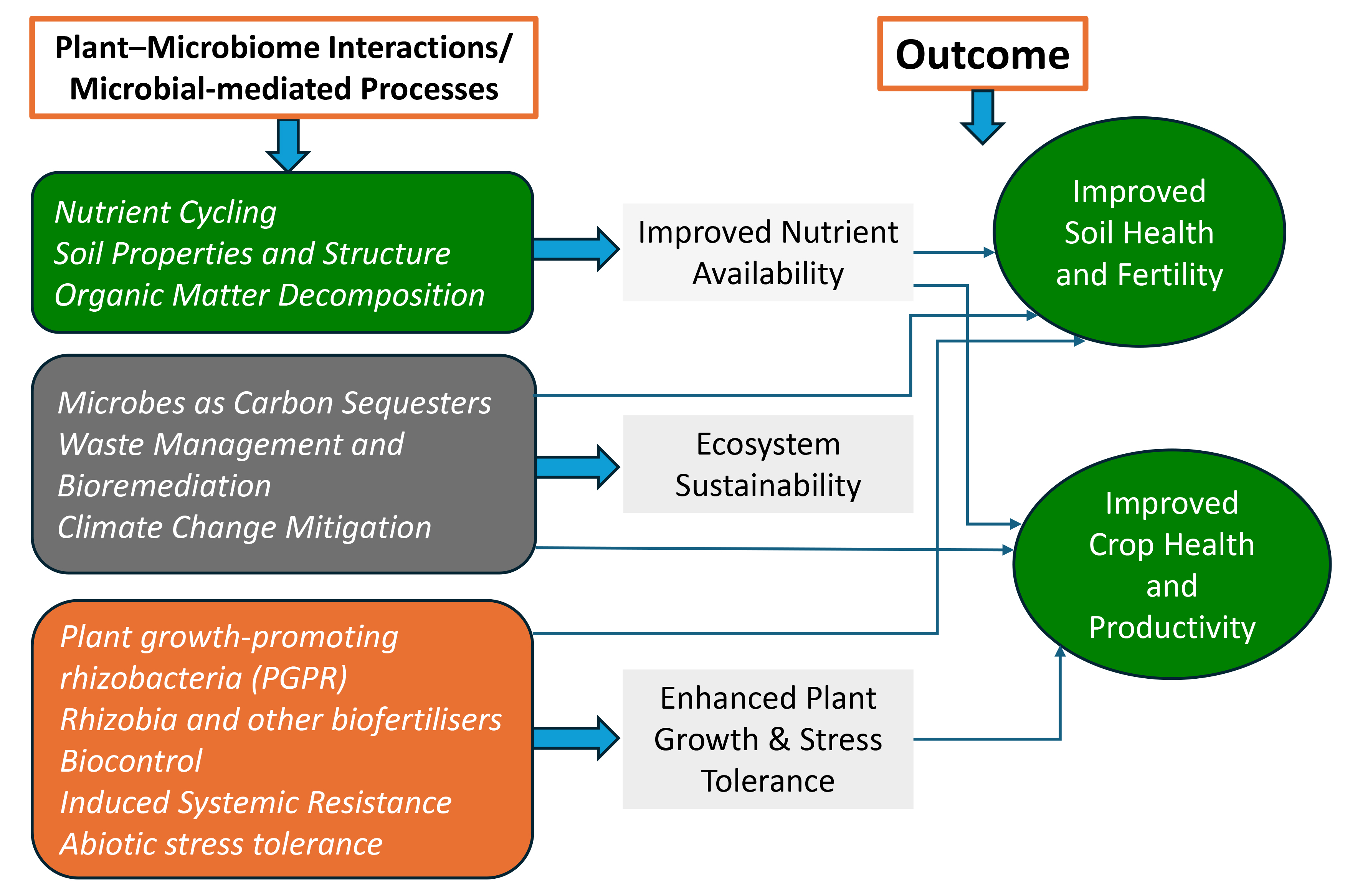
- Soil-Plant Health and Fertility through Microbial Partnerships: Microbes are fundamental to maintaining soil structure, nutrient availability, and overall soil health, forming the foundation of sustainable agriculture. Plants depend on these microbial functions to optimise root growth, nutrient uptake, and long-term productivity, forming the foundation of sustainable agriculture.
- Ecosystem Sustainability through Microbial Functions: Microbial communities contribute significantly to carbon sequestration, methane reduction, and ecosystem resilience, positioning them as key players in climate change mitigation strategies. In plants, these processes are enhanced through root exudates and canopy–soil feedback that fuel microbial activity.
- Plant Health, Productivity and Food Security through Microbial Innovations: The microbial 3Bs—biofertilisers, biostimulants, and bioprotectants—offer sustainable alternatives to chemical inputs, enhancing crop yields while reducing environmental impact. When tailored to crop species and integrated with plant growth stages, these tools deliver maximum yield and quality benefits.
- Advancing Standardisation and Stewardship in Microbial Agriculture: Emerging technologies in metagenomics, synthetic biology, and precision agriculture are unlocking new potential in microbial applications. These advances will allow real-time optimisation of plant–microbe interactions in field conditions.
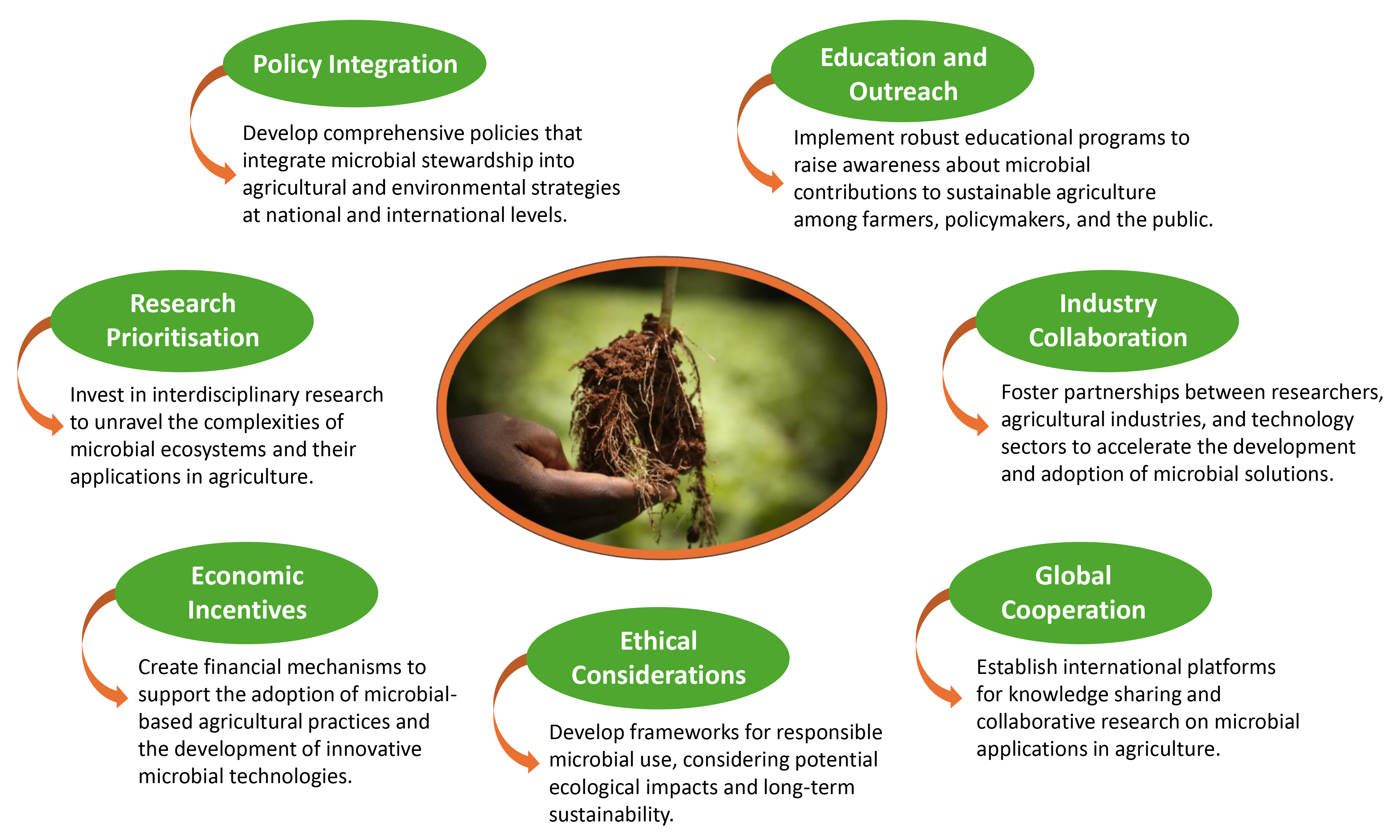
6.2. Challenges and Opportunities
- Standardisation: Developing globally accepted standards for microbial product quality and efficacy.
- Scalability: Translating laboratory successes to field-scale applications, especially ensuring that field conditions, plant varieties, and local agronomy align with microbial requirements.
- Knowledge Gaps: Understanding complex microbial interactions in diverse ecosystems with a specific focus on how these interactions vary among plant species and genotypes.
- Regulatory Frameworks: Establishing adaptive policies that balance innovation with safety, including safeguards to prevent disruption of beneficial plant–microbe symbioses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sharma, S.B.; Wightman, J.A. Vision Infinity for Food Security: Some Whys, Why Nots and Hows; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Godfrey, H.; Beddington, J.R.; Crute, I.R. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E. Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14, 32. [Google Scholar] [CrossRef]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F. Earth Beyond Six of Nine Planetary Boundaries. Sci. Adv. 2023, 9, 2458. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the Plant Microbiome: Looking Back and Future Perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Locey, K.J.; Lennon, J.T. Scaling Laws Predict Global Microbial Diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Parangusadoss, K.; Pasumalaiarasu, S.; Habeeb Mohamed, V.B.; Sankaranarayanan, A. Significance and Contribution of Microbial Biodiversity to Various Biotechnological and Industrial Sectors. In Industrial Microbiology and Biotechnology: An Insight into Current Trends; Springer Nature: Singapore, 2024; pp. 25–34. [Google Scholar] [CrossRef]
- Solanki, M.K.; Mandal, A.; Medeiros, F.H.V.D.; Awasthi, M.K. Editorial: Emerging Frontiers of Microbial Functions in Sustainable Agriculture. Front. Microbiol. 2022, 13, 1128267. [Google Scholar] [CrossRef]
- Ammar, E.E.; Rady, H.A.; Khattab, A.M.; Amer, M.H.; Mohamed, S.A.; Elodamy, N.I.; Al Farga, A.; Aioub, A.A.A. A Comprehensive Overview of Eco-friendly Bio-fertilizers Extracted from Living Organisms. Environ. Sci. Pollut. Res. 2023, 30, 113119–113137. [Google Scholar] [CrossRef]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive Effects of Crop Diversity on Productivity Driven by Changes in Soil Microbial Composition. Front. Microbiol. 2021, 12, 660749. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil Biodiversity and Soil Community Composition Determine Ecosystem Multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Pareek, N.; Arya, P.; Upadhayay, V.K.; Kumar Jugran, A.; Kumar Mishra, P.; Goel, R. Credibility Assessment of Cold Adaptive Pseudomonas jesenni MP1 and P. palleroniana N26 on Growth, Rhizosphere Dynamics, Nutrient Status, and Yield of the Kidney Bean Cultivated in Indian Central Himalaya. Front. Plant Sci. 2023, 14, 1042053. [Google Scholar] [CrossRef]
- Morton, J.B. Evolutionary Relationships Among Arbuscular Mycorrhizal Fungi in the Endogonaceae. Mycologia 1990, 82, 192–207. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The Mutualistic Interaction Between Plants and Arbuscular Mycorrhizal Fungi. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Neuenkamp, L.; Moora, M.; Öpik, M.; Davison, J.; Gerz, M.; Männistö, M.; Jairus, T.; Vasar, M.; Zobel, M. The Role of Plant Mycorrhizal Type and Status in Modulating the Relationship Between Plant and Arbuscular Mycorrhizal Fungal Communities. New Phytol. 2018, 220, 1236–1247. [Google Scholar] [CrossRef]
- Sukmasari, M.D.; Dani, U.; Wijaya, A.A. Arbuscular Mycorrhiza Inoculation for Increasing the Tolerance Index and Productivity of Soybean on Marginal Soils. IOP Conf. Ser. Earth Environ. Sci. 2021, 748, 012043. [Google Scholar] [CrossRef]
- Jansa, J.; Finlay, R.; Wallander, H.; Smith, F.A.; Smith, S.E. Role of Mycorrhizal Symbioses in Phosphorus Cycling. In Soil Biology; Springer: Berlin, Germany, 2011; pp. 137–168. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate-solubilizing Bacteria, and Silicon to P Pptake by Plants. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Augé, R.M.; Stodola, A.J.W.; Tims, J.E.; Saxton, A.M. Moisture Retention Properties of a Mycorrhizal Soil. Plant Soil 2001, 230, 87–97. [Google Scholar] [CrossRef]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of Plant Water Uptake by an Arbuscular Mycorrhizal Fungus: A Gordian Knot of Roots and Hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The Critical Role of Arbuscular Mycorrhizal Fungi to Improve Drought Tolerance and Nitrogen Use Efficiency in Crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.M.; Jones, D.L.; Chadwick, D.R.; Qi, X.; Cotta, S.R.; Araújo, V.L.V.P.; Matteoli, F.P.; Lacerda-Júnior, G.V.; Pereira, A.P.A.; Fernandes, P.I.; et al. Can Arbuscular Mycorrhizal Fungi and Rhizobacteria Facilitate 33P Uptake in Maize Plants under Water Stress? Microbiol. Res. 2023, 271, 127350. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular Mycorrhiza and Nitrogen: Implications for Individual Plants Through to Ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Praveen, R.; Ashwin, R.; Joanna, D.; Bagyaraj, D.J. Arbuscular Mycorrhizal Fungi as Biofertilizers for Sustainable Agriculture. In Biofertilizers: Agricultural Uses; Nova Science Publishers: Hauppauge, NY, USA, 2023. [Google Scholar]
- Ashwin, R.; Bagyaraj, D.J.; Mohan Raju, B. Ameliorating the Drought Stress Tolerance of a Susceptible Soybean Cultivar, MAUS 2 Through Dual Inoculation with Selected Rhizobia and AM Fungus. Fungal Biol. Biotechnol. 2023, 10, 10. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Wang, G. Inoculation with Arbuscular Mycorrhizal Fungi Improves Plant Biomass and Nitrogen and Phosphorus Nutrients: A Meta-analysis. BMC Plant Biol. 2024, 24, 960. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of Microbial Communities in the Formation and Decomposition of Soil Organic Matter. In Soil Microbiology and Sustainable Crop Production; Springer: Dordrecht, The Netherlands, 2010; pp. 81–118. [Google Scholar]
- Cui, J.; Holden, N.M. The Relationship Between Soil Microbial Activity and Microbial Biomass, Soil Structure and Grassland Management. Soil Tillage Res. 2015, 146, 32–38. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V. Multifarious Effect of ACC Deaminase and EPS Producing Pseudomonas sp. and Serratia marcescens to Augment Drought Stress Tolerance and Nutrient Status of Wheat. World J. Microbiol. Biotechnol. 2021, 37, 198. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mardatin, N.F.; Leifheit, E.F.; Antunes, P.M. Mycelium of Arbuscular Mycorrhizal Fungi Increases Soil Water Repellency and is Sufficient to Maintain Water-Stable Soil Aggregates. Soil Biol. Biochem. 2010, 42, 1189–1191. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Ashwin, R.; Praveen, R. Arbuscular Mycorrhizal Fungi and Their Role in Soil Health. In Sustainable Soil Management: Beyond Food Production; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2023; pp. 272–292. [Google Scholar]
- Gopalakrishnan, S.; Srinivas, V.; Chand, U.; Pratyusha, S.; Samineni, S. Streptomyces Consortia-Mediated Plant Growth-promotion and Yield Performance in Chickpea. 3 Biotech 2022, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Srinivas, V.; Alekhya, G.; Prakash, B.; Kudapa, H.; Rathore, A.; Rajeev Kumar, V. The Extent of Grain Yield and Plant Growth Enhancement by Plant Growth-Promoting Broad-Spectrum Streptomyces sp. in Chickpea. Springerplus 2015, 4, 31. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Rehman, S.U.; Oetting, J.N. Unrevealing the Potential of Microbes in Decomposition of Organic Matter and Release of Carbon in the Ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Wani, S.P.; Pathak, P.; Jangawad, L.S.; Eswaran, H.; Singh, P. Improved Management of Vertisols in Semiarid Tropics for Increased Productivity and Soil Carbon Sequestration. Soil Use Manag. 2003, 19, 217–222. [Google Scholar] [CrossRef]
- Komala, T.; Khun, T.C. Biological Carbon Dioxide Sequestration Potential of Bacillus pumilus. Sains Malays 2014, 43, 1149–1156. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological Precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Rossi, F.; Li, H.; Liu, Y.; De Philippis, R. Cyanobacterial Inoculation (Cyanobacterisation): Perspectives for the Development of a Standardized Multifunctional Technology for Soil Fertilization and Desertification Reversal. Earth-Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Büdel, B.; Williams, W.J.; Reichenberger, H. Annual Net Primary Productivity of a Cyanobacteria-dominated Biological Soil Crust in the Gulf Savannah, Queensland, Australia. Biogeosciences 2018, 15, 491–505. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Román, J.R.; Roncero-Ramos, B.; Erickson, T.E.; Merritt, D.J.; Aguila-Carricondo, P.; Canton, D. Cyanobacteria Inoculation Enhances Carbon Sequestration in Soil Substrates Used in Dryland Restoration. Sci. Total Environ. 2018, 636, 1149–1154. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Bhaduri, D.; Chauhan, S.; Chandra, R.; Raverkar, K.P.; Pareek, N. Comparative Evaluation of Three Contrasting Land Use Systems for Soil Carbon, Microbial and Biochemical Indicators in North-Western Himalaya. Ecol. Eng. 2017, 103, 21–30. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in Ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef]
- Mukasa Mugerwa, T.T.; McGee, P.A. Potential Effect of Melanised Endophytic Fungi on Levels of Organic Carbon within an Alfisol. Soil Res. 2017, 55, 245. [Google Scholar] [CrossRef]
- Mason, A.R.G.; Salomon, M.J.; Lowe, A.J.; Cavagnaro, T.R. Microbial Solutions to Soil Carbon Sequestration. J. Clean. Prod. 2023, 417, 137993. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic Matter and Water-Stable Aggregates in Soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Liu, X. Erosion Reduces Soil Microbial Diversity, Network Complexity and Multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Zarei Darki, B. Quality Improvement of An Erosion-Prone Soil Through Microbial Enrichment. Soil Tillage Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Ham, S.M.; Chang, I.; Noh, D.H.; Kwon, T.-H.; Muhunthan, B. Improvement of Surface Erosion Resistance of Sand by Microbial Biopolymer Formation. J. Geotech. Geoenviron. Eng. 2018, 144, 06018004. [Google Scholar] [CrossRef]
- Noh, D.H.; Ajo-Franklin, J.B.; Kwon, T.H.; Muhunthan, B. P and S Wave Responses of Bacterial Biopolymer Formation in Unconsolidated Porous Media. J. Geophys. Res. Biogeosci 2016, 121, 1158–1177. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, M. Runoff and Soil Loss from Revegetated Grasslands in The Hilly Loess Plateau region, China: Influence of Biocrust Patches and Plant Canopies. J. Hydrol. Eng. 2013, 18, 387–393. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, N.; Weber, B.; Xu, M. Response of Biological Soil Crusts to Raindrop Erosivity and Underlying Influences in The Hilly Loess Plateau region, China. Biodivers. Conserv. 2014, 23, 1669–1686. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Zarei Darki, B.; Homaee, M. Controlling Rainfall-Induced Soil Loss from Small Experimental Plots Through Inoculation of Bacteria and Cyanobacteria. Catena 2017, 152, 40–46. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, R.; Li, T.; Meng, F.; Fu, Q.; Li, M.; Liu, X. Mechanism of Microbial Inhibition of Rainfall Erosion in Black Soil Area, as a Soil Structure Builder. Soil Tillage Res. 2023, 233, 105819. [Google Scholar] [CrossRef]
- Bollag, J.-M.; Mertz, T.; Otjen, L. Role of Microorganisms in Soil Bioremediation. In Bioremediation through Rhizosphere Technology; American Chemical Society: Washington, DC, USA, 1994; pp. 2–10. [Google Scholar]
- Kumar, A.; Sharma, S. (Eds.) Microbes and Enzymes in Soil Health and Bioremediation, 1st ed.; Springer: Singapore, 2021. [Google Scholar]
- Wani, S.P.; Chander, G.; Vineela, C.K. Vermicomposting: Recycling Wastes into Valuable Manure for Sustained Crop Intensification in the Semi-Arid Tropics. In Bioresources for Sustainable Plant Nutrient Management; Satish Serial Publishing House: Delhi, India, 2014; pp. 123–151. [Google Scholar]
- Chander, G.; Wani, S.P.; Gopalakrishnan, S.; Mahapatra, A.; Chaudhury, S.; Pawar, C.S.; Srinivas, I. Microbial Consortium Culture and Vermicomposting Technologies for Recycling On-Farm Wastes and Food Production. Int. J. Recycl. Org. Waste Agric. 2018, 7, 99–108. [Google Scholar] [CrossRef]
- Epstein, E. The Science of Composting; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Bioremediation of Soils by Plant–Microbe Systems. Int. J. Green Energy 2004, 1, 301–312. [Google Scholar] [CrossRef]
- Savitha, J.; Sriharsha, D.V. Microbes for Restoration of Degraded Ecosystems. In Biosorption of Heavy Metals by Microbes and Agro Wastes—An Ecofriendly Inexpensive Approach for Remediation of Heavy Metal Polluted Environment; Bagyaraj, D.J., Jamaluddin, Eds.; NIPA Publishers (Co-Published with CRC Press): New Delhi, India, 2019; pp. 37–74. [Google Scholar]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of Environmental Wastes: The Role of Microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Rafeeq, H.; Qamar, S.A.; Nguyen, T.A.; Bilal, M.; Iqbal, H.M.N. Microbial Degradation of Environmental Pollutants. In Biodegradation and Biodeterioration at the Nanoscale; Iqbal, H.M.N., Bilal, M., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 509–528. [Google Scholar]
- Arun, A.; Raja, P.P.; Arthi, R.; Ananthi, M.; Kumar, K.S.; Eyini, M. Polycyclic Aromatic Hydrocarbons (PAHs) Biodegradation by Basidiomycetes Fungi, Pseudomonas Isolate, and Their Cocultures: Comparative in vivo and in silico Approach. Appl. Biochem. Biotechnol. 2008, 151, 132–142. [Google Scholar] [CrossRef]
- Daims, H.; Taylor, M.W.; Wagner, M. Wastewater Treatment: A Model System for Microbial Ecology. Trends Biotechnol. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Ferrera, I.; Sánchez, O. Insights into Microbial Diversity in Wastewater Treatment Systems: How Far Have We Come? Biotechnol. Adv. 2016, 34, 790–802. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Microbes and Wastewater Treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–255. [Google Scholar]
- Food and Agriculture Organization of the United Nations. AQUASTAT-FAO’s Global Information System on Water and Agriculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Tilak, A.S.; Wani, S.P.; Patil, M.D.; Datta, A. Evaluating Wastewater Treatment Efficiency of Two Field Scale Subsurface Flow Constructed Wetlands. Curr. Sci. 2016, 110, 1764–1772. [Google Scholar] [CrossRef]
- Wani, S.P. Soil Carbon Budgeting and Trading to Benefit Rural Poor; ICRISAT: Patancheru, India, 2008. [Google Scholar]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Mangan, N.; Brenner, M. Systems Analysis of the CO2 Concentrating Mechanism in Cyanobacteria. eLife 2014, 3, e02043, Erratum in eLife 2015, 4, e06400. https://doi.org/10.7554/eLife.06400. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Cargill, R.I.M.; Van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal Mycelium as a Global Carbon Pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular Mechanisms Associated with Microbial Biostimulant-Mediated Growth Enhancement, Priming and Drought Stress Tolerance in Maize Plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Lim, J.; Wehmeyer, H.; Heffner, T.; Aeppli, M.; Gu, W.; Kim, P.J.; Horn, M.A.; Ho, A. Resilience of Aerobic Methanotrophs in Soils; Spotlight on the Methane Sink Under Agriculture. FEMS Microbiol. Ecol. 2024, 100, fiae008. [Google Scholar] [CrossRef]
- Mohite, J.A.; Khatri, K.; Pardhi, K.; Manvi, S.S.; Jadhav, R.; Rathod, S.; Rahalkar, M.C. Exploring the Potential of Methanotrophs for Plant Growth Promotion in Rice Agriculture. Methane 2023, 2, 361–371. [Google Scholar] [CrossRef]
- Ministry of Agriculture; Farmers Welfare. Pioneering Breakthrough Toward Net Zero Emissions in Agriculture: ICAR-NRRI Scientists Develop Novel formulation of Methane-Oxidising Bacteria Isolated from Sundarban Mangroves for Climate Change Mitigation. 2024. Available online: https://www.pib.gov.in/PressReleasePage.aspx?PRID=2044598(accessed on 4 September 2025).
- Senthilraja, K.; Venkatesan, S.; Udhaya Nandhini, D.; Dhasarathan, M.; Prabha, B.; Boomiraj, K.; Mohan Kumar, S.; Bhuvaneswari, K.; Raveendran, M.; Geethalakshmi, V. Mitigating Methane Emission from the Rice Ecosystem through Organic Amendments. Agriculture 2023, 13, 1037. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Azevedo, L.C.B.; Bertini, S.C.B.; Ferreira, A.S.; Rodovalho, N.S.; Ferreira, L.F.R.; Kumar, A. Microbial Contribution to the Carbon Flux in the Soil: A Literature Review. Rev. Bras. Ciência Solo 2024, 48, e0230065. [Google Scholar] [CrossRef]
- Beattie, G.A.; Edlund, A.; Esiobu, N.; Gilbert, J.; Nicolaisen, M.H.; Jansson, J.K.; Jensen, P.; Keiluweit, M.; Lennon, J.T.; Martiny, J.; et al. Soil Microbiome Interventions for Carbon Sequestration and Climate Mitigation. mSystems 2025, 10, e01129-24. [Google Scholar] [CrossRef]
- Xu, Q.; Li, L.; Guo, J.; Guo, H.; Liu, M.; Guo, S.; Kuzyakov, Y.; Ling, N.; Shen, Q. Active Microbial Population Dynamics and Life Strategies Drive the Enhanced Carbon Use Efficiency in High-Organic Matter Soils. MBio 2024, 15, e00177-24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Unlocking Microbial Insights for Pandemic Preparedness and Sustainability. LinkedIn. 2023. Available online: https://www.linkedin.com/pulse/unlocking-microbial-insights-pandemic-preparednessshashi-sharma (accessed on 25 January 2024).
- Lorimer, J. Parasites, Ghosts and Mutualists: A Relational Geography of Microbes for Global Health. Trans. Inst. Br. Geogr. 2017, 42, 544–558. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G., Jr. One Health—Cycling of Diverse Microbial Communities as a Connecting Force for Soil, Plant, Animal, Human and Ecosystem Health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef]
- Jutla, A.; Filippelli, G.M.; McMahon, K.D.; Tringe, S.G.; Colwell, R.R.; Nguyen, H.; Imperiale, M.J. One Health, Climate Change, and Infectious Microbes: A Joint Effort Between AGU and ASM to Understand Impacts of Changing Climate and Microbes on Human Well-Being Across Scales. MSphere 2024, 9, e0003524. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Bruns, M.A.; Casadevall, A.; Criddle, C.S.; Eloe-Fadrosh, E.; Karl, D.M.; Nguyen, N.K.; Zhou, J. Microbes and Climate Change: A Research Prospectus for the Future. MBio 2022, 13, e0080022. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic Analysis of Microbial Communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef]
- Ferrer, M.; Beloqui, A.; Timmis, K.N.; Golyshin, P.N. Metagenomics for Mining New Genetic Resources of Microbial Communities. J. Mol. Microbiol. Biotechnol. 2009, 16, 109–123. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gao, W.; Gao, X.; Zhu, L.; Gao, S.; Sun, R.; Feng, Z.; Wu, D.; Liu, Z.; Zhu, R.; Jiao, N. Multimodal Metagenomic Analysis Reveals Microbial Single Nucleotide Variants as Superior Biomarkers for Early Detection of Colorectal Cancer. Gut Microbes 2023, 15, 2245562. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Dixon, R. Biotechnological Solutions to the Nitrogen Problem. Curr. Opin. Biotechnol. 2014, 26, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Kanimozhi, S. Plant Growth Promoting Rhizobacteria (PGPR)—Prospective and Mechanisms: A Review. J. Pure Appl. Microbiol. 2018, 12, 733–749. [Google Scholar] [CrossRef]
- Wani, S.P.; Gopalakrishnan, S. Plant Growth-Promoting Microbes for Sustainable Agriculture. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer: Singapore, 2019; pp. 19–45. [Google Scholar]
- Maheshwari, D.K. (Ed.) Bacteria in Agrobiology: Plant Probiotics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Subbarao, N.S. Field Response of Legumes in India to Inoculation and Fertilizer Applications. In Symbiotic Nitrogen Fixation in Plants; PS Nutman, Ed.; Cambridge Univ Press: London, UK, 1976; pp. 265–288. [Google Scholar]
- Subbarao, N.S. Annual Report on All India Coordinated Research Project on Biological Nitrogen Fixation; ICAR-Indian Agricultural Research Institute: New Delhi, India, 1978. [Google Scholar]
- Wani, S.P.; Rupela, O.P.; Lee, K.K. Sustainable Agriculture in The Semiarid Tropics Through Biological Nitrogen Fixation in Grain Legumes. Plant Soil 1995, 174, 29–49. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Singh, J.; Pareek, N.; Raghav, A. FE-SEM/EDX based Zinc Mobilization Analysis of Burkholderia cepacia and Pantoea rodasii and Their Functional Annotation in Crop Productivity, Soil Quality, and Zinc Biofortification of Paddy. Front. Microbiol. 2022, 13, 852192. [Google Scholar] [CrossRef]
- Chandra, R.; Pareek, N.; Raverkar, K.P. Evaluation of Multi-Functional Rhizobium sp. Isolates of Urdbean (Vigna mungo L.) as Bio-Inoculant for Enhancing Productivity and Soil Health. J. Indian Soc. Soil Sci. 2019, 67, 103–109. [Google Scholar]
- Bakaeva, M.; Chetverikov, S.; Timergalin, M.; Feoktistova, A.; Rameev, T.; Chetverikova, D.Y.; Kenjieva, A.; Starikov, S.; Sharipov, D.; Hkudaygulov, G. PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress. Plants 2022, 11, 3289. [Google Scholar] [CrossRef]
- Batool, S.; Asghar, H.N.; Shehzad, M.A.; Yasin, S.; Sohaib, M.; Nawaz, F.; Akhtar, G.; Mubeen, K.; Zahir, Z.A.; Uzair, M. Zinc-Solubilizing Bacteria-Mediated Enzymatic and Physiological Regulations Confer Zinc Biofortification in Chickpea (Cicer arietinum L.). J. Soil Sci. Plant Nutr. 2021, 21, 2456–2471. [Google Scholar] [CrossRef]
- Karnwal, A. Pseudomonas spp., a Zinc-Solubilizing Vermicompost Bacteria with Plant Growth-Promoting Activity Moderates Zinc Biofortification in Tomato. Int. J. Veg. Sci. 2021, 27, 398–412. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.D.S.; Reis, A.R.D.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Vidal, M.; Baldani, J.I.; Meneses, C. Gluconacetobacter diazotrophicus Changes The Molecular Mechanisms of Root Development in Oryza sativa L. Growing Under Water Stress. Int. J. Mol. Sci. 2020, 21, 333. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-Arid Agroecosystem and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2017, 8, 2580. [Google Scholar] [CrossRef]
- Srinivas, V.; Gopalakrishnan, S.; Kamidi, J.P.; Chander, G. Effect of Plant Growth-Promoting Streptomyces sp. on Plant Growth and Yield of Tomato and Chilli. APJAS 2020, 6, 65–70. [Google Scholar]
- Gopalakrishnan, S.; Srinivas, V.; Anil Kumar, V.; Samineni, S.; Rathore, A. Influence of Diazotrophic Bacteria on Nodulation, Nitrogen Fixation, Growth Promotion and Yield Traits in Five Cultivars of Chickpea. Biocatal. Agric. Biotechnol. 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Prakash, B.; Arumugam, S.; Vijayabharathi, R.; Rupela, O.; Kudapa, H.; Krishnamohan, K.; Varshney, R.K. Evaluation of Streptomyces Strains Isolated from Herbal Vermicompost for Their Plant Growth-Promotion Traits in Rice. Microbiol. Res. 2014, 169, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Srinivas, V.; Sameer Kumar, C.V. Plant Growth-Promotion Traits of Streptomyces sp. in Pigeonpea. Legum. Perspect. 2016, 11, 43–44. Available online: https://oar.icrisat.org/9238/1/Plant%20growth-promotion%20traits%20of%20Streptomycessp.pdf(accessed on 4 September 2025).
- Srinivas, V.; Naresh, N.; Pratyusha, S.; Ankati, S.; Govindaraj, M.; Gopalakrishnan, S. Enhancing Pearl Millet Hybrids Performance for Yield and Nutrients Through Plant Growth-Promoting Streptomyces spp.: An Agronomic Biofortification Approach. Crop Pasture Sci. 2022, 73, 484–493. [Google Scholar] [CrossRef]
- Sathya, A.; Vijayabharathi, R.; Srinivas, V.; Gopalakrishnan, S. Plant Growth-Promoting Actinobacteria on Chickpea Seed Mineral Density: An Upcoming Complementary Tool for Sustainable Biofortification Strategy. 3Biotech 2016, 6, 138. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Sree Vidya, M.; Rathore, A. Plant Growth-Promoting Activities of Streptomyces spp. in Sorghum and Rice. Springerplus 2013, 2, 574. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Etesami, H.; Santoyo, G. Trichoderma: A Multifunctional Agent in Plant Health and Microbiome Interactions. BMC Microbiol. 2025, 25, 434. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular Mycorrhizal Fungi (AMF) Enhanced the Growth, Yield, Fiber Quality and Phosphorus Regulation in Upland Cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.; Dakora, F.D. Rhizobia as a Source of Plant Growth-Promoting Molecules: Potential Applications and Possible Operational Mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Raverkar, K.P.; Konde, B.K. Effect of Rhizobium and Azospirillum lipoferum Inoculation on the Nodulation, Yield and Nitrogen Uptake of Peanut Cultivars. Plant Soil 1988, 106, 249–252. [Google Scholar] [CrossRef]
- Thilagar, G.; Bagyaraj, D.J.; Rao, M.S. Selected Microbial Consortia Developed for Chilly Reduces Application of Chemical Fertilizers by 50% under Field Conditions. Sci. Hortic. 2016, 198, 27–35. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [PubMed]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 1, 963401. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Lee Díaz, A.S.; Macheda, D.; Saha, H.; Ploll, U.; Orine, D.; Biere, A. Tackling the Context-Dependency of Microbial-Induced Resistance. Agronomy 2021, 11, 1293. [Google Scholar] [CrossRef]
- Donadio, S.; Monciardini, P.; Alduina, R.; Mazza, P.; Chiocchini, C.; Cavaletti, L.; Sosio, M.; Puglia, A.M. Microbial Technologies for the Discovery of Novel Bioactive Metabolites. J. Biotechnol. 2002, 99, 187–198. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and Facts About Antibiotics: Where We are Now and Where We are Heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Monciardini, P.; Iorio, M.; Maffioli, S.; Sosio, M.; Donadio, S. Discovering New Bioactive Molecules from Microbial Sources. Microb. Biotechnol. 2014, 7, 209–220. [Google Scholar] [CrossRef]
- Kusakabe, A.; Molnár, I.; Stock, S.P. Photorhabdus-Derived Secondary Metabolites Reduce Root Infection by Meloidogyne incognita in Cowpea. Plant Dis. 2023, 107, 3383–3388. [Google Scholar] [CrossRef]
- Helepciuc, F.-E.; Todor, A. Making the Best of Research Investment in Pathogens Control Through Biocontrol. How is Research Correlated with Agricultural Microbial Biological Control Product Availability? PLoS Pathog. 2023, 19, e1011071. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, A.V.; Joshi, S.; Kumar, M. Antifungal and Enzyme Activity of Endophytic Fungi Isolated from Ocimum sanctum and Aloe vera. Afr. J. Microbiol. Res. 2015, 9, 1783–1788. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Qadri, M.; Short, S.; Gast, K.; Hernandez, J.; Wong, A.C.N. Microbiome Innovation in Agriculture: Development of Microbial Based Tools for Insect Pest Management. Front. Sustain. Food Syst. 2020, 4, 547751. [Google Scholar] [CrossRef]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Malviya, D.; Singh, P.; Singh, U.B.; Paul, S.; Kumar Bisen, P.; Rai, J.P.; Verma, R.L.; Fiyaz, R.A.; Kumar, A.; Kumari, P.; et al. Arbuscular Mycorrhizal Fungi-Mediated Activation of Plant Defense Responses in Direct Seeded Rice (Oryza sativa L.) against Root-Knot Nematode Meloidogyne graminicola. Front. Microbiol. 2023, 14, 1104490. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Ruiu, L. Plant-Growth-Promoting Bacteria (PGPB) against Insects and Other Agricultural Pests. Agronomy 2020, 10, 861. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Rajendran, V.; Arumugam, S.; Sharma, H.C.; Vadlamudi, S.; Bhimineni, R.K.; Gonzalez, S.V.; Melø, T.M.; Simic, N. Insecticidal Activity of a Novel Fatty Acid Amide Derivative from Streptomyces species against Helicoverpa armigera. Nat. Prod. Res. 2016, 30, 2760–2769. [Google Scholar] [CrossRef]
- Sathya, A.; Vijayabharathi, R.; Kumari, B.R.; Srinivas, V.; Sharma, H.C.; Sathyadevi, P.; Gopalakrishnan, S. Assessment of a Diketopiperazine, Cyclo (Trp-Phe) from Streptomyces griseoplanus SAI-25 against Cotton Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Appl. Entomol. Zool 2016, 51, 11–20. [Google Scholar] [CrossRef]
- Bull, D.L.; Ivie, G.W.; MacConnell, J.G.; Gruber, V.F.; Ku, C.C.; Arison, B.H.; Stevenson, J.M.; VandenHeuvel, W.J. Fate of Avermectin B1a in Soil and Plants. J. Agric. Food Chem. 1984, 32, 94–102. [Google Scholar] [CrossRef]
- Monfort, W.S.; Kirkpatrick, T.L.; Long, D.L.; Rideout, S. Efficacy of a Novel Nematicidal Seed Treatment against Meloidogyne incognita on Cotton. J. Nematol. 2006, 38, 245–249. [Google Scholar]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.; Pereira, M.G.; Carvalho, T.S.; Silva, R.A.; Ferreira, E.P. Responses of Soil Microbial Biomass and Enzyme Activity to Herbicides Imazethapyr and Flumioxazin. Sci. Rep. 2020, 10, 7694. [Google Scholar] [CrossRef]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G.; Ramaraj, G.; Ponnusamy, P.; Sekar, J.; Ramalingam, P.V. Biocontrol Efficiency of Native Plant Growth Promoting Rhizobacteria against Rhizome Rot Disease of Turmeric. Biol. Control 2019, 129, 55–64. [Google Scholar]
- Oni, F.E.; Olorunleke, O.F.; Hofte, M. Phenazines and Cyclic Lipopeptides Produced by Pseudomonas sp. CMR12a Are Involved in the Biological Control of Pythium myriotylum on Cocoyam (Xanthosoma sagittifolium). Biol. Control 2019, 129, 109–114. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus thuringiensis Strains to Identify New Potential Biocontrol Agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Chae, D.H.; Kim, D.R.; Cheong, M.S.; Lee, Y.B.; Kwak, Y.S. Investigating the Induced Systemic Resistance Mechanism of 2,4-Diacetylphloroglucinol (DAPG) Using DAPG Hydrolase-Transgenic Arabidopsis. Plant Pathol. J. 2020, 36, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Chinchilla, D.; Tan, C.; Mène-Saffrané, L.; L’Haridon, F.; Weisskopf, L. Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains against Phytophthora infestans. Microorganisms 2020, 8, 1114. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Govindasamy, V.; Annapurna, K. Role of Antibiosis in Suppression of Charcoal Rot Disease by Soybean Endophyte Paenibacillus sp. HKA-15. Curr. Microbiol. 2007. [Google Scholar] [CrossRef]
- Cai, F.; Yang, C.; Ma, T.; Osei, R.; Jin, M.; Zhang, C.; Wang, Y. An Endophytic Paenibacillus polymyxa hg18 and Its Biocontrol Potential against Fusarium oxysporum f. sp. cucumerinum. Biol. Control 2024, 188, 105380. [Google Scholar] [CrossRef]
- Dhar, P.G.; Mangar, P.; Saha, A.; Saha, D. Evaluation of the Biocontrol Efficacy of a Serratia marcescens Strain Indigenous to Tea Rhizosphere for the Management of Root Rot Disease in Tea. PLoS ONE 2018, 13, e0191761. [Google Scholar] [CrossRef]
- Sarwar, A.; Latif, Z.; Zhang, S.; Hao, J.; Bechthold, A. A Potential Biocontrol Agent Streptomyces violaceusniger AC12AB for Managing Potato Common Scab. Front. Microbiol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Wang, R.; Yu, X.; Yin, Y.; Norvienyeku, J.; Khan, R.A.A.; Zhang, M.; Ren, S.; Chen, J.; Liu, T. Biocontrol of Cucumber Fusarium Wilt by Trichoderma asperellum FJ035 Dependent on Antagonism and Spatiotemporal Competition with Fusarium oxysporum. Biol. Control 2023, 186, 105334. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Chen, J.; Tian, Y. Cellulase from Trichoderma harzianum Interacts with Roots and Triggers Induced Systemic Resistance to Foliar Disease in Maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef]
- Islam, S.M.N.; Chowdhury, M.Z.H.; Mim, M.F.; Momtaz, M.B.; Islam, T. Biocontrol Potential of Native Isolates of Beauveria bassiana against Cotton Leafworm Spodoptera litura (Fabricius). Sci. Rep. 2023, 13, 8331. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Albaghdady, M.D.; Xiao, Y.; Xiao, X.; Mo, C.; Tian, T.; Wan, G. Endophytic Metarhizium anisopliae Is a Potential Biocontrol Agent against Wheat Fusarium Head Blight Caused by Fusarium graminearum. J. Plant Pathol. 2021, 103, 875–885. [Google Scholar] [CrossRef]
- Elhalag, K.M.; Nasr-Eldin, M.A.; Huang, Q.; Rabab, A.M.; Ahmad, A.A. Lytic Phages Isolated from Egypt for Biocontrol of Potato Soft Rot Caused by Pectobacterium carotovorum. Biol. Control 2024, 189, 105444. [Google Scholar]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Srinivas, V.; Sharma, M. Deciphering the Tri-Dimensional Effect of Endophytic Streptomyces sp. on Chickpea for Plant Growth Promotion, Helper Effect with Mesorhizobium ciceri and Host-Plant Resistance Induction against Botrytis cinerea. Microb. Pathog. 2018, 122, 98–107. [Google Scholar] [CrossRef]
- Sravani, A.; Vadlamudi, S.; Sambangi, P.; Gopalakrishnan, S. Streptomyces Consortia-Mediated Plant Defense against Fusarium Wilt and Plant Growth-Promotion in Chickpea. Microb. Pathog. 2021, 157, 104961. [Google Scholar]
- Gopalakrishnan, S.; Sharma, R.; Srinivas, V.; Naresh, N.; Mishra, S.P.; Ankati, S.; Pratyusha, S.; Govindaraj, M.; Gonzalez, S.V.; Nervik, S.; et al. Identification and Characterization of a Streptomyces albus Strain and Its Secondary Metabolite Organophosphate against Charcoal Rot of Sorghum. Plants 2020, 9, 1727. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Alkassab, A.T.; Collison, E.; Odemer, R.; Vojtech, E.; Pistorius, J. Overview of the Testing and Assessment of Effects of Microbial Pesticides on Bees: Strengths, Challenges and Perspectives. Apidologie 2021, 52, 1256–1277. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kailoo, S.; Khan, R.T.; Rafiq, A.; Shah, M.D.; Jan, R.; Riyaz-Ul-Hassan, S. Harnessing Fungal Endophytes for Natural Management: A Biocontrol Perspective. Front. Microbiol. 2023, 14, 1280258. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mikaelyan, A.; Coates, B.S.; Lorenzen, M. The Genome of Arsenophonus sp. and Its Potential Contribution in the Corn Planthopper, Peregrinus maidis. Insects 2024, 15, 113. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Chitra, C.; Mathivanan, N. Metabolites of Plant Growth-Promoting Rhizobacteria for the Management of Soilborne Pathogenic Fungi in Crops. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Singh, H.B., Keswani, C., Reddy, M.S., Eds.; Springer: Singapore, 2019; pp. 293–315. [Google Scholar]
- Valette, M.; Rey, M.; Gerin, F.; Dubois, E.; Gibon, Y.; Citerne, S.; Cottaz, S.; Briand, M.; Dorey, S.; Ballini, E.; et al. A Common Metabolomic Signature is Observed upon Inoculation of Rice Roots with Various Rhizobacteria. J. Integr. Plant Biol. 2020, 62, 228–246. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Verma, S.; Azevedo, L.C.B.; Pandey, J.; Khusharia, S.; Kumari, M.; Kumar, D.; Kaushalendra; Bhardwaj, N.; Teotia, P.; Kumar, A. Microbial Intervention: An Approach to Combat the Postharvest Pathogens of Fruits. Plants 2022, 11, 3452. [Google Scholar] [CrossRef]
- Markets and Markets. Agricultural Biologicals Market by Function, Product Type (Microbials, Macrobials, Semio-chemicals, Natural Products), Mode of Application (Foliar Spray, Soil & Seed Treatment), Crop Type (Cereals & Grains, Fruits) & Region—Global Forecast to 2028; Markets and Markets: Pune, India, 2023. [Google Scholar]
- Feng, H.; Edwards, N.; Anderson, C.M.H.; Althaus, M.; Duncan, R.P.; Hsu, Y.-C.; Luetje, C.W.; Price, D.R.G.; Wilson, A.C.C.; Thwaites, D.T. Trading Amino Acids at the Aphid–Buchnera Symbiotic Interface. Proc. Natl. Acad. Sci. USA 2019, 116, 16003–16011. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.; Vega, F.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut Microbiota Mediate Caffeine Detoxification in the Primary Insect Pest of Coffee. Nat. Commun. 2015, 6, 7618, Erratum in Nat. Commun. 2023, 14, 6306. https://doi.org/10.1038/s41467-023-42217-2. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Strassert, J.F.H.; Tokuda, G.; Brune, A. The Fibre-Associated Cellulolytic Bacterial Community in the Hindgut of Wood-Feeding Higher Termites (Nasutitermes spp.). Environ. Microbiol. 2014, 16, 2711–2722. [Google Scholar] [CrossRef]
- Schwarz, M.; Tokuda, G.; Osaki, H.; Mikaelyan, A. Reevaluating Symbiotic Digestion in Cockroaches: Unveiling the Hindgut’s Contribution to Digestion in Wood-Feeding Panesthiinae (Blaberidae). Insects 2023, 14, 768. [Google Scholar] [CrossRef]
- Schwarz, M.; Beza-Beza, C.F.; Mikaelyan, A. Wood Fibers Are a Crucial Microhabitat for Cellulose- and Xylan-Degrading Bacteria in the Hindgut of the Wood-Feeding Beetle Odontotaenius disjunctus. Front. Microbiol. 2023, 14, 1173696. [Google Scholar] [CrossRef]
- Birkemoe, T.; Jacobsen, R.M.; Sverdrup-Thygeson, A.; Biedermann, P.H.W. Insect–Fungus Interactions in Dead Wood Systems. In Saproxylic Insects; Ulyshen, M.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 377–427. [Google Scholar]
- Shikano, I.; Rosa, C.; Tan, C.-W.; Felton, G.W. Tritrophic Interactions: Microbe-Mediated Plant Effects on Insect Herbivores. Annu. Rev. Phytopathol. 2017, 55, 313–331. [Google Scholar] [CrossRef]
- Aslam, F.; Ali, B. Halotolerant Bacterial Diversity Associated with Suaeda fruticosa (L.) Forssk. Improved Growth of Maize under Salinity Stress. Agronomy 2018, 8, 131. [Google Scholar] [CrossRef]
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Santos, M.A.; Souza, R.J.; Fronza, D.; Vendruscolo, E.P. Can Co-Inoculation of Bradyrhizobium and Azospirillum Alleviate Adverse Effects of Drought Stress on Soybean (Glycine max L. Merrill)? Arch. Microbiol. 2019, 201, 325–335. [Google Scholar] [CrossRef]
- Inthama, P.; Pumas, P.; Pekkoh, J.; Leelakriangsak, M.; Boonmak, C.; Mongkolsuk, S.; Lumyong, S. Plant Growth and Drought Tolerance Promoting Bacterium for Bioremediation of Paraquat Pesticide Residues in Agricultural Soils. Front. Microbiol. 2021, 12, 604662. [Google Scholar] [CrossRef] [PubMed]
- Raverkar, K.P.; Dwivedi, A.; Tilak, K.V.B.R. Vesicular-Arbuscular Mycorrhizal Associations in Glycine max (L.) Merrill Improve Symbiotic Nitrogen Fixation under Water Stress. In Current Trends in Mycorrhizal Research; Jalali, B.L., Chand, H., Eds.; University of California Press: Oakland, CA, USA, 1990; pp. 167–171. [Google Scholar]
- Armada, E.; Roldán, A.; Azcón, R. Differential Activity of Autochthonous Bacteria in Controlling Drought Stress in Native Lavandula and Salvia Plant Species under Drought Conditions in Natural Arid Soil. Microb. Ecol. 2014, 67, 410–420. [Google Scholar] [CrossRef]
- Ghosh, D.; Sen, S.; Mohapatra, S. Modulation of Proline Metabolic Gene Expression in Arabidopsis thaliana under Water-Stressed Conditions by a Drought-Mitigating Pseudomonas putida Strain. Ann. Microbiol. 2017, 67, 655–668. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Hamayun, M.; Joo, G.-J.; Shahzad, R.; Kim, J.-G.; Lee, I.-J. Plant Growth Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Carreiras, J.; Cruz Silva, A.; Fonseca, B.; Brígido, C. Improving Grapevine Heat Stress Resilience with Marine Plant Growth Promoting Rhizobacteria Consortia. Microorganisms 2023, 11, 856. [Google Scholar] [CrossRef]
- Bal, H.B.; Adhya, T.K. Alleviation of Submergence Stress in Rice Seedlings by Plant Growth-Promoting Rhizobacteria with ACC Deaminase Activity. Front. Sustain. Food Syst. 2021, 5, 606158. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; de Russo Godoy, F.M.; Duré, L.M.M.; Fernandes-Junior, P.I.; Baldani, J.I.; Paggi, G.M.; Zanoelo, F.F.; Brasil, M.S. Potential of Bacterial Strains Isolated from Ironstone Outcrops Bromeliads to Promote Plant Growth under Drought Conditions. Curr. Microbiol. 2021, 78, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Berjak, P. Unifying Perspectives of Some Mechanisms Basic to Desiccation Tolerance across Life Forms. Seed Sci. Res. 2006, 16, 1–15. [Google Scholar] [CrossRef]
- Timmusk, S.; Wagner, E.G.H. The Plant-Growth-Promoting Rhizobacterium Paenibacillus polymyxa Induces Changes in Arabidopsis thaliana Gene Expression: A Possible Connection between Biotic and Abiotic Stress Responses. Mol. Plant Microbe Interact. 1999, 12, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Ansary, M.H.; Rahmani, H.A.; Ardakani, M.R. Effect of Pseudomonas fluorescent on Proline and Phytohormonal Status of Maize (Zea mays L.) under Water Deficit Stress. Ann. Biol. Res. 2012, 3, 1054–1062. [Google Scholar]
- Kaushal, M.; Wani, S.P. Plant-Growth-Promoting Rhizobacteria: Drought Stress Alleviators to Ameliorate Crop Production in Drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
| Microorganism | Crop System | Agricultural Practice | Reported Effect Size | Key Outcomes | References |
|---|---|---|---|---|---|
| Type I & II Methanotrophs | Rice | Alternate wetting & drying (AWD) | ↓ CH4 emissions by 30–70%; methanotrophic activity significantly ↑ during dry phases | Reduced GHG emissions | [80] |
| Methanotrophic Bioinoculants | Rice, wetland crops | Inoculation with methanotroph consortia | CH4 ↓ 20–40%; ↑ plant growth (PGPR traits) | Grain yield ↑ up to 38% | [81] |
| Methanotrophic bacteria | Rice | Inoculation (MT-22) with water-management optimisation | ↓ CH4 ~10–12% (field trials) | Reduced GHG emissions | [82] |
| Blue-green algae + Azolla | Rice | Blue-green algae @ 10 kg ha−1 (T2), Azolla @ 1 tonnes per hectare | CH4 emission ↓ 37.9% over the control | Reduced GHGemissions | [83] |
| Cyanobacteria (biocrust/soil) | Dryland soils | Application of cyanobacteria as inoculants | ↑ Soil C sequestration; ↑ aggregate stability | Dryland restoration | [84] |
| Arbuscular mycorrhizal fungi (AMF) | Wheat, maize | Reduced tillage, organic amendments | ↑ SOC stabilization | Aggregate formation ↑ by 15–30% | [85] |
| Microbial consortia (bioinoculants) | Rice, maize | Inoculation with carbon-sequestering strains | Potential SOC ↑ 12–25% | Carbon sequestration and climate mitigation | [86] |
| Actinomycetes & Saprophytic fungi | Diverse cropping systems | Compost addition, residue retention | ↑ Carbon use efficiency (CUE) and microbial necromass, SOC ↑ by 8–15% | Improved soil health | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.B.; Raverkar, K.P.; Wani, S.P.; Bagyaraj, D.J.; Kannepalli, A.; Kandula, D.R.W.; Mikaelyan, A.; Ansari, M.A.; Stock, S.P.; Davies, K.G.; et al. Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture. Microorganisms 2025, 13, 2839. https://doi.org/10.3390/microorganisms13122839
Sharma SB, Raverkar KP, Wani SP, Bagyaraj DJ, Kannepalli A, Kandula DRW, Mikaelyan A, Ansari MA, Stock SP, Davies KG, et al. Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture. Microorganisms. 2025; 13(12):2839. https://doi.org/10.3390/microorganisms13122839
Chicago/Turabian StyleSharma, Shashi B., Kiran P. Raverkar, Suhas P. Wani, Davis Joseph Bagyaraj, Annapurna Kannepalli, Diwakar R. W. Kandula, Aram Mikaelyan, Minshad A. Ansari, S. Patricia Stock, Keith G. Davies, and et al. 2025. "Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture" Microorganisms 13, no. 12: 2839. https://doi.org/10.3390/microorganisms13122839
APA StyleSharma, S. B., Raverkar, K. P., Wani, S. P., Bagyaraj, D. J., Kannepalli, A., Kandula, D. R. W., Mikaelyan, A., Ansari, M. A., Stock, S. P., Davies, K. G., & Sharma, R. (2025). Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture. Microorganisms, 13(12), 2839. https://doi.org/10.3390/microorganisms13122839







