Screening of Protease-Producing Microorganisms and Optimization of Fermentation Processes for the Efficient Preparation of Broussonetia papyrifera Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents, Microbial Strains, and Culture Media
2.2. Primary and Secondary Screening of Protease-Producing Microorganisms
2.3. Re-Screening of Isolates Suitable for the Preparation of B. papyrifera Feed
2.4. Molecular Identification of Isolated Strains
2.5. Single-Factor Experiments for Improving the Quality of Fermented B. papyrifera Feed
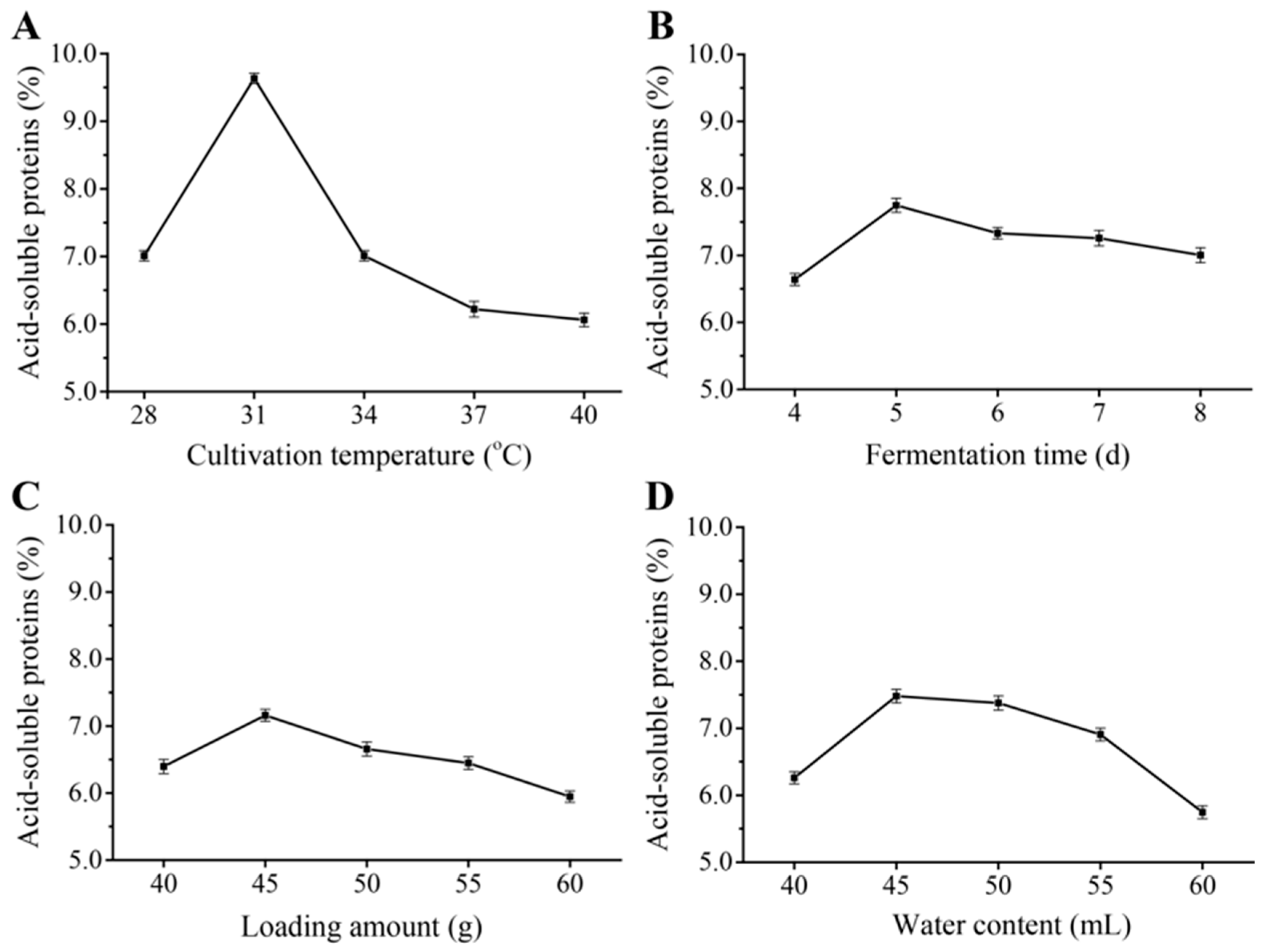
2.5.1. Effect of Cultivation Temperature on the Acid-Soluble Protein Content
2.5.2. Influence of Fermentation Time on the Acid-Soluble Protein Content
2.5.3. Influence of Loading Amount on Acid-Soluble Protein Content
2.5.4. Effect of Water Content on Acid-Soluble Protein Content
2.6. Orthogonal Experimental Design of Fermentation Conditions
2.7. Statistical Analyses
2.8. Nucleotide Sequence Accession Numbers
3. Results
3.1. Primary and Secondary Screening of Protease-Harboring Microorganisms
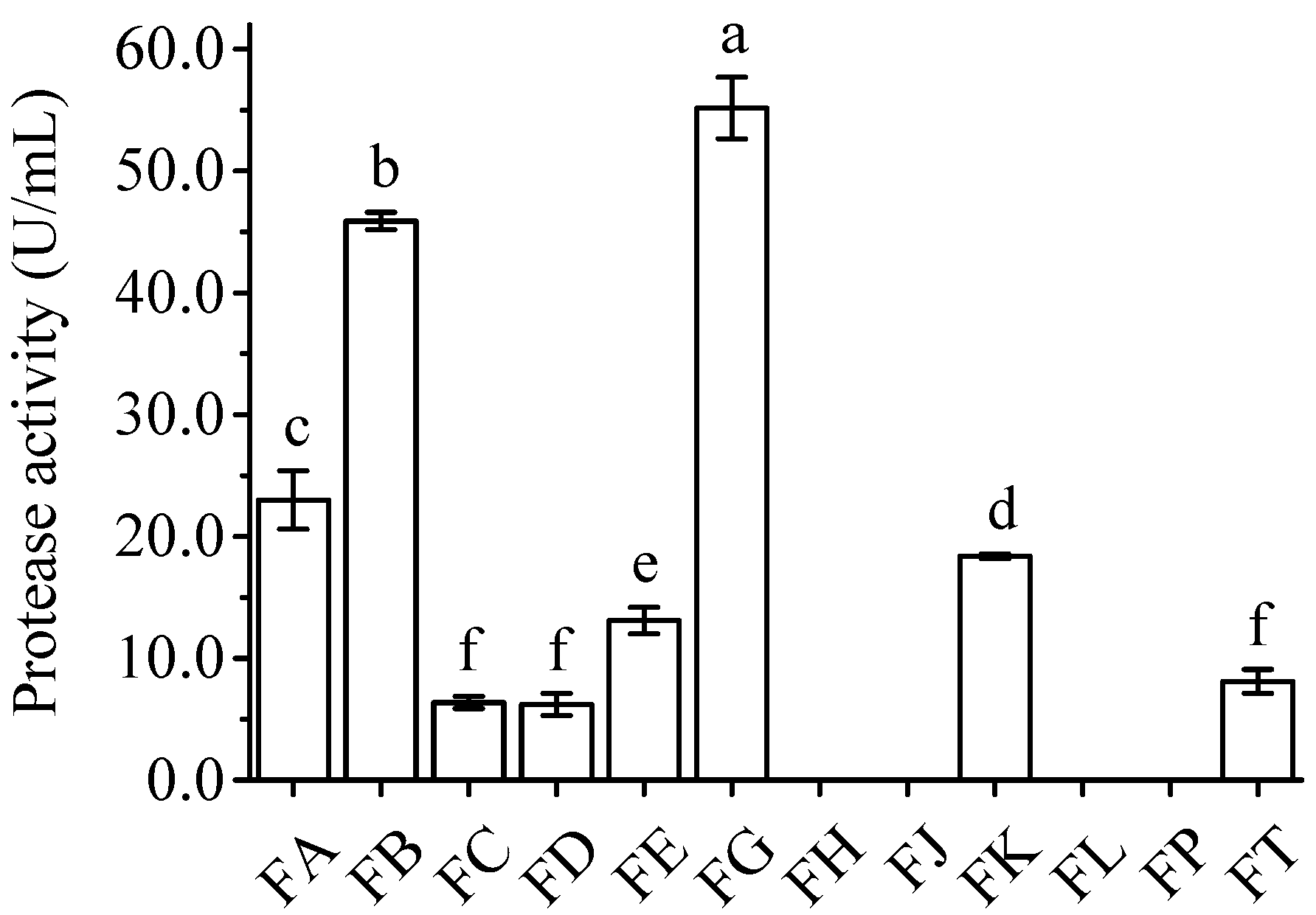
3.2. Re-Screening of Appropriate Strains for the Efficient Preparation of B. papyrifera Feed
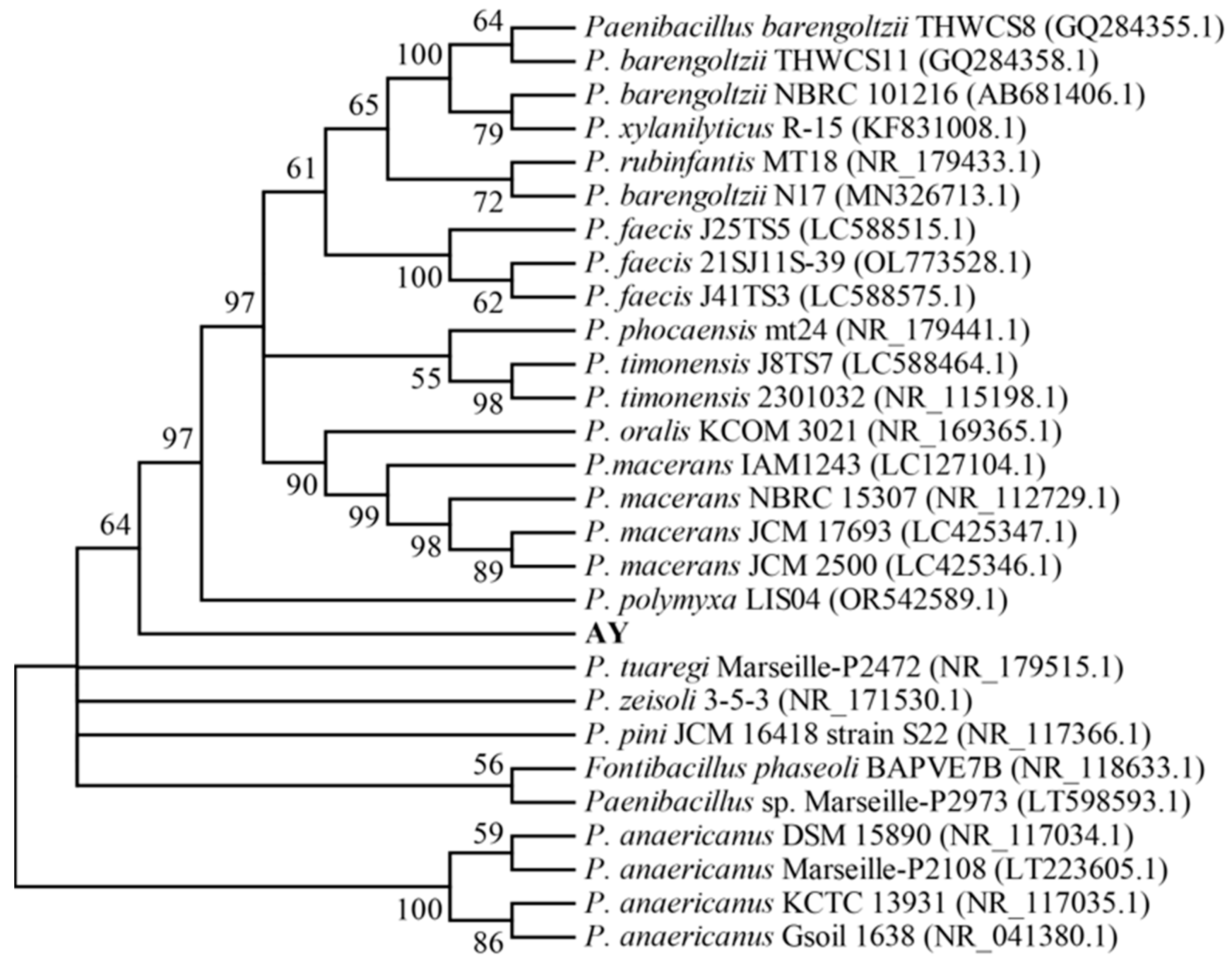
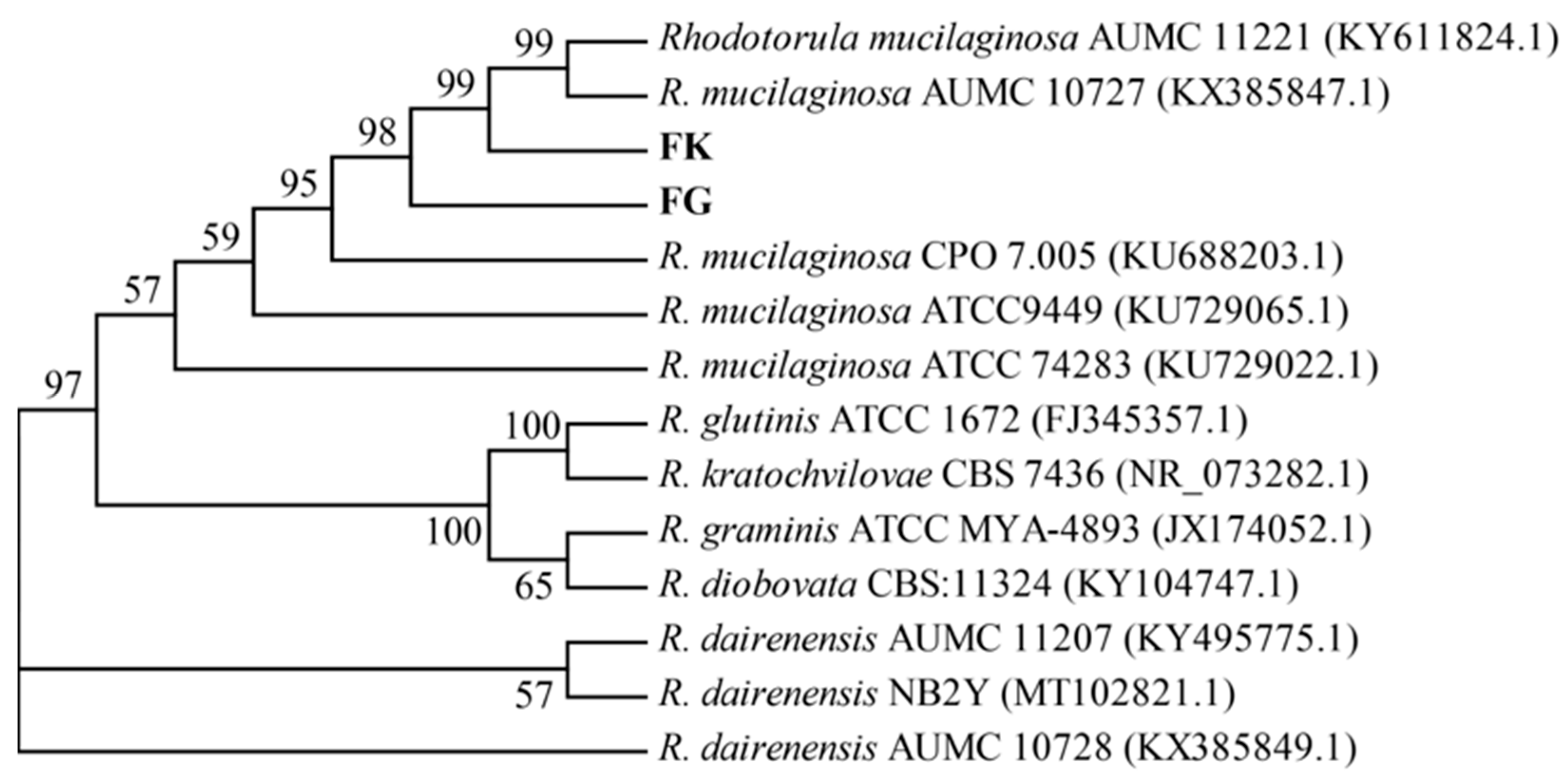
3.3. Molecular Identification
3.4. Single-Factor Experiments Using Paenibacillus sp. AY and R. mucilaginosa FG for Optimization of B. papyrifera Feed Preparation Process
3.5. Orthogonal Experimental Design for Optimizing B. papyrifera Feed Preparation Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Wang, Z.; Zhu, X. New reflections on food security and land use strategies based on the evolution of Chinese dietary patterns. Land Use Policy 2023, 126, 106520. [Google Scholar] [CrossRef]
- Yang, S.; Cui, X. Large-scale production: A possible way to the balance between feed grain security and meat security in China. J. Agric. Food Res. 2023, 14, 100745. [Google Scholar] [CrossRef]
- Niu, Y.; Xie, G.; Xiao, Y.; Liu, J.; Wang, Y.; Luo, Q.; Zou, H.; Gan, S.; Qin, K.; Huang, M. Spatiotemporal patterns and determinants of grain self-sufficiency in China. Foods 2021, 10, 747. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, X.; Du, H.; Li, Q.; Zhang, Z.; Qiu, M.; Yu, C. A review: Achievements and new obstacles in China’s food security revealed by grain and animal meat production. IOP Conf. Ser. Earth Environ. Sci. 2021, 705, 012025. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, R.; Chen, Y. Estimation of supply-demand gap of feed grain in China. J. Shandong Agric. Sci. 2022, 54, 158–165. [Google Scholar]
- Sime, A.G.; Duguma, B.S. The role of non-conventional feeds in small ruminant production and their implication for feed cost reduction in Ethiopia—A mini review. Vet. Med. Sci. 2025, 11, e70554. [Google Scholar] [CrossRef] [PubMed]
- Lavania, P.; Bairva, K.C. Utilization of non-conventional feeds for enhancing the animal production. Just Agric. 2025, 2, 1–11. [Google Scholar]
- Peng, X.; Liu, H.; Chen, P.; Tang, F.; Hu, Y.; Wang, F.; Pi, Z.; Zhao, M.; Chen, N.; Chen, H. A chromosome-scale genome assembly of paper mulberry (Broussonetia papyrifera) provides new insights into its forage and papermaking usage. Mol. Plant 2019, 12, 661–677. [Google Scholar] [CrossRef]
- Verma, K.; Deep, P.; Srivastava, V.; Dwivedi, J.; Verma, S. Pharmacognostical properties and medicinal uses of Broussonetia papyrifera (Moraceae): A review. Int. J. Pharm. Sci. Rev. Res. 2022, 74, 96–101. [Google Scholar] [CrossRef]
- Li, Y.; Huang, R.; Zhang, W.; Chen, Q.; Wang, Q.; Ye, J.; Xu, F. Medicinal potential of Broussonetia papyrifera: Chemical composition and biological activity analysis. Plants 2025, 14, 523. [Google Scholar] [CrossRef]
- Nguyen, L.T.H. Biological activities of paper mulberry (Broussonetia papyrifera): More than a skin-lightening agent. Cosmetics 2022, 9, 112. [Google Scholar] [CrossRef]
- Fan, X.; Yu, W.; Wang, Q.; Yang, H.; Tan, D.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.; et al. Protective effect of Broussonetia papyrifera leaf polysaccharides on intestinal integrity in a rat model of diet-induced oxidative stress. Int. J. Biol. Macromol. 2024, 268, 131589. [Google Scholar] [CrossRef]
- Li, C.-W.; Li, S.-M.; Chen, J.-J. Isolation, characterization, and biological evaluation of bioactive components from the stem bark of Broussonetia papyrifera: Antioxidant properties and apoptosis induction in breast cancer cells. Bioorg. Chem. 2025, 163, 108778. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Li, S.; Sun, Y.; Hou, G.; Huang, R.; Zhang, F. The effect of Broussonetia papyrifera silage on the growth performance, blood physiological parameters, serum biochemical parameters, immune response, antioxidant capacity, and rumen bacteria of Kazakh lamb. Animals 2025, 15, 78. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shen, S.B.; Cheng, B.; Zhou, T.T.; Wu, X.; Niu, K.M.; Peng, Y. Dietary supplementation with fermented Broussonetia papyrifera enhances tissue mineral deposition and intestinal microbiome in finishing pigs. J. Anim. Feed Sci. 2025, 34, 42–54. [Google Scholar] [CrossRef]
- Lai, G.-L.; Lin, Y.-R.; Yang, T.-J.; Chu, Y.-T.; Nan, F.-H.; Hu, Y.-F. Effects of feed supplementation with fermented Broussonetia papyrifera leaves on non-specific immune responses, resistance to Vibrio parahaemolyticus, growth, intestinal histology, and microbiota of white shrimp (Penaeus vannamei). Fish Shellfish. Immunol. 2025, 167, 110901. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, B.; Qu, H.; Cheng, J.; Feng, Y.; Liu, L.; Ma, Q. Evaluating the effects of replacing alfalfa with Broussonetia papyrifera branch/leaf powder on growth and serum indicators in Dezhou donkeys. Animals 2024, 14, 123. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, D.; Lei, Y.; Li, M.; Chen, Y.; Wang, J.; He, X.; Zhao, Y.; Chen, C.; Zhang, X. Altering the bacterial community: Sour soup decreased CO2 production and improved the fermentation quality of Broussonetia papyrifera, Tritriale and mixed silages. Front. Microbiol. 2025, 16, 1606628. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.; Zhang, X.; Wang, P.; Xiang, L.; Fang, Y.; Wang, Y.; Xie, F. Advances of fermentation technology with paper mulberry feedstuff. China Feed 2020, 13, 6–14. [Google Scholar] [CrossRef]
- Masi, C.; Gemechu, G.; Tafesse, M. Isolation, screening, characterization, and identification of alkaline protease-producing bacteria from leather industry effluent. Ann. Microbiol. 2021, 71, 24. [Google Scholar] [CrossRef]
- GB/T 23527.1-2023; Quality Requirements for Enzyme Preparations—Part 1: Protease Preparations. State Administration for Market Regulation; China National Standardization Administration: Beijing, China, 2023.
- NY/T 3801-2020; Determination of Acid-Soluble Protein in Feed Materials. Standards Press of China: Beijing, China, 2020.
- GB/T 12143-2008; General Analysis Method for Beverages. Standardization Administration of China: Beijing, China, 2009.
- Luo, A.-G.; Wang, Y.-Y.; Xue, S.-S.; Zhao, J.; Hao, J.-W.; Shi, S.-L.; Hu, B.-F. Screening, identification, and optimization of enzyme-producing conditions for cellulose-degrading bacteria in distillery lees. Appl. Sci. 2023, 13, 7693. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Q.-W.; Cheng, S.-Y.; Cong, X.; Xu, F. Research progress on fermentation technology of Broussonetia papyrifera silage and its application in animal production. Feed Res. 2024, 47, 162–165. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Y.; Fang, Y.; Yan, H.; Cao, L.; Gao, Z.; Wang, P.; Wei, Y. Effects of fermentation conditions on the content of acid soluble protein in Broussonetia papyrifera feed. Feed Res. 2021, 44, 75–78. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Zhou, L.; Abouelezz, K.; Momenah, M.A.; Bajaber, M.A.; Baazaoui, N.; Taha, T.F.; Awad, A.E.; Alamoudi, S.A.; Beyari, E.A.; Alanazi, Y.F.; et al. Dietary Paenibacillus polymyxa AM20 as a new probiotic: Improving effects on IR broiler growth performance, hepatosomatic index, thyroid hormones, lipid profile, immune response, antioxidant parameters, and caecal microorganisms. Poultry Sci. 2024, 103, 103239. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yuan, Y.; Jin, B.; Zhang, Z.; Murtaza, B.; Zhao, H.; Li, X.; Wang, L.; Xu, Y. Feasibility insights into the application of Paenibacillus pabuli E1 in animal feed to eliminate non-starch polysaccharides. Front. Microbiol. 2023, 14, 1205767. [Google Scholar] [CrossRef]
- Lee, A.-R.; Kim, S.-H.; Kim, S.-K. Effects of supplementation with a novel strain of Paenibacillus konkukensis on laying performance, tibial characteristics, and caecal microbiota in laying hens. Ital. J. Anim. Sci. 2025, 24, 827–841. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, X.; Ying, M.; Tu, B.; Zhao, K.; Hu, D.; Wang, P.; Liu, J.; Zeng, Y. A strain of Paenibacillus polysaccharolyticus XY5 promotes fiber component degradation in a co-fermentation system of mulberry leaves and distillers’ grains. Lett. Appl. Microbiol. 2025, 78, ovaf058. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Ravi, G.; Venkata Dasu, V.; Pakshirajan, K. A comparative evaluation of batch and fed-batch cultures for enhanced lipid, carotenoid, and β-carotene production by Rhodotorula mucilaginosa. Prep. Biochem. Biotechnol. 2025, 55, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Meng, L.; Li, J.; Li, L.; Zeng, Q.; Wang, R.; Wang, D.; Tong, T.; Liu, Y.; Yang, H. Effects of Rhodotorula mucilaginosa on growth, antioxidant, and immune function, and Toll/Imd and JAK-STAT signaling pathways in red claw crayfish (Cherax quadricanatus). Aquac. Nutr. 2025, 2025, 4904293. [Google Scholar] [CrossRef] [PubMed]
- Kheyrandish, S.; Rastgar, A.; Hamidi, M.; Sajjadi, S.M.; Sarab, G.A. Evaluation of anti-tumor effect of the exopolysaccharide from new cold-adapted yeast, Rhodotorula mucilaginosa sp. GUMS16 on chronic myeloid leukemia K562 cell line. Int. J. Biol. Macromol. 2022, 206, 21–28. [Google Scholar] [CrossRef]
- Eliwa, D.; El-Bouseary, M.M.; Farghali, M.H.; El-Masry, T.A.; Ragab, A.E. Investigation of antibacterial and wound healing activities of the extract of Rhodotorula mucilaginosa endophyte isolated from cucumber leaves. Sci. Rep. 2025, 15, 31730. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Ding, S.; Gao, Z.; Xiang, L.; Fang, Y.; Hu, H.; Lv, W. Experimental study on the production of protein feed by compound bacteria solid fermentation of Broussonetia papyrifera. Feed Ind. 2020, 41, 34–39. [Google Scholar] [CrossRef]
| Levels | Factors | ||
|---|---|---|---|
| Fermentation Temperature (A) | Fermentation Time (B) | Water Content (C) | |
| 1 | 28 | 4 | 40 |
| 2 | 31 | 5 | 45 |
| 3 | 34 | 6 | 50 |
| Isolates | Diameter of Transparent Zone (D, mm) | Diameter of Colony (d, mm) | Ratio (D/d) | Activity of Proteases (U/mL) |
|---|---|---|---|---|
| AH | 7.5 | 1.1 | 6.82 | 20.05 ± 2.84 d |
| AK | 12.2 | 1.5 | 8.13 | 44.70 ± 3.52 b |
| AL | 10.3 | 1.0 | 10.30 | 62.82 ± 3.24 a |
| AM | 11.6 | 1.2 | 9.67 | 65.21 ± 1.27 a |
| AS | 12.7 | 2.0 | 6.35 | 67.01 ± 2.96 a |
| AT | 11.5 | 1.5 | 7.67 | 30.03 ± 1.01 c |
| AV | 11.6 | 1.6 | 7.25 | 48.49 ± 2.11 b |
| AW | 13.4 | 1.6 | 8.38 | 62.03 ± 0.99 a |
| AY | 10.1 | 1.5 | 6.73 | 21.95 ± 0.14 d |
| Isolates | Acid-Soluble Protein Content (%) | Ammonia Nitrogen Content (mg/100 g) |
|---|---|---|
| Control | 5.96 ± 0.09 i | 95.2 ± 0.3 i |
| AH | 8.41 ± 0.01 c | 115.6 ± 0.4 e |
| AK | 7.61 ± 0.13 d | 108.5 ± 0.7 f |
| AL | 7.00 ± 0.06 g | 129.5 ± 1.3 b |
| AM | 7.24 ± 0.13 g | 115.5 ± 1.1 e |
| AS | 8.78 ± 0.10 b | 125.8 ± 0.9 c |
| AT | 7.83 ± 0.13 de | 119.0 ± 1.2 d |
| AV | 7.23 ± 0.06 g | 133.0 ± 0.7 a |
| AW | 9.06 ± 0.06 a | 105.4 ± 1.4 fh |
| AY | 7.55 ± 0.06 df | 106.8 ± 1.1 f |
| B. subtilis AS 1.398 | 6.42 ± 0.07 h | 109.4 ± 1.5 fg |
| Isolates | Acid-Soluble Protein Content (%) | Ammonia Nitrogen Content (mg/100 g) |
|---|---|---|
| Control | 4.76 ± 0.01 e | 166.6 ± 1.3 f |
| FA | 5.89 ± 0.04 d | 179.3 ± 1.5 e |
| FB | 6.26 ± 0.21 c | 181.5 ± 1.7 d |
| FE | 6.61 ± 0.09 abc | 197.2 ± 0.9 c |
| FG | 6.82 ± 0.04 a | 207.4 ± 1.1 ab |
| FK | 6.48 ± 0.07 bc | 210.8 ± 0.7 a |
| S. cerevisiae S288C | 6.79 ± 0.03 a | 204.0 ± 1.2 b |
| A. oryzae RIB40 | 6.39 ± 0.01 c | 200.6 ± 1.1 bc |
| Number | A (°C) | B (d) | C (mL) | Acid-Soluble Protein Content (%) |
|---|---|---|---|---|
| 1 | 28 | 4 | 40 | 6.99 ± 0.03 a |
| 2 | 28 | 5 | 45 | 7.08 ± 0.02 ab |
| 3 | 28 | 6 | 50 | 7.13 ± 0.03 c |
| 4 | 31 | 4 | 45 | 7.54 ± 0.03 e |
| 5 | 31 | 5 | 50 | 7.63 ± 0.02 a |
| 6 | 31 | 6 | 40 | 7.58 ± 0.01 b |
| 7 | 34 | 4 | 50 | 6.84 ± 0.02 d |
| 8 | 34 | 5 | 40 | 6.88 ± 0.03 e |
| 9 | 34 | 6 | 45 | 6.79 ± 0.03 f |
| k1 | 7.07 | 7.12 | 7.15 | |
| k2 | 7.58 | 7.2 | 7.14 | |
| k3 | 6.84 | 7.17 | 7.2 | |
| R | 0.71 | 0.06 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Li, X.; Zhang, K.; Tang, C.; Li, D.; Kan, Y.; Yao, L. Screening of Protease-Producing Microorganisms and Optimization of Fermentation Processes for the Efficient Preparation of Broussonetia papyrifera Feed. Microorganisms 2025, 13, 2722. https://doi.org/10.3390/microorganisms13122722
Dong Z, Li X, Zhang K, Tang C, Li D, Kan Y, Yao L. Screening of Protease-Producing Microorganisms and Optimization of Fermentation Processes for the Efficient Preparation of Broussonetia papyrifera Feed. Microorganisms. 2025; 13(12):2722. https://doi.org/10.3390/microorganisms13122722
Chicago/Turabian StyleDong, Zixing, Xuehui Li, Kun Zhang, Cunduo Tang, Dandan Li, Yunchao Kan, and Lunguang Yao. 2025. "Screening of Protease-Producing Microorganisms and Optimization of Fermentation Processes for the Efficient Preparation of Broussonetia papyrifera Feed" Microorganisms 13, no. 12: 2722. https://doi.org/10.3390/microorganisms13122722
APA StyleDong, Z., Li, X., Zhang, K., Tang, C., Li, D., Kan, Y., & Yao, L. (2025). Screening of Protease-Producing Microorganisms and Optimization of Fermentation Processes for the Efficient Preparation of Broussonetia papyrifera Feed. Microorganisms, 13(12), 2722. https://doi.org/10.3390/microorganisms13122722







